Abstract
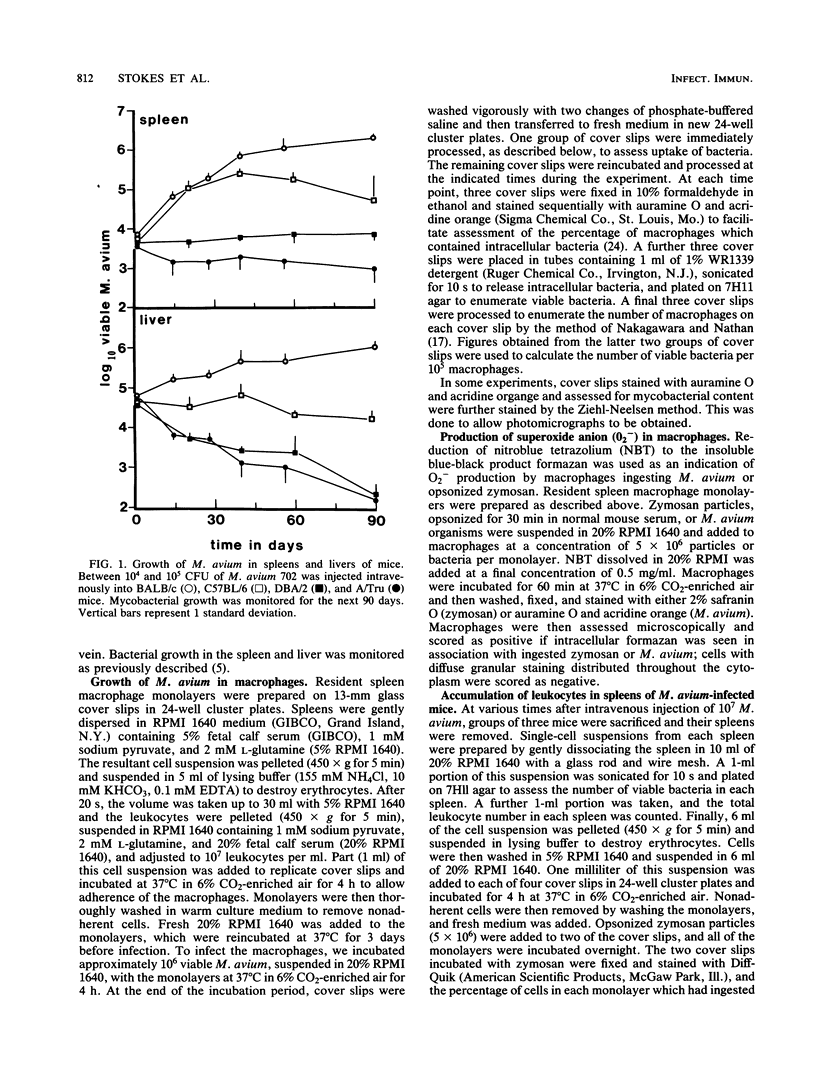
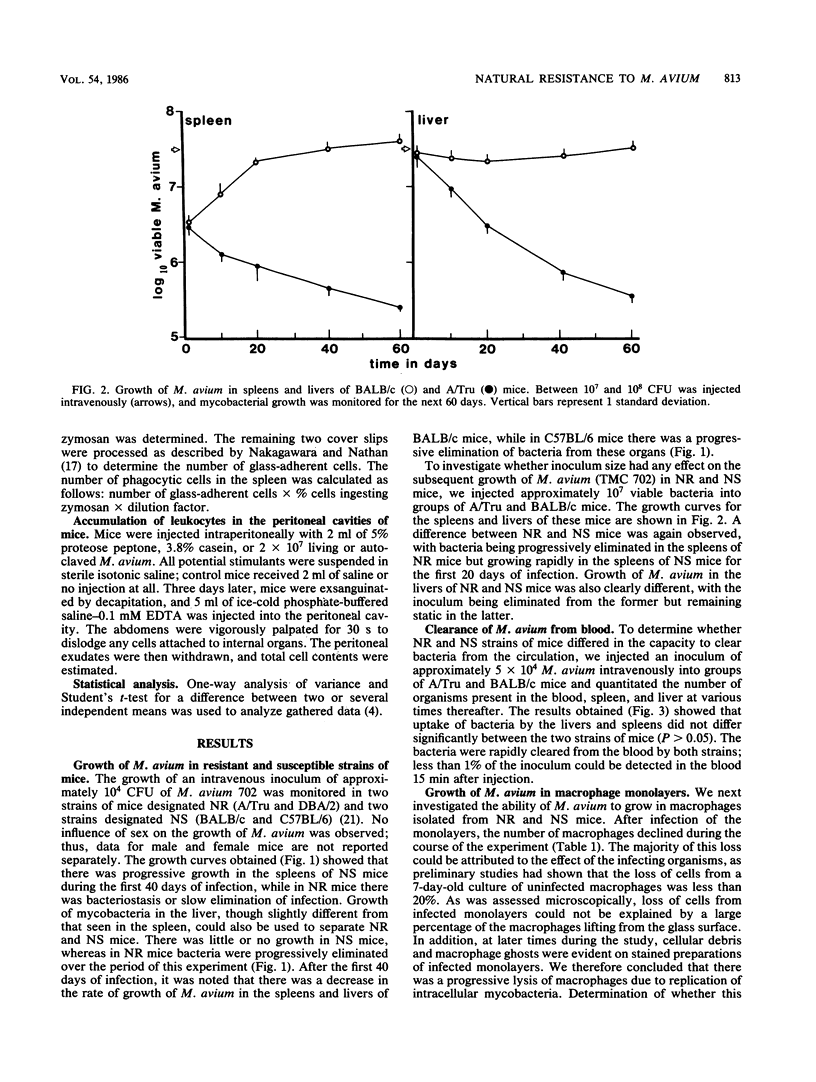
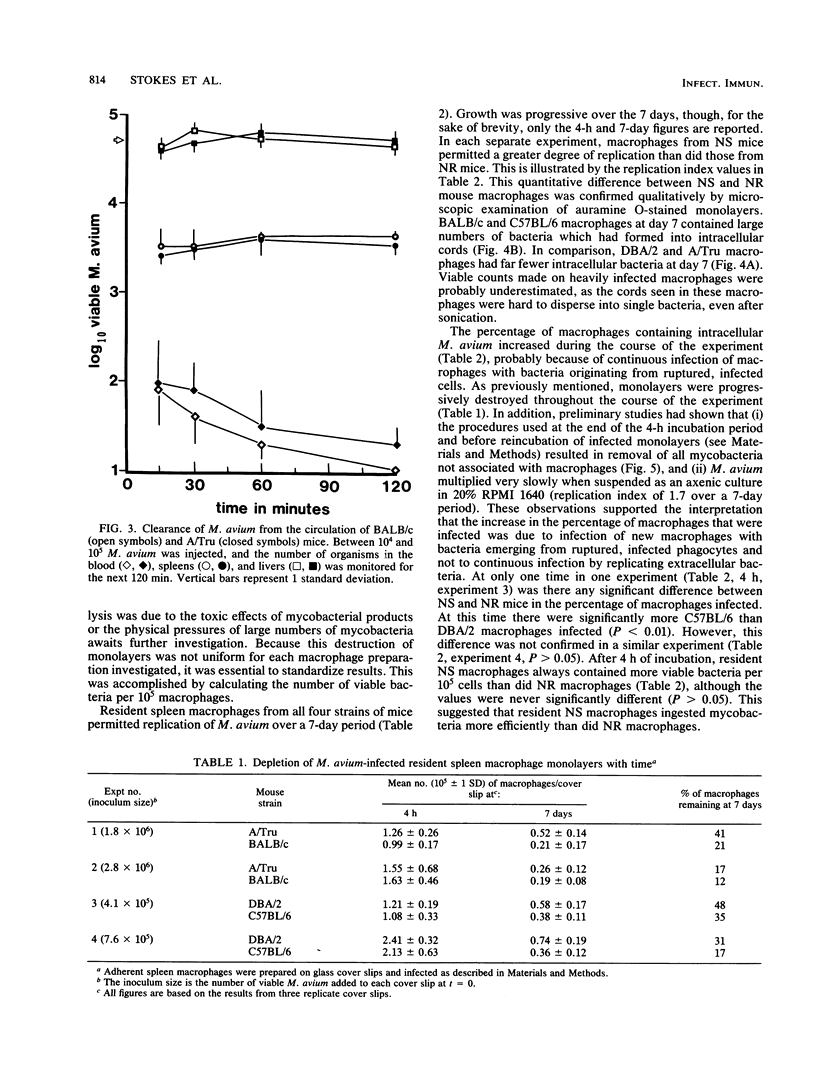
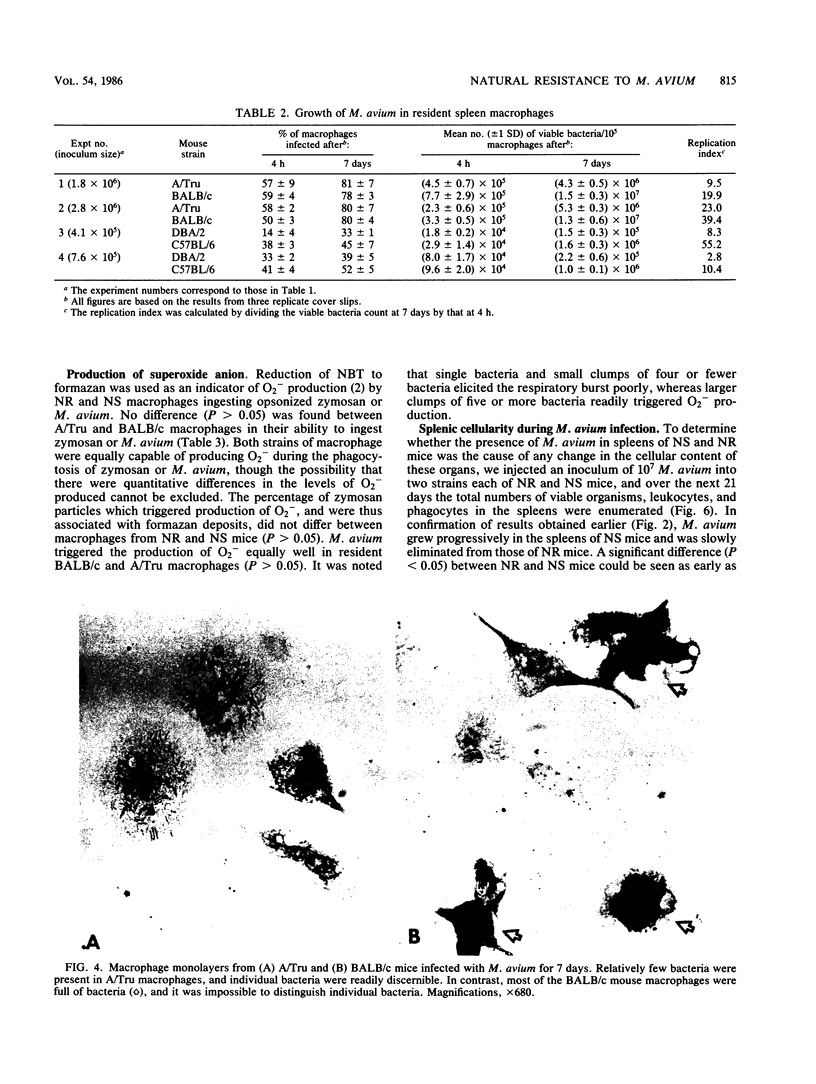
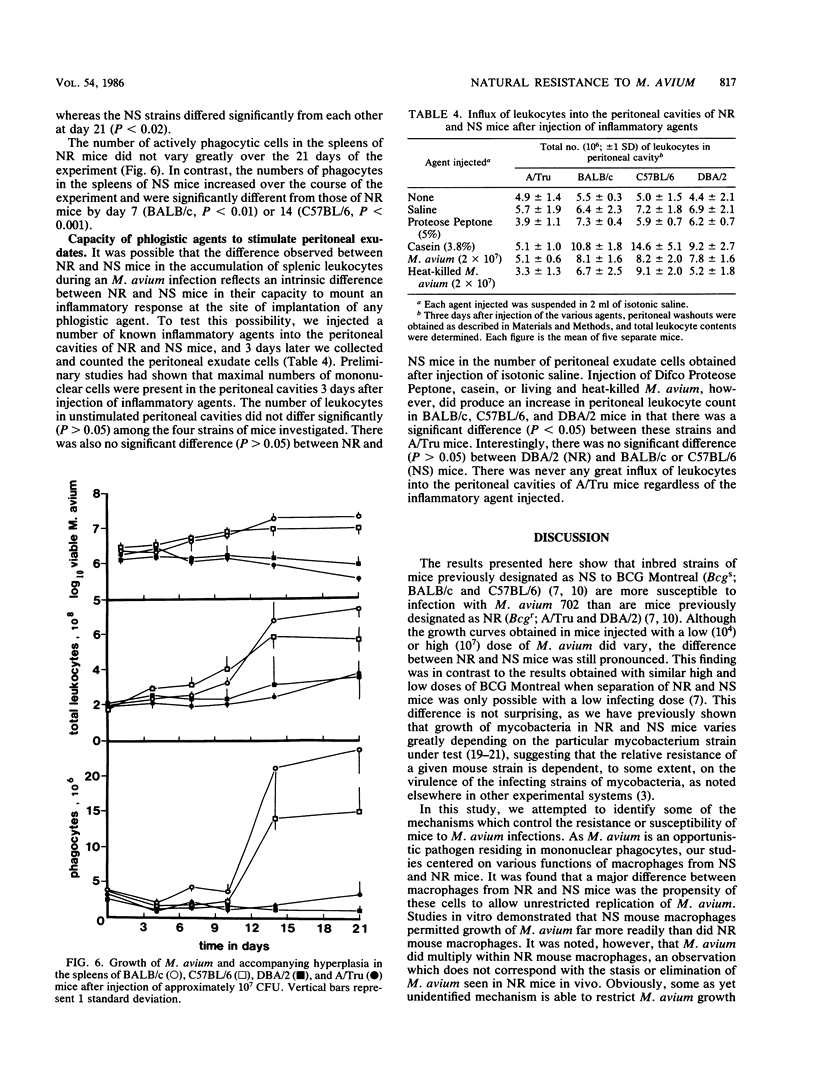
The growth of Mycobacterium avium 702 in the spleens and livers of four inbred strains of mice varied such that the mice could be separated into naturally susceptible (BALB/c and C57BL/6) and naturally resistant (A/Tru and DBA/2) strains. This phenomenon was independent of the size of the infecting inoculum of bacteria in that both low (10(4))- and high (10(7))-dose inocula of M. avium grew progressively in susceptible strains and were eliminated from the target organs of resistant strains. Resistance and susceptibility were also demonstrated in in vitro preparations of macrophages from these strains of mice. Over a 7-day period, replication of M. avium in susceptible mouse macrophages was far greater than that in resistant macrophages. Evidence was obtained to suggest that toxic oxygen metabolites were not responsible for this difference. Though no difference was found in the rate of clearance of M. avium from the blood of susceptible or resistant mice, resident macrophages from susceptible mice ingested more M. avium in vitro than did resident macrophages from resistant animals. Growth of M. avium in spleens of susceptible mice induced a large influx of phagocytes, whereas this was not observed in resistant mice. In contrast to this it was found that, after injection of a variety of inflammatory agents, influx of leukocytes into the peritoneal cavity could not be used to distinguish susceptible and resistant strains of mice.
Full text
PDF
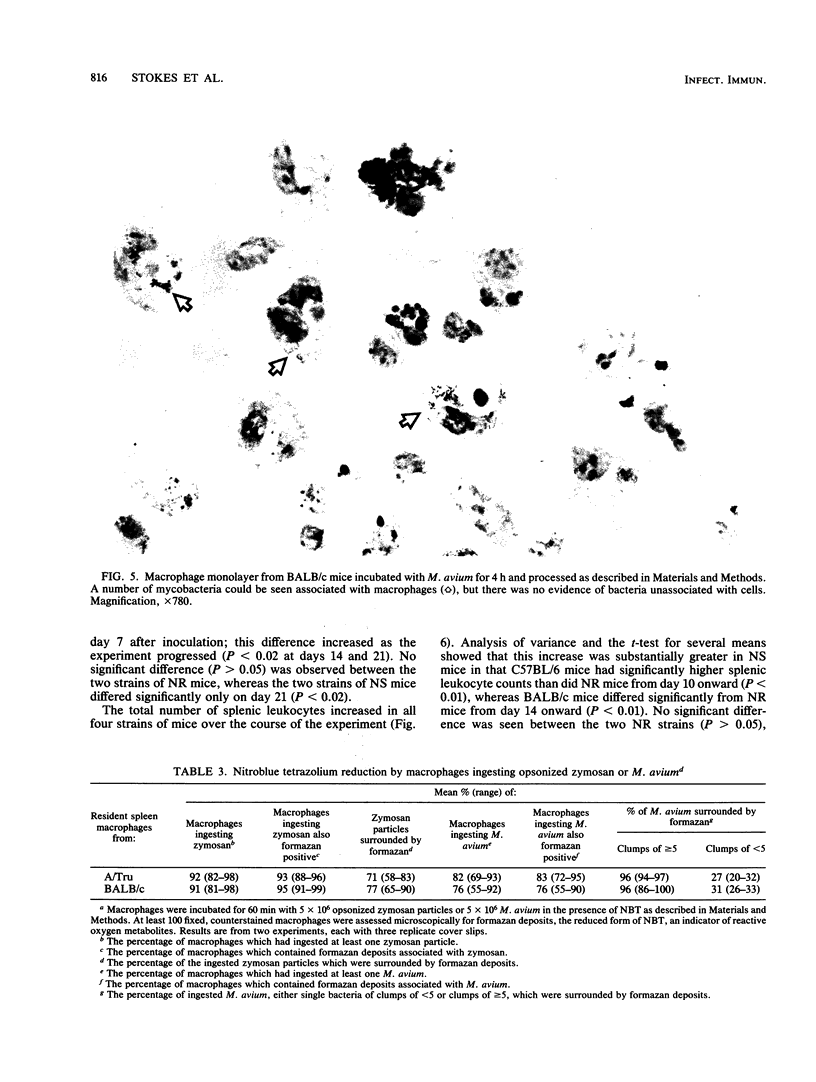


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976 Aug;48(2):309–313. [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nakamura R. M., Takahashi H., Tokunaga T. Genetic control of resistance to Mycobacterium intracellulare infection in mice. Infect Immun. 1984 Oct;46(1):135–140. doi: 10.1128/iai.46.1.135-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Cellular mechanisms of genetically controlled host resistance to Mycobacterium bovis (BCG). J Immunol. 1983 Oct;131(4):1966–1972. [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- Haidaris C. G., Bonventre P. F. A role for oxygen-dependent mechanisms in killing of Leishmania donovani tissue forms by activated macrophages. J Immunol. 1982 Aug;129(2):850–855. [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. The susceptibility of strains of Mycobacterium tuberculosis to catalase-mediated peroxidative killing. J Gen Microbiol. 1980 Dec;121(2):381–386. doi: 10.1099/00221287-121-2-381. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B. How macrophages kill tubercle bacilli. J Med Microbiol. 1983 Feb;16(1):1–12. doi: 10.1099/00222615-16-1-1. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Demonstration of acquired resistance in Bcgr inbred mouse strains infected with a low dose of BCG montreal. Clin Exp Immunol. 1984 Apr;56(1):81–88. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Weidner E., Krahenbuhl J. L. Phagosome acidification blocked by intracellular Toxoplasma gondii. 1985 May 30-Jun 5Nature. 315(6018):416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Smithwick R. W., David H. L. Acridine orange as a fluorescent counterstain with the auramine acid-fast stain. Tubercle. 1971 Sep;52(3):226–231. doi: 10.1016/0041-3879(71)90045-6. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]




