Abstract
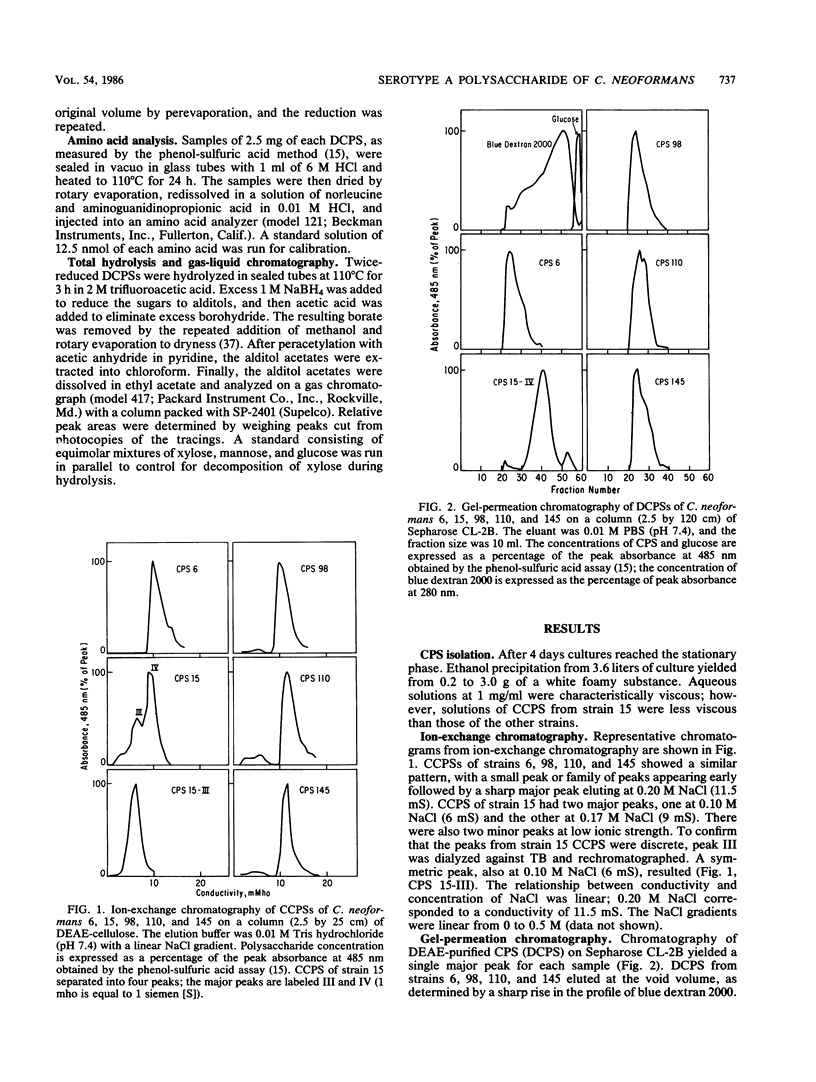
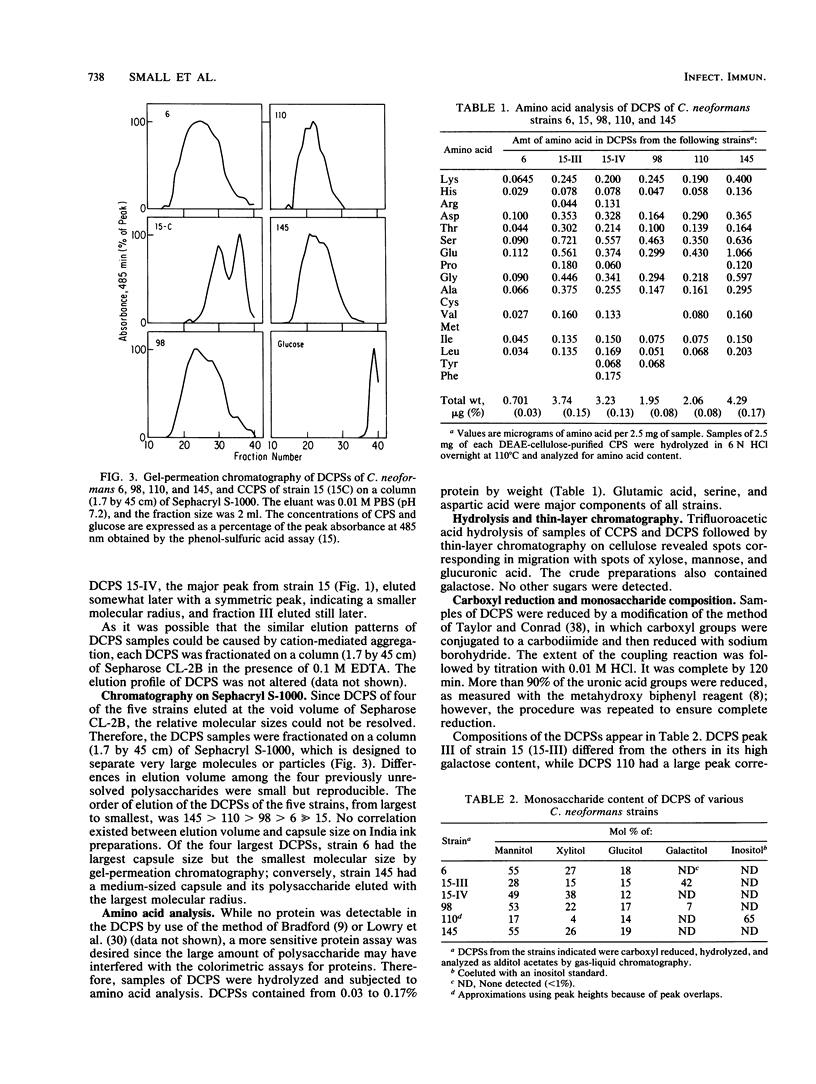
The capsule of Cryptococcus neoformans is an important virulence factor. In this investigation capsular polysaccharides (CPSs) were isolated by ethanol precipitation from culture filtrates of C. neoformans serotype A strains 6, 15, 98, 110, and 145. Capsule sizes on India ink examination ranged from barely perceptible (strain 15) to greater than the diameter of the yeast cell (strain 6); the others were intermediate in size. On ion-exchange chromatography on DEAE-cellulose each CPS eluted at 0.2 M NaCl; CPS of strain 15 had two major peaks, designated III and IV. On gel-permeation chromatography CPSs of strains 6, 98, 110, and 145 eluted at the void volume of Sepharose CL-2B in the presence or 0.1 M EDTA, while the CPS of strain 15 eluted in two peaks. Sephacryl S-1000 resolved CPSs of all five strains in the following order, from largest to smallest molecular size: 145 greater than 110 greater than 98 greater than 6 much greater than 15. All five CPSs contained mannose, xylose, and glucuronic acid, while the carboxyl-reduced CPS of strain 110 also contained a large percentage of an inositol-like compound. The CPS of strain 110 contained approximately 30% uronic acid by weight, while the others had 15 to 20%. The composition of peak IV from the CPS of strain 15 resembled those of the other strains; peak III of strain 15 contained a substantial amount of galactose. Each CPS contained less than 0.2% protein by weight. The significant differences in molecular size and sugar composition among CPSs of these strains of C. neoformans serotype A may partially explain strain differences in virulence and biological properties of the organism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. E., Kwon-Chung K. J., Howard D. H. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am J Epidemiol. 1977 Jun;105(6):582–586. doi: 10.1093/oxfordjournals.aje.a112423. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. Capsular polysaccharides from a parent strain and from a possible, mutant strain of Cryptococcus neoformans serotype A. Carbohydr Res. 1981 Sep 16;95(2):237–248. doi: 10.1016/s0008-6215(00)85580-9. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C--II. Mol Immunol. 1979 Jul;16(7):531–532. doi: 10.1016/0161-5890(79)90081-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C. Immunochemistry. 1978 Sep;15(9):673–679. doi: 10.2196/40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. Structural studies on the major, capsular polysaccharide from Cryptococcus bacillisporus serotype B. Carbohydr Res. 1980 Jun;82(1):103–111. doi: 10.1016/s0008-6215(00)85524-x. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. The structure of the capsular polysaccharide from Cryptococcus neoformans serotype D. Carbohydr Res. 1979 Aug;73:183–192. doi: 10.1016/s0008-6215(00)85488-9. [DOI] [PubMed] [Google Scholar]
- Blandamer A., Danishefsky I. Investigations on the structure of the capsular polysaccharide from Cryptococcus neoformans type B. Biochim Biophys Acta. 1966 Apr 25;117(2):305–313. doi: 10.1016/0304-4165(66)90081-x. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. 3. Inhibition of phagocytosis. J Bacteriol. 1968 Jan;95(1):5–8. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaskes S., Tyndall R. L. Pigment production by Cryptococcus neoformans from para- and ortho-Diphenols: effect of the nitrogen source. J Clin Microbiol. 1975 Jun;1(6):509–514. doi: 10.1128/jcm.1.6.509-514.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak R., Reiss E., Slodki M. E., Plattner R. D., Blumer S. O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol Immunol. 1980 Aug;17(8):1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Dykstra M. A., Friedman L., Murphy J. W. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977 Apr;16(1):129–135. doi: 10.1128/iai.16.1.129-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engster M., Abraham R. Cecal response to different molecular weights and types of carrageenan in the guinea pig. Toxicol Appl Pharmacol. 1976 Nov;38(2):265–282. doi: 10.1016/0041-008x(76)90134-4. [DOI] [PubMed] [Google Scholar]
- Fromtling R. A., Shadomy H. J., Jacobson E. S. Decreased virulence in stable, acapsular mutants of cryptococcus neoformans. Mycopathologia. 1982 Jul 23;79(1):23–29. doi: 10.1007/BF00636177. [DOI] [PubMed] [Google Scholar]
- GOODMAN J. W., KABAT E. A. Immunochemical studies on cross-reactions of antipneumococcal sera. III. The effect of variation in molecular weight on the cross-reactivity of dextran with type II antipneumococcal serum. J Immunol. 1960 Oct;85:342–346. [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Virulence and growth rates of Cryptococcus neoformans in mice. Ann N Y Acad Sci. 1960 Aug 27;89:156–162. doi: 10.1111/j.1749-6632.1960.tb20138.x. [DOI] [PubMed] [Google Scholar]
- Ikeda R., Nishikawa A., Shinoda T., Fukazawa Y. Chemical characterization of capsular polysaccharide from Cryptococcus neoformans serotype A-D. Microbiol Immunol. 1985;29(10):981–991. doi: 10.1111/j.1348-0421.1985.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Kagaya K., Yamada T., Miyakawa Y., Fukazawa Y., Saito S. Characterization of pathogenic constituents of Cryptococcus neoformans strains. Microbiol Immunol. 1985;29(6):517–532. doi: 10.1111/j.1348-0421.1985.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Reiss E., Cherniak R. Concomitant but not causal association between surface charge and inhibition of phagocytosis by cryptococcal polysaccharide. Infect Immun. 1980 Aug;29(2):295–300. doi: 10.1128/iai.29.2.295-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E. Distribution of alpha and alpha mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978 Oct;108(4):337–340. doi: 10.1093/oxfordjournals.aje.a112628. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Popkin T. J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982 Jun;150(3):1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTMAN M. L. Capsule synthesis by Cryptococcus neoformans. Trans N Y Acad Sci. 1958 May;20(7):623–648. doi: 10.1111/j.2164-0947.1958.tb00625.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane D. A., MacGregor I. R., VanRoss M., Cella G., Kakkar V. V. Molecular weight dependence of the anticoagulant properties of heparin: intravenous and subcutaneous administration of fractionated heparins to man. Thromb Res. 1979;16(5-6):651–662. doi: 10.1016/0049-3848(79)90209-3. [DOI] [PubMed] [Google Scholar]
- Mitchell T. G., Friedman L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect Immun. 1972 Apr;5(4):491–498. doi: 10.1128/iai.5.4.491-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C., Polacheck I., Kwon-Chung K. J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982 Jun;36(3):1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A., Taylor I. E. Extracellular glycoprotein from virulent and avirulent Cryptococcus species. Infect Immun. 1981 Mar;31(3):911–918. doi: 10.1128/iai.31.3.911-918.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Tripp C., Ruiz A., Bulmer G. S. Culture of Cryptococcus neoformans in the nonencapsulated state. Mycopathologia. 1981 Dec 11;76(3):129–131. doi: 10.1007/BF00437192. [DOI] [PubMed] [Google Scholar]
- Turner S. H., Cherniak R., Reiss E. Fractionation and characterization of galactoxylomannan from Cryptococcus neoformans. Carbohydr Res. 1984 Feb 15;125(2):343–349. doi: 10.1016/0008-6215(84)85172-1. [DOI] [PubMed] [Google Scholar]



