Abstract
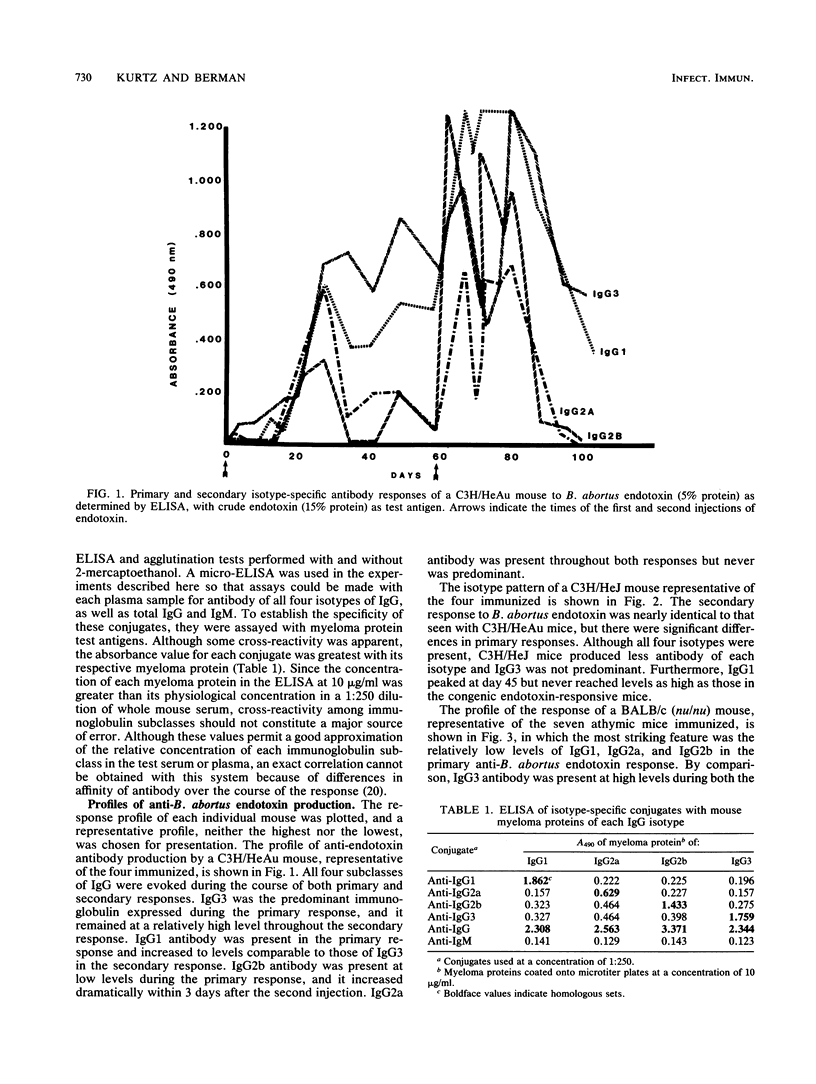
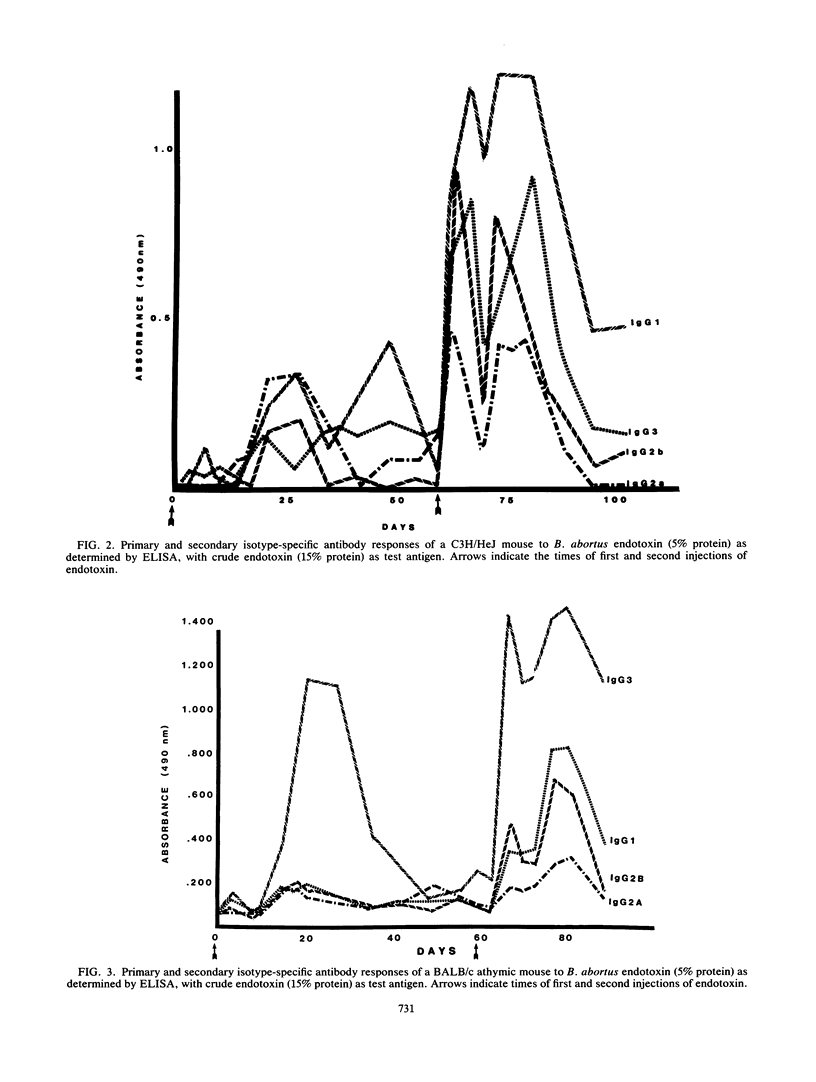
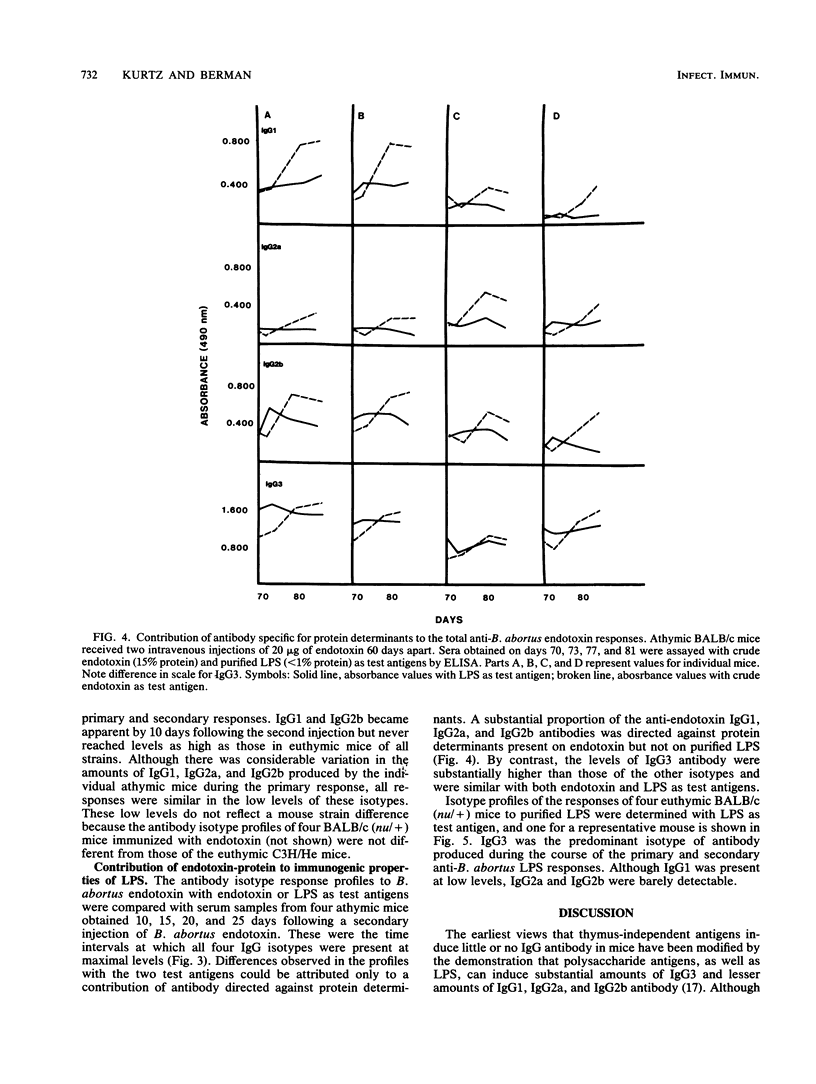
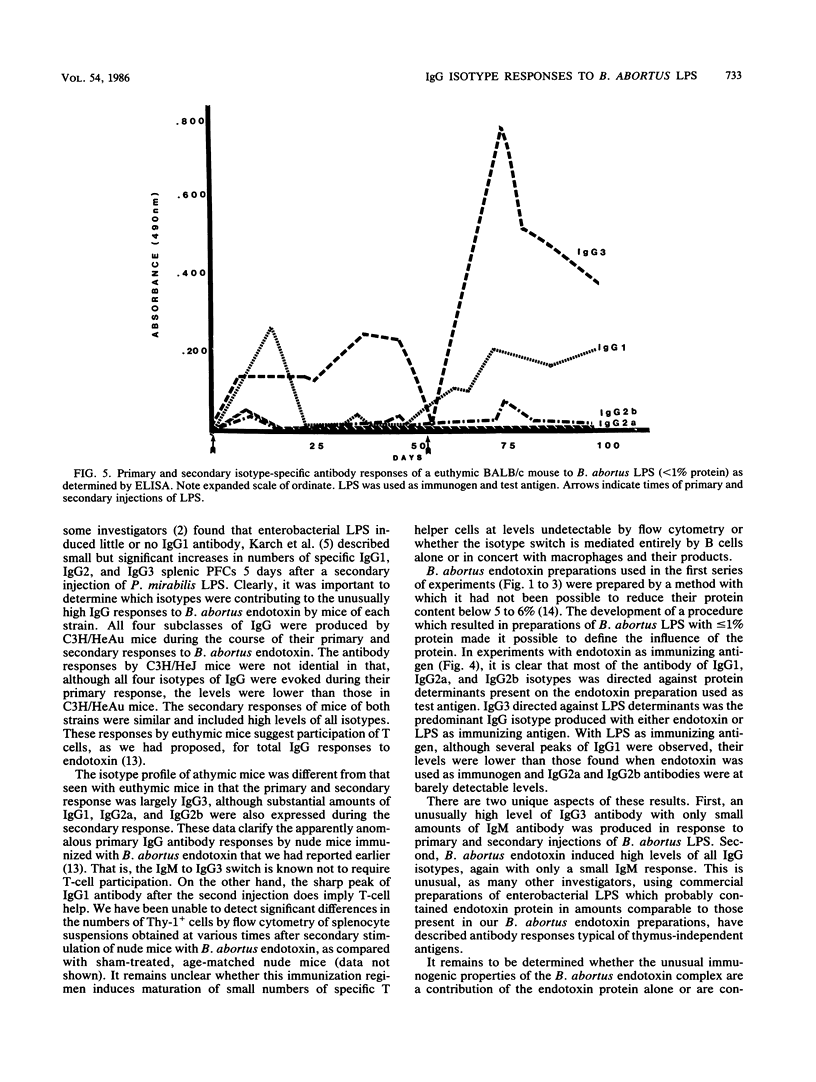
Brucella abortus endotoxin preparations, containing approximately 5 to 6% protein, induce strong immune and adjuvant immunoglobulin G (IgG) responses as compared with Escherichia coli endotoxin preparations, with equivalent amounts of protein, which induce responses in which IgM antibody predominates. Using an enzyme-linked immunoassay with isotype-specific conjugates, we found that antibody of all four subclasses of IgG were evoked during the course of the immune responses of C3H/HeAu mice to B. abortus endotoxin. Secondary responses of endotoxin-hyporesponsive C3H/HeJ mice were similar to those seen in C3H/HeAu mice, although lower levels of antibody were produced during their primary responses. The primary responses of BALB/c athymic mice consisted almost entirely of IgG3, and IgG1 appeared following a second injection. The effects of lipopolysaccharide (LPS)-associated protein on the immunogenic properties of B. abortus endotoxin were examined by comparing responses to endotoxin with those to a purified B. abortus LPS containing less than 1% protein. The endotoxin evoked strong primary and secondary responses in which antibody directed to LPS determinants consisted mainly of IgG3 and those to the protein determinants were largely IgG1 antibody. Primary and secondary responses to purified LPS consisted mainly of IgG3 antibody. The potential mechanism of the contribution of protein to the immunogenic properties of the endotoxin as well as possible immune mechanisms involved in these responses are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Hiernaux J. R., Stashak P. W., Rudbach J. A. Cyclic development of immunological memory to bacterial lipopolysaccharide. Infect Immun. 1985 Apr;48(1):1–6. doi: 10.1128/iai.48.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danneman P. J., Michael J. G. Adjuvant and immunogenic properties of bacterial lipopolysaccharide in IgE and IgG antibody formation in mice. Cell Immunol. 1976 Mar 1;22(1):128–139. doi: 10.1016/0008-8749(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Karch H., Gmeiner J., Nixdorff K. Alteration of the immunoglobulin G subclass responses in mice to lipopolysaccharide: effects of nonbacterial proteins and bacterial membrane phospholipids or outer membrane proteins of Proteus mirabilis. Infect Immun. 1983 Apr;40(1):157–165. doi: 10.1128/iai.40.1.157-165.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb V. L., Jones L. M., Schurig G. G., Berman D. T. Enzyme-linked immunosorbent assay for bovine immunoglobulin subclass-specific response to Brucella abortus lipopolysaccharides. Infect Immun. 1979 Oct;26(1):240–247. doi: 10.1128/iai.26.1.240-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongini P. K., Stein K. E., Paul W. E. T cell regulation of IgG subclass antibody production in response to T-independent antigens. J Exp Med. 1981 Jan 1;153(1):1–12. doi: 10.1084/jem.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T., Boettcher L. A. Biological activities of Brucella abortus lipopolysaccharides. Infect Immun. 1981 Jan;31(1):362–370. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T. Brucella abortus lipopolysaccharide is mitogenic for spleen cells of endotoxin-resistant C3H/HeJ mice. J Immunol. 1979 Dec;123(6):2915–2919. [PubMed] [Google Scholar]
- Moreno E., Kurtz R. S., Berman D. T. Induction of immune and adjuvant immunoglobulin G responses in mice by Brucella lipopolysaccharide. Infect Immun. 1984 Oct;46(1):74–80. doi: 10.1128/iai.46.1.74-80.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Isolation, purification, and partial characterization of Brucella abortus matrix protein. Infect Immun. 1983 Jan;39(1):394–402. doi: 10.1128/iai.39.1.394-402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta I., Portnoï D., Truffa-Bachi P. Induction and differentiation of B memory cells by a thymus-independent antigen, trinitrophenylated lipopolysaccharide. Cell Immunol. 1981 Jan 15;57(2):327–338. doi: 10.1016/0008-8749(81)90091-5. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


