Abstract
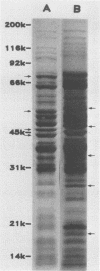
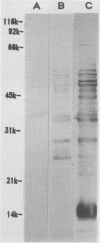
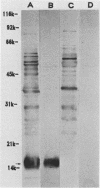
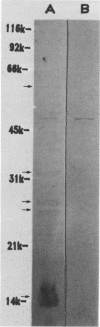
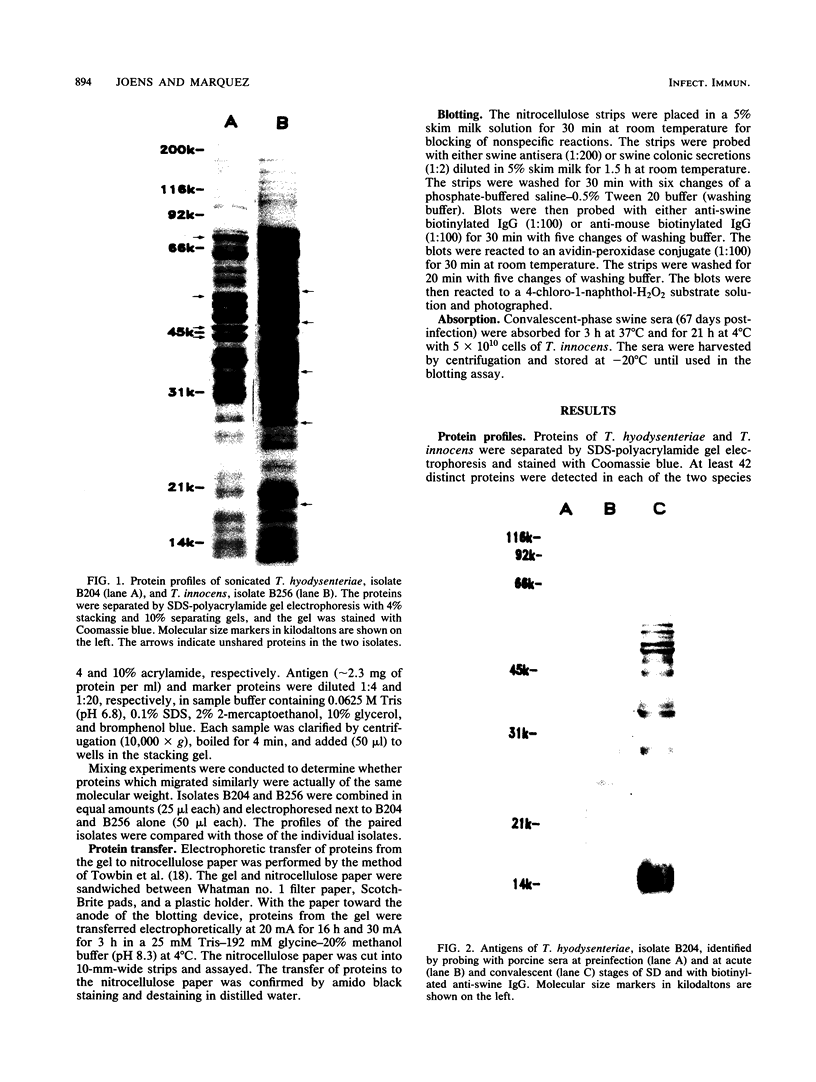
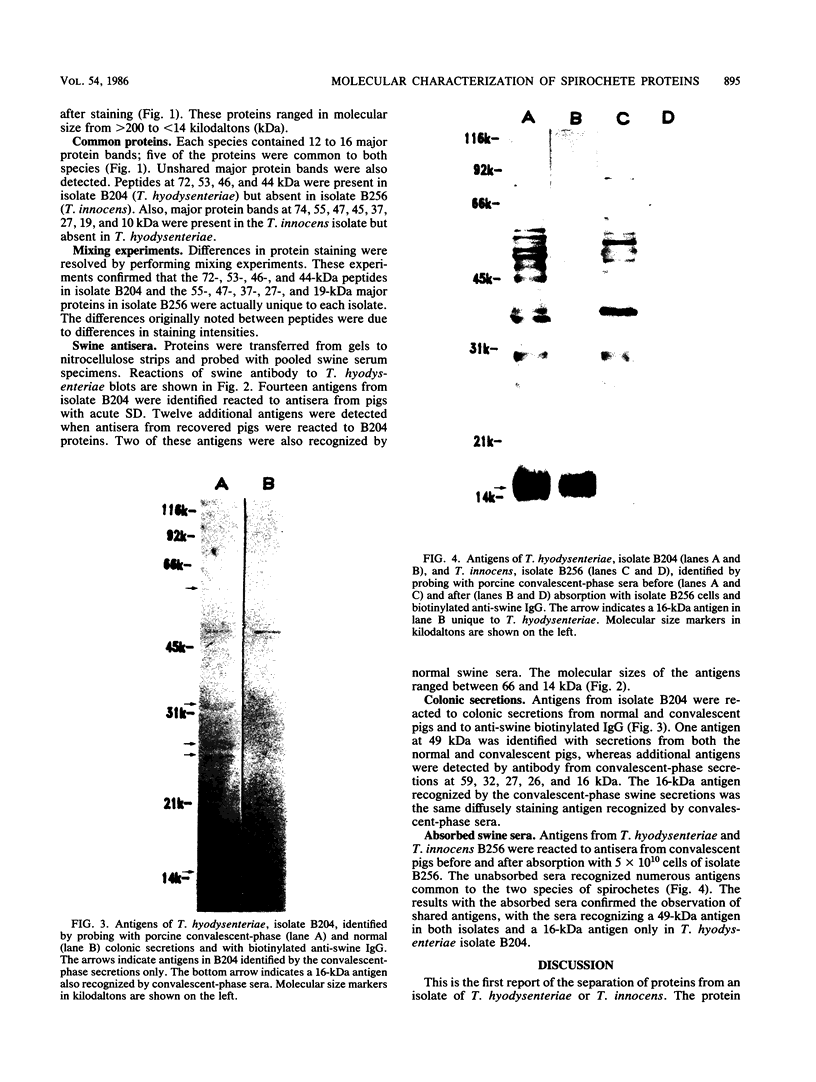
Sonicated preparations of Treponema hyodysenteriae and Treponema innocens were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis. Treponemal proteins were electrophoresed on a 10% polyacrylamide slab gel in a discontinuous Tris-glycine system and either stained with Coomassie blue dye or transferred electrophoretically at 20 mA for 16 h and 30 mA for 3 h to nitrocellulose paper. Staining of the gels revealed at least 42 distinct T. hyodysenteriae and T. innocens proteins, with molecular sizes ranging from greater than 100 to 14 kilodaltons (kDa). Each species contained 12 to 16 major protein bands; five of the proteins were common to both species. Fourteen major antigens were identified in T. hyodysenteriae isolate B204 by using serum specimens from pigs in the acute stage of swine dysentery. Twelve additional antigens were detected in isolate B204 when convalescent-phase serum specimens were reacted to the blot. A wide band at 16 kDa was identified with convalescent-phase serum specimens in T. hyodysenteriae but not in T. innocens. This 16-kDa antigen was also identified in T. hyodysenteriae with colonic secretions from convalescent pigs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. J., Alexander T. J., Lysons R. J. Diagnosis of swine dysentery: spirochaetes which may be confused with Treponema hyodysenteriae. Vet Rec. 1976 Dec 18;99(25-26):498–500. doi: 10.1136/vr.99.25-26.498. [DOI] [PubMed] [Google Scholar]
- Hunter D., Saunders C. N. Diagnosis of swine dysentery using an absorbed fluorescent antiserum. Vet Rec. 1977 Oct 8;101(15):303–304. doi: 10.1136/vr.101.15.303. [DOI] [PubMed] [Google Scholar]
- Joens L. A., DeYoung D. W., Glock R. D., Mapother M. E., Cramer J. D., Wilcox H. E., 3rd Passive protection of segmented swine colonic loops against swine dysentery. Am J Vet Res. 1985 Nov;46(11):2369–2371. [PubMed] [Google Scholar]
- Joens L. A., Glock R. D., Kinyon J. M. Differentiation of Treponema hyodysenteriae from T innocens by enteropathogenicity testing in the CF1 mouse. Vet Rec. 1980 Dec 6;107(23):527–529. [PubMed] [Google Scholar]
- Joens L. A., Harris D. L., Baum D. H. Immunity to Swine dysentery in recovered pigs. Am J Vet Res. 1979 Oct;40(10):1352–1354. [PubMed] [Google Scholar]
- Joens L. A., Harris D. L., Kinyon J. M., Kaeberle M. L. Microtitration agglutination for detection of Treponema hyodysenteriae antibody. J Clin Microbiol. 1978 Sep;8(3):293–298. doi: 10.1128/jcm.8.3.293-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Nord N. A., Kinyon J. M., Egan I. T. Enzyme-linked immunosorbent assay for detection of antibody to Treponema hyodysenteriae antigens. J Clin Microbiol. 1982 Feb;15(2):249–252. doi: 10.1128/jcm.15.2.249-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Whipp S. C., Glock R. D., Neussen M. E. Serotype-specific protection against Treponema hyodysenteriae infection in ligated colonic loops of pigs recovered from swine dysentery. Infect Immun. 1983 Jan;39(1):460–462. doi: 10.1128/iai.39.1.460-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L., Glock R. D. Enteropathogenicity of various isolates of Treponema hyodysenteriae. Infect Immun. 1977 Feb;15(2):638–646. doi: 10.1128/iai.15.2.638-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Picard B., Massicotte L., Saheb S. A. Effet du ribonucléate de sodium sur la croissance et l'activité hémolytique de Treponema hyodysenteriae. Experientia. 1979 Apr 15;35(4):484–486. doi: 10.1007/BF01922721. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]