Abstract
The midsagittal corpus callosum (CC) cross-sectional area subdivisions have been used as early and sensitive markers of human brain white matter connectivity, development, natural aging and disease. Despite the simplicity and conspicuity of the appearance of the CC on anatomical magnetic resonance imaging (MRI), the published quantitative MRI literature on its regional sex and age trajectories are contradictory. The availability of noninvasive quantitative methods to assess the CC regions across the human lifespan would help clarify its contribution to behavior and cognition. In this report, we extended the utility of a recently described semi-automated diffusion tensor imaging (DTI) tissue segmentation method to utilize the high orientation contrast of the CC on DTI. Using optimized DTI methods on a cohort of 121 right-handed children and adults aged 6–68 years, we examined the CC areas and corresponding DTI metrics of the different functionally specialized sectors of the CC. Both the area and fractional anisotropy metrics followed inverted U-shaped curves, while the mean and radial diffusivities followed U-curve reflecting white matter progressive and regressive myelination dynamics that continue into young adulthood.
Keywords: Diffusion tensor imaging, corpus callosum, child, adult, brain development, aging, lifespan, Witelson Corpus Callosum Subdivisions
1. Introduction
The corpus callosum (CC) is the largest interhemispheric fiber network in the human brain (De Lacoste et al., 1985; Cook, 1986; Clarke et al., 1989; Gazzaniga, 2000). The corpus callosum is composed of region-dependent fiber density and myelination levels that reflect its functional specialization (Aboitiz et al., 1992; Highley et al., 1999; Tomasch, 1954; Zaidel and Iacoboni, 2003). The CC has been used as an early and sensitive marker of brain development (Clarke et al., 1989; Georgy et al., 1993; Pandya et al., 1971; Rakic and Yakovlev, 1968), hemispheric lateralization (Witelson and Goldsmith, 1991; Westerhausen et al., 2004; Putnam et al., 2008), function (Bengtsson et al., 2005; Bonzano et al, 2008; Fryer et al, 2008; Klass et al, 1999; Luders et al., 2007; Muetzel et al., 2008; Ringo et al., 1994; Schulte et al., 2005), development (Durston et al., 2001; Rajapakse et al., 1996; Keshavan et al., 2002) and natural aging (Allen et al., 1991; Biegon et al., 1994; Cowell et al., 1992; Johnson et al., 1994; Lebel et al, 2008; Mclaughlin et al., 2007; Rauch and Jinkins, 1994; Stadlbauer et al., 2008). The CC has also been implicated as a surrogate marker of a host of developmental (Alexander et al., 2007; Cascio et al., 2006; Giedd et al., 1994; Highley et al., 1999; Machado et al., 2007; Overmeyer et al., 2000; Plessen et al., 2006; Rotarska-Jagiela et al., 2008; von Plessen et al., 2002), neurodegenerative (Biegon et al., 1994; Cader et al., 2007; Evangelou et al., 2000; Georgy et al., 1993; Hampel et al., 1998; Hasan et al., 2005; Head et al. 2004) and acquired pathologies (Ewing-Cobbs et al., 2006; Estruch et al., 1997; Gupta et al., 2006; Jackowski et al., 2008; Moeller et al., 2005; Pfefferbaum et al., 2007; Wilde et al., 2006; Yu et al., 2008).
Involved in integrating cortico-cortical communication (Cook, 1986; De Lacoste et al., 1985; Lamantia and Rakic 1990; Ringo et al., 1994; Zaidel and Iacoboni, 2003; Wahl et al., 2007), the CC has been subdivided based on its microstructural and functional specialization using different approaches (Alexander et al., 2007; Aboitiz et al., 1992; Cascio et al., 2006; Head et al., 2004; Highley et al., 1999; Hofer and Frahm, 2006; Huang et al. 2005; Rotarska-Jagiela et al., 2008; Shin et al., 2005; Sullivan et al., 2002; Westerhausen et al., 2004; Witelson, 1989; Xu et al., 2006). The seven midsagittal callosal subdivision convention (CC1-CC7) seems to be the most common approach in quantitative magnetic resonance imaging (MRI) studies (Levin et al., 2000; Rajapakse et al., 1996; Overmeyer et al., 2000; von Plessen et al., 2002; Witelson and Goldsmith, 1991). The rostrum (CC1), genu (CC2), and rostral body (CC3) are associated with the units comprising prefrontal and frontal lobe structures. The anterior midbody (CC4), posterior midbody (CC5), isthmus (CC6) and splenium (CC7) are associated with sensorimotor, midtemporal, and occipital parcellation units, respectively (Aboitiz et al., 1992; Witelson, 1989).
The human CC has been the subject of hundreds of articles and reviews regarding its heterogeneous structure and function as well as differences related to sex, development and aging (Bishop and Wahlston 1997; Cook, 1986; Dubb et al., 2003; Zaidel and Iacoboni, 2003). Aside from some discrepancies, the majority of independent MRI publications on children (Alexander et al., 2007; Rajapakse et al., 1996; De Bellis et al., 2001; Lenroot et al., 2007), adults (Davatzikos and Resnick, 1998; Doraiswamy et al, 1991; Johnson et al., 1994; Keshavan et al., 2002; Mitchell et al. 2003; Salat et al., 1997; Sullivan et al., 2001) and lifespan (Allen et al., 1991; Cowell et al. 1992; Hasan et al., 2008; Hayakawa et al., 1989; Pujol et al., 1993; Rauch and Jinkins, 1994; McLaughlin et al., 2007) would predict that the entire CC (eCC) cross-sectional area growth curve follows an inverted U-curve across the human lifespan.
Specific lifespan studies based on quantification of MRI measures of CC development are scant. Major conclusions regarding the CC aging trajectories and gender relations require validation from large populations that include both children and adults. In particular, simultaneous estimation of the corpus callosum regional areas and the corresponding microstructural diffusion tensor metrics across the lifespan would help provide an important baseline for the interpretation of data collected from patients (Hasan et al., 2008).
There have been several quantitative diffusion tensor imaging (DTI) studies of the human corpus callosum using region-of-interest (Chepuri et al, 2002; Pierpaoli et al., 1996; Hasan et al., 2005), fiber tracking (Huang et al., 2005; Xu et al., 2002;), and voxel based morphometric methods (Bengtsson et al., 2005). These studies include samples ranging from in utero (Bui et al., 2006) to preterm (Partridge et al., 2004) and term infants (Dubois et al., 2006), children and adolescents (Hermoye et al., 2006; Mukherjee et al., 2001; Snook et al., 2005;), and adults (Abe et al., 2002; Chepuri et al., 2002; Hasan et al., 2005; Salat et al., 2005; Ota et al., 2006; Sullivan et al., 2006; Westerhausen et al., 2004). To the best of our knowledge, there are three DTI reports on the quantification of the CC across the human lifespan from 5–70 years. These studies showed nonlinear regional age-trajectories of the tensor anisotropy (Hasan et al., 2004; Hasan et al., 2008; McLaughlin et al., 2007).
The main goal of this work is to extend the utility of a DTI-based tissue segmentation methodology described recently (Hasan et al., 2007a; Hasan et al., 2008) to the midsagittal corpus callosum subregions. In addition, we applied the validated methods on a cohort of children and adults to demonstrate spatiotemporal development and gender relations of the CC areas along with the corresponding DTI metrics such as fractional anisotropy and radial diffusivity. We hypothesized that the midsagittal corpus callosum regional development and aging trajectories are best characterized by nonlinear curves across the human lifespan that would consolidate results of prior studies examining the impact of development and aging on the CC area based on conventional MRI and quantitative DTI measurements.
2. Results
2.1 DTI based regional CC Comparisons
Group Mean Values and Nonlinear Trends of Regional CCA, FA, Radial and Mean Diffusivity
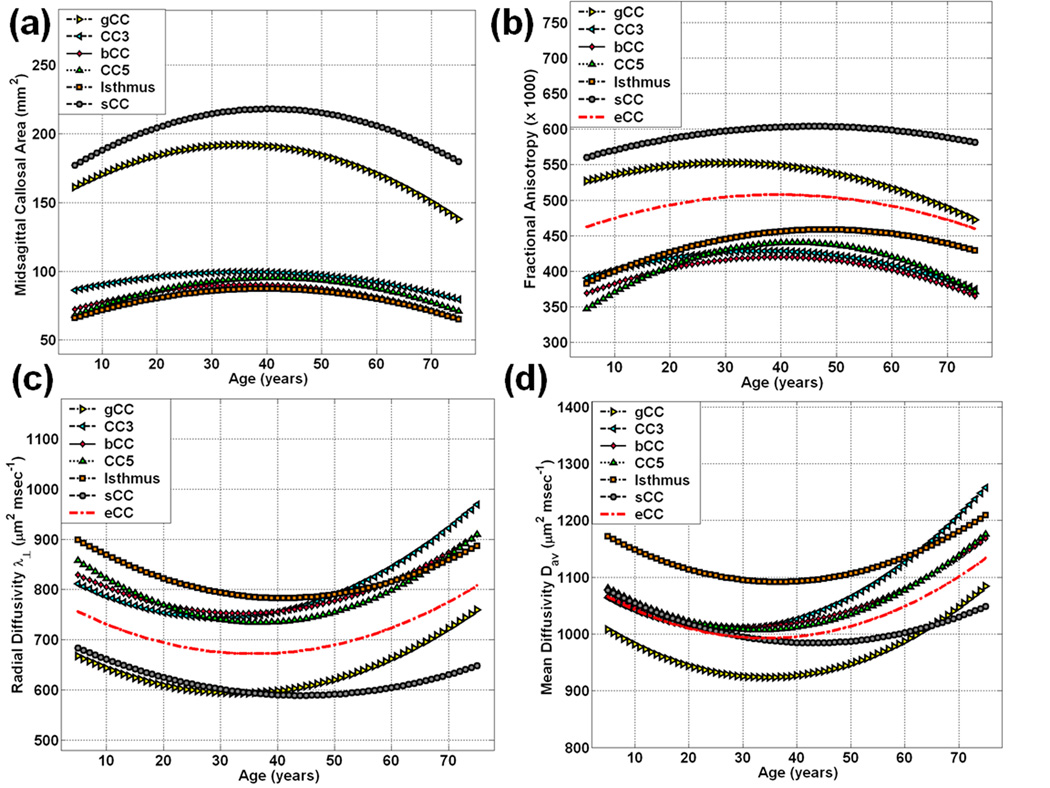
Table 1 summarizes the group mean values of the CC2-CC7& eCC midsagittal areas and corresponding FA, radial and mean diffusivities on both children and adults. Note that comparisons of the mean values between children and adults may provide misleading results due to the fact that simple averaging does not account for nonlinear development and aging trends. To illustrate, Table 2–Table 4 summarize the quadratic curve best fits of the CCA, FA, radial and mean diffusivities. The best fit parameters were used to generate Figure 1 which shows clearly for (a) CCA (b) FA (c) radial and (d) mean diffusivities that across the lifespan, simple averaging and comparisons of data collected on children and adults need to be replaced with statistical models that incorporate the linear and quadratic age effects.
Table 1.
The mean values and standard errors of the DTI-based midsagittal corpus callosum cross sectional (a) areas (mm2) and corresponding (b) fractional anisotropy, (c) radial diffusivities, and (d) mean diffusivities of the CC2-CC7 segments and entire CC.
| Average Regional Corpus Callosum Midsagittal | |||||
|---|---|---|---|---|---|
| Areas and Corresponding DTI Metrics (µ±σ) | |||||
| Area (mm2) | FA (× 1000) | Radial Diffusivity (µm2 msec−1) | Mean Diffusivity (µm2 msec−1) | ||
| CC2 | Child | 173.7±33.8 | 535.2±42.2 | 639.6±59.9 | 975.1±69.7 |
| Adult | 184.8±37.1 | 544.5±38.6 | 612.4±61.4 | 943.3±64.3 | |
| All | 180.8±36.2 | 541.2±40.0 | 622.0±62.0 | 954.6±67.7 | |
| C vs. A (p) | 0.11 | 0.22 | 0.02 | 0.01 | |
| CC3 | Child | 90.6±21.2 | 406.0±42.8 | 778.0±86.9 | 1034.4±91.8 |
| Adult | 97.3±20.3 | 420.9±29.3 | 772.7±83.0 | 1041.7±96.2 | |
| All | 94.9±20.8 | 415.6±35.3 | 774.6±84.1 | 1039.1±94.3 | |
| C vs. A (p) | 0.09 | 0.026 | 0.74 | 0.69 | |
| CC4 | Child | 79.3±14.3 | 388.8±48.7 | 794.7±89.0 | 1040.5±87.1 |
| Adult | 86.7±12.6 | 410.2±35.8 | 773.6±79.4 | 1032.9±90.0 | |
| All | 84.1±13.6 | 402.6±41.9 | 781.1±83.2 | 1035.6±8.7 | |
| C vs. A (p) | 0.004 | 0.007 | 0.18 | 0.66 | |
| CC5 | Child | 77.2±16.2 | 378.8±58.3 | 805.8±132.8 | 1043.7±129.6 |
| Adult | 90.6±14.3 | 424.6±36.0 | 761.7±71.3 | 1031.3±82.0 | |
| All | 85.8±16.2 | 408.3±50.1 | 777.4±99.4 | 1035.7±101.1 | |
| C vs. A (p) | <0.0000001 | 4e-007 | 0.02 | 0.52 | |
| CC6 | Child | 74.6±16.7 | 407.4±67.0 | 848.5±120.5 | 1129.5±118.5 |
| Adult | 82.9±15.1 | 443.9±46.6 | 808.2±95.4 | 1114.4 ±105.6 | |
| All | 79.9±16.1 | 430.9±57.2 | 822.5±106.3 | 1119.8 ±110.1 | |
| C vs. A (p) | 0.006 | 0.0006 | 0.046 | 0.47 | |
| CC7 | Child | 191.8±32.7 | 573.7±43.5 | 652.7±65.6 | 1045.1±71.8 |
| Adult | 210.8±28.8 | 96.6±30.1 | 605.6±54.3 | 1000.6±72.9 | |
| All | 204.1±31.5 | 588.5±36.9 | 622.3±62.5 | 1016.4±75.3 | |
| C vs. A (p) | 0.001 | 0.0009 | 0.00004 | 0.002 | |
| Midsagittal Whole CC | Child | 697.0±99.9 | 478.6±33.8 | 721.5±57.1 | 1033.7±59.4 |
| Adult | 764.5±83.51 | 499.7±21.7 | 691.0±49.6 | 1011.7±56.9 | |
| All | 740.5±95.0 | 492.1±28.3 | 701.8±54.2 | 1019.5±58.5 | |
| C vs. A (p) | 0.0001 | 0.00001 | 0.003 | 0.048 | |
A = Adult; C = Child.
Table 2.
DTI-based callosal areas (mm2) (CC2-CC7 and weighted average across the entire midsagittal CC) fit statistics on males and females.
| Corpus Callosum Midsagittal Areas (mm2) Quadratic | |||||
|---|---|---|---|---|---|
| Least Squares Fit: y=β0+β1*age+β2*age2+η | |||||
| R2 | β0±SD (p) | β1±SD (p) | β2±SD (p) | ||
| CC2 | M | 0.087 | 147.2±17.1 (p*) | 2.80±1.26 (0.03) | −0.042±0.019 (0.03) |
| F | 0.065 | 151.0±15.0 (p*) | 2.15±1.04 (0.04) | −0.029±0.015 (0.06) | |
| M&F | 0.071 | 150.0±11.1 (p*) | 2.39±0.79 (0.003) | −0.034±0.013 (0.004) | |
| M vs. F (p) | 0.87 | 0.69 | 0.61 | ||
| CC3 | M | 0.094 | 78.2±9.7 (p*) | 1.63±0.72 (0.02) | −0.028±0.007 (0.0001) |
| F | 0.032 | 82.5±8.7 (p*) | 0.60±0.60 (0.32) | −0.006±0.009 (0.48) | |
| M&F | 0.037 | 81.9±6.5 (p*) | 0.97±0.46 (0.04) | −0.013±0.007 (0.05) | |
| M vs. F | 0.74 | 0.28 | 0.18 | ||
| CC4 | M | 0.126 | 68.5±6.4 (p*) | 1.282±0.47 (0.009) | −0.019±0.007 (0.01) |
| F | 0.202 | 64.1±5.2 (p*) | 1.32±0.36 (0.0005) | −0.017±0.005 (0.002) | |
| M&F | 0.152 | 66.5±4.0 (p*) | 1.28±0.29 (0.0001) | −0.017±0.004 (0.0001) | |
| M vs. F | 0.60 | 0.94 | 0.81 | ||
| CC5 | M | 0.251 | 58.9±6.7 (p*) | 2.05±0.50 (0.0001) | −0.028±0.007 (0.0001) |
| F | 0.302 | 59.1±6.0 (p*) | 1.57±0.41 (0.0001) | −0.017±0.006 (0.007) | |
| M&F | 0.250 | 59.8±4.5 (p*) | 1.73±0.32 (0.0001) | −0.021±0.005 (0.0001) | |
| M vs. F | 0.98 | 0.46 | 0.23 | ||
| CC6 | M | 0.116 | 61.2±7.2 (p*) | 1.37±0.53 (0.01) | −0.019±0.008 (0.02) |
| F | 0.197 | 57.3±6.4 (p*) | 1.49±0.44 (0.001) | −0.017±0.006 (0.009) | |
| M&F | 0.152 | 59.3±4.7 (p*) | 1.41±0.34 (0.0001) | −0.018±0.005 (0.001) | |
| M vs. F | 0.69 | 0.89 | 0.88 | ||
| CC7 | M | 0.112 | 175.2±13.6 (p*) | 2.49±1.01 (0.02) | −0.038±0.015 (0.01) |
| F | 0.276 | 153.7±12.2 (p*) | 2.89±0.84 (0.001) | −0.030±0.012 (0.02) | |
| M&F | 0.147 | 165.0±9.3 (p*) | 2.62±0.66 (0.0001) | −0.032±0.010 (0.001) | |
| M vs. F | 0.24 | 0.76 | 0.67 | ||
| Midsagittal Whole CC | M | 0.224 | 602.6±39.4 (p*) | 11.30±2.92 (0.0003) | −0.166±0.044 (0.0004) |
| F | 0.289 | 574.2±35.7 (p*) | 10.30±2.47 (0.0001) | −0.120±0.036 (0.001) | |
| M&F | 0.225 | 591.7±26.6 (p*) | 10.45±1.9 (0.00001) | −0.136±0.028 (0.00001) | |
| M vs. F | 0.59 | 0.80 | 0.42 | ||
p*<0.000001, M=Males, F=Females.
Table 4.
Radial diffusivity (µm2 msec−1) of (CC2-CC7 and weighted average across the entire midsagittal CC) fit statistics on males and females.
| Midsagittal Corpus Callosum | |||||
|---|---|---|---|---|---|
| Radial Diffusivity (µm2 msec−1) | |||||
| Quadratic Least Squares Fit: y=β0+β1*age+β2*age2+η | |||||
| R2 | β0±SD (p) | β1±SD (p) | β2±SD (p) | ||
| CC2 | M | 0.258 | 720.5±25.9 (p*) | −8.096±1.921 (0.0001) | 0.121± 0.029 (0.0001) |
| F | 0.125 | 678.6±25.2 (p*) | −4.908±1.746 (0.007) | 0.076±0.025 (0.004) | |
| M&F | 0.174 | 696.4±17.9 (p*) | −6.220±1.281 (0.0001) | 0.094±0.019 (0.0001) | |
| M vs. F (p) | 0.25 | 0.22 | 0.24 | ||
| CC3 | M | 0.214 | 873.6±40.1 (p*) | −9.224±2.971 (0.003) | 0.156±0.044 (0.001) |
| F | 0.109 | 818.3±31.0 (p*) | −4.762±2.148 (0.030) | 0.080±0.031 (0.01) | |
| M&F | 0.149 | 841.4±24.7 (p*) | −6.55±1.764 (0.0001) | 0.110±0.026 (0.000) | |
| M vs. F | 0.28 | 0.23 | 0.16 | ||
| CC4 | M | 0.170 | 906.0±41.4 (p*) | −9.768±3.065 (0.002) | 0.149±0.046 (0.002) |
| F | 0.047 | 820.6±30.8 (p*) | −3.751±2.135 (0.08) | 0.054±0.031 (0.09) | |
| M&F | 0.094 | 857.8±25.2 (p*) | −6.230±1.801 (0.001) | 0.092±0.027 (0.001) | |
| M vs. F | 0.10 | 0.11 | 0.09 | ||
| CC5 | M | 0.132 | 928.3±54.6 (p*) | −11.271±4.041 (0.007) | 0.158±0.060 (0.011) |
| F | 0.166 | 877.7±30.9 (p*) | −7.303±2.135 (0.001) | 0.096±0.031 (0.003) | |
| M&F | 0.137 | 899.0±29.4 (p*) | −8.912±2.100 (0.0001) | 0.121±0.031 (0.0001) | |
| M vs. F | 0.42 | 0.39 | 0.36 | ||
| CC6 | M | 0.075 | 947.6±60.8 (p*) | −8.636±4.502 (0.06) | 0.113±0.067 (0.1) |
| F | 0.171 | 923.1±32.3 (p*) | −6.506±2.232 (0.005) | 0.075±0.033 (0.025) | |
| M&F | 0.105 | 933.1±32.0 (p*) | −7.345±2.287 (0.002) | 0.090±0.034 (0.009) | |
| M vs. F | 0.72 | 0.67 | 0.61 | ||
| CC7 | M | 0.131 | 708.1±29.7 (p*) | −5.613±2.200 (0.01) | 0.072±0.033 (0.03) |
| F | 0.308 | 711.7±21.4 (p*) | −5.429±1.483 (0.001) | 0.056±0.022 (0.01) | |
| M&F | 0.209 | 709.4±17.7 (p*) | −5.443±1.264 (0.0001) | 0.062±0.019 (0.001) | |
| M vs. F | 0.92 | 0.94 | 0.68 | ||
| Weighted Average @ Midsagittal Whole CC | M | 0.250 | 804.9±26.3 (p*) | −8.066±1.948 (0.0001) | 0.120±0.029 (0.0001) |
| F | 0.244 | 772.5±17.3 (p*) | −5.222±1.199 (0.0001) | 0.069±0.017 (0.0001) | |
| M&F | 0.227 | 785.7±15.2 (p*) | −6.343±1.083 (0.0001) | 0.088±0.016 (0.0001) | |
| M vs. F | 0.31 | 0.22 | 0.13 | ||
p*<0.000001, M=Males, F=Females.
Figure 1.
Graphical summary of the fitted curves of the CC2-CC7 (genu = gCC = CC2; bCC = CC4; isthmus = CC6; splenium = sCC = CC7) and entire CC (eCC) on the entire 121 males and females (a) midsagittal areas (b) fractional anisotropy (c) radial diffusivity and (d) mean diffusivity (see Table 1 for the average values on both children and adults).
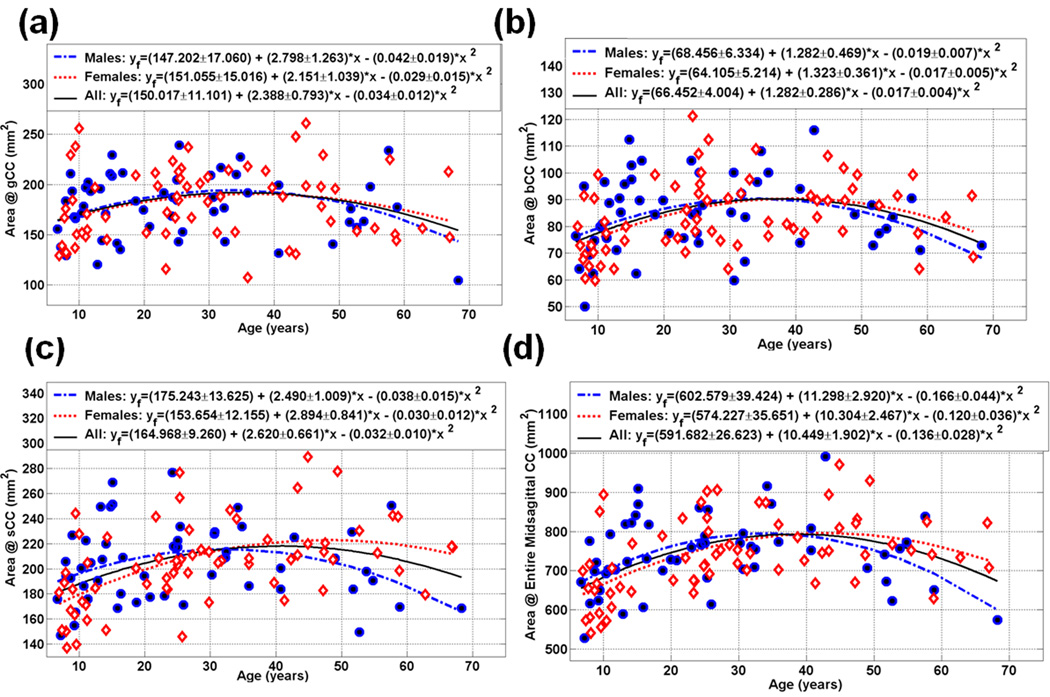
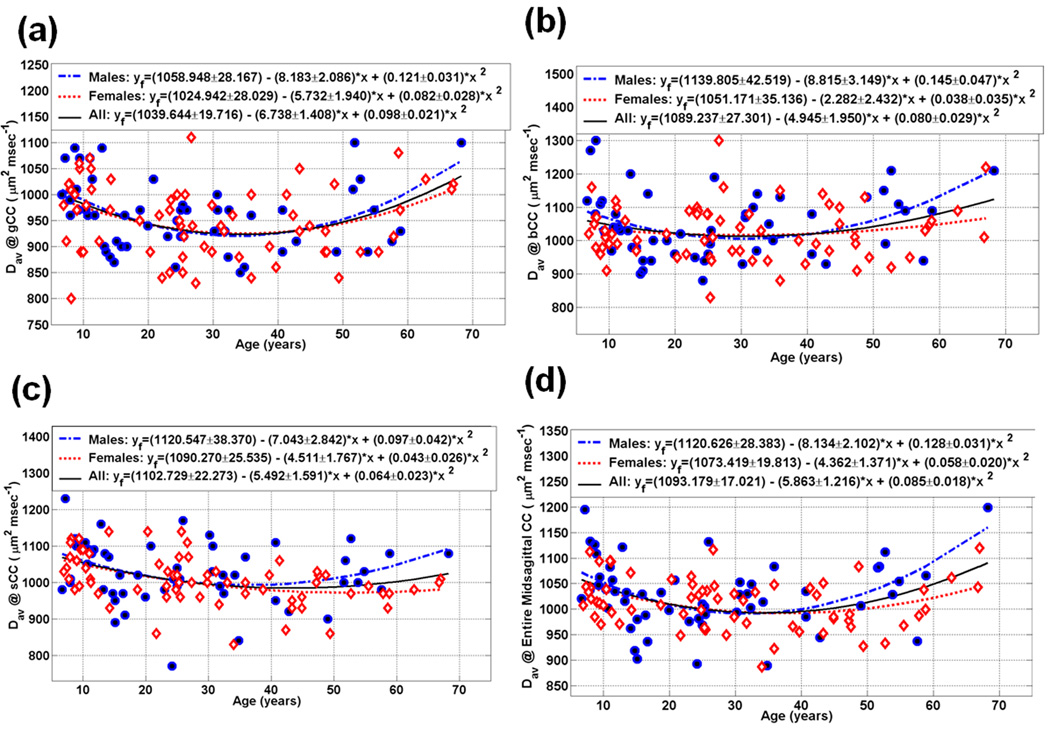
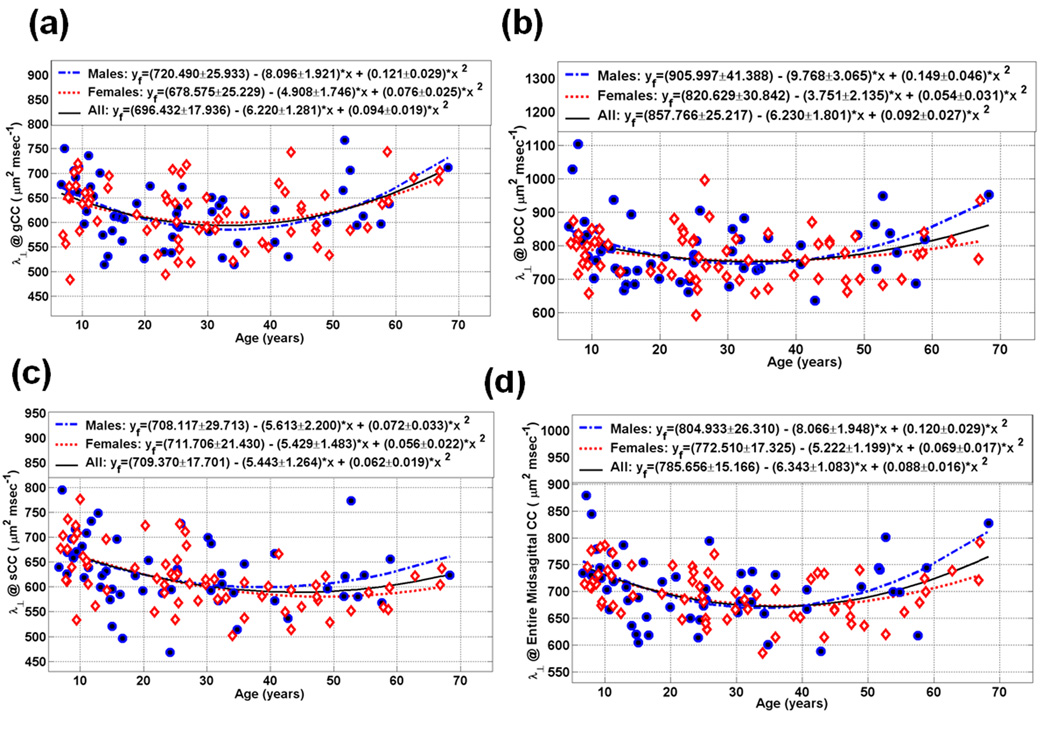
Table 2–Table 5 and Figure 2–Figure 5 summarize our main quantitative results in regards to the callosal subregional areas (CC2-CC7& eCC) and the corresponding DTI metrics’ (FA, radial and mean diffusivities) heterogeneity and their dependence on age and sex using the quadratic models described in the Methods. Note that our measurements are best fit with quadratic curves as function of age (Figure 1; Table 2–Table 4; Figure 2–Figure 4). The fit parameters and age at peak (mean, standard deviation, and significance) statistics for males, females, males vs. females and the pooled sample (males and females) are provided in the Table 2–Table 4 for the segments CC2-CC7 and the entire CC. The entire CC refers to the sum of all CC midsagittal areas and their corresponding area weighted-average DTI metrics.
Table 5.
Mean diffusivity (µm2 msec−1) of (CC2-CC7 and weighted average across the entire midsagittal CC) fit statistics on males and females.
| Corpus Callosum Midsagittal | |||||
|---|---|---|---|---|---|
| Mean Diffusivity (µm2 msec−1) | |||||
| Quadratic Least Squares Fit: y=β0+β1*age+β2*age2+η | |||||
| R2 | β0±SD (p) | β1±SD (p) | β2±SD (p) | ||
| CC2 | M | 0.230 | 1058.9±28.2 (p*) | −8.183±2.086 (0.0001) | 0.121±0.031 (0.0001) |
| F | 0.122 | 1024.9±28.0 (p*) | −5.732±1.940 (0.007) | 0.082±0.028 (0.004) | |
| M&F | 0.163 | 1039.6±19.7 (p*) | −6.738±1.408 (0.0001) | 0.098±0.021 (0.0001) | |
| M vs. F (p) | 0.39 | 0.39 | 0.36 | ||
| CC3 | M | 0.237 | 1126.6±43.5 (p*) | −8.889±3.222 (0.008) | 0.162±0.048 (0.001) |
| F | 0.102 | 1066.0±35.6 (p*) | −4.151±2.467 (0.10) | 0.077±0.036 (0.04) | |
| M&F | 0.150 | 1091.6±27.7 (p*) | −6.047±1.977 (0.003) | 0.110±0.029 (0.0001) | |
| M vs. F | 0.28 | 0.25 | 0.16 | ||
| CC4 | M | 0.164 | 1139.8±42.5 (p*) | −8.815±3.149 (0.007) | 0.145±0.047 (0.003) |
| F | 0.020 | 1051.2±35.1 (p*) | −2.282±2.432 (0.35) | 0.038±0.035 (0.29) | |
| M&F | 0.065 | 1089.2±27.3 (p*) | −4.945±1.950 (0.001) | 0.080±0.029 (0.006) | |
| M vs. F | 0.11 | 0.10 | 0.07 | ||
| CC5 | M | 0.092 | 1144.0±54.9 (p*) | −8.837±4.066 (0.03) | 0.139±0.061 (0.03) |
| F | 0.053 | 1084.8±35.4 (p*) | −4.515±2.449 (0.07) | 0.067±0.036 (0.06) | |
| M&F | 0.067 | 1110.3±31.1 (p*) | −6.278±2.221 (0.006) | 0.095±0.033 (0.004) | |
| M vs. F | 0.37 | 0.36 | 0.36 | ||
| CC6 | M | 0.065 | 1239.7±64.9 (p*) | −8.886±4.803 (0.07) | 0.136±0.072 (0.06) |
| F | 0.063 | 1167.8±32.8 (p*) | −3.786±2.268 (0.10) | 0.044±0.033 (0.19) | |
| M&F | 0.048 | 1199.0±34.2 (p*) | −5.853±2.443 (0.02) | 0.080±0.036 (0.03) | |
| M vs. F | 0.32 | 0.34 | 0.24 | ||
| CC7 | M | 0.109 | 1120.5±38.4 (p*) | −7.043±2.842 (0.02) | 0.097±0.042 (0.03) |
| F | 0.212 | 1090.3±25.5 (p*) | −4.511±1.767 (0.01) | 0.043±0.026 (0.01) | |
| M&F | 0.137 | 1102.7±22.3 (p*) | −5.492±1.591 (0.001) | 0.064±0.023 (0.01) | |
| M vs. F | 0.51 | 0.45 | 0.28 | ||
| Weighted Average @ Midsagittal Whole CC | M | 0.243 | 1120.6±28.4 (p*) | −8.134±2.102 (0.0001) | 0.128±0.031 (0.0001) |
| F | 0.143 | 1073.4±19.8 (p*) | −4.362±1.371 (0.002) | 0.058±0.020 (0.005) | |
| M&F | 0.166 | 1093.2±17.0 (p*) | −5.863±1.216 (0.0001) | 0.085±0.018 (0.0001) | |
| M vs. F | 0.18 | 0.14 | 0.07 | ||
Figure 2.
Scatter plot of the measured and fitted data of the midsagittal area (mm2) as function of age for the (a) gCC (b) bCC (c) sCC and (d) the entire CC (Note the quadratic dependence of CCA vs. age; see also Table 2).
Figure 5.
Scatter plot of the measured and fitted data of the midsagittal callosal subdivisions mean diffusivity as function of age for the (a) gCC (b) bCC (c) sCC, and (d) the entire CC (see Table 5)
Figure 4.
Scatter plot of the measured and fitted data of the midsagittal callosal subdivisions radial diffusivity as function of age for the (a) gCC (b) bCC (c) sCC, and (d) the entire CC (see Table 4).
2.2 Regional Callosal Area Heterogeneity, Age, and Sex Effects
Table 2 summarizes the main results of our work on the fitted callosal midsagittal areas (in mm2) and the entire CC. As a representative graphical illustration, Fig. 2 (a, b, c, and d) show the genu (CC2 = gCC), anterior midbody (CC4 = bCC), splenium (CC7 = sCC) and the entire corpus callosum (eCC) age trajectories for both males and females, respectively.
2.3 Regional Callosal Fractional Anisotropy Heterogeneity, Age and Sex Effects
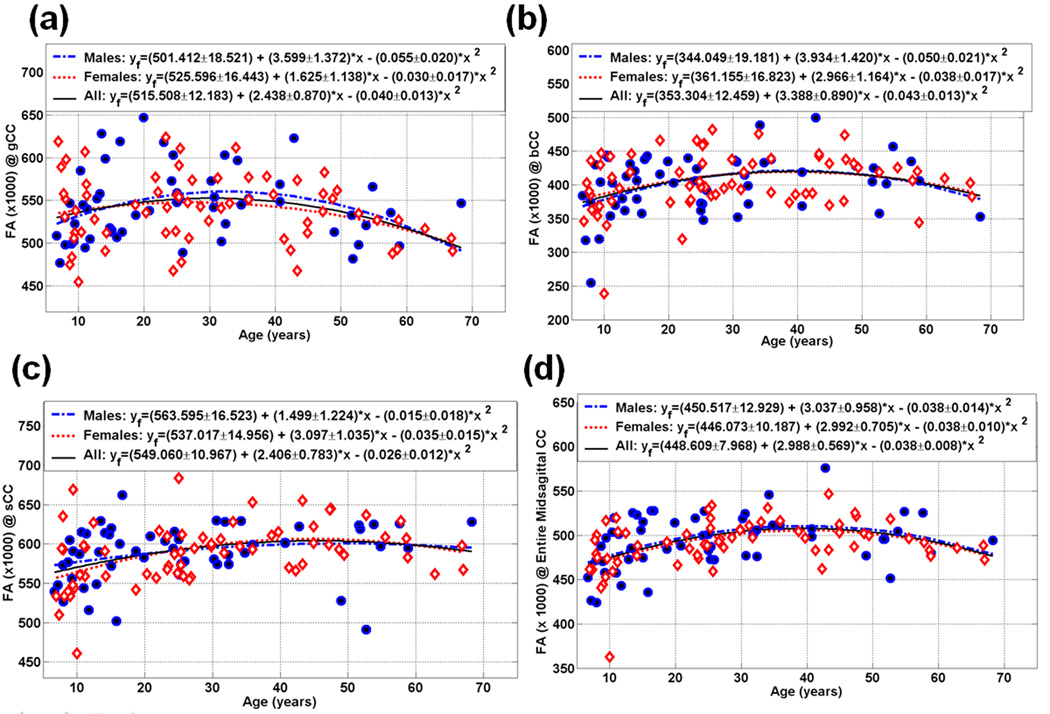
Table 3 details the age trajectory and age at peak for the callosal FA of the midsagittal areas and the entire CC. Figure 3 (a, b, c, and d) show the FA of the genu (CC2 = gCC), anterior midbody (CC4 = bCC), splenium (CC7 = sCC) and entire CC (eCC) age trajectories, respectively. The FA callosal trajectories followed an inverted U curve for both males and females. Note the diffusion tensor anisotropy heterogeneity trend FA(CC7) > FA(CC2) > FA (CC4) (see Figure 3). For example, a statistical comparison of FA(CC5) and FA(CC7) of the intercept, linear and quadratic parameters in Table 5 provides p=0.0000, 0.01 and 0.03, respectively. A detailed quantitative listing of all possible paired comparisons on all variables in this study is not provided in the current manuscript (see Figure 1 for a graphical summary)
Table 3.
Fractional anisotropy of (CC2-CC7 and weighted average across the entire midsagittal CC) fit statistics on males and females.
| Corpus Callosum Midsagittal | |||||
|---|---|---|---|---|---|
| Fractional Anisotropy (× 1000) | |||||
| Quadratic Least Squares Fit: y=β0+β1*age+β2*age2+η | |||||
| R2 | β0±SD (p) | β1±SD (p) | β2±SD (p) | ||
| CC2 | M | 0.121 | 501.4±18.5 (p*) | 3.599±1.372 (0.01) | −0.055±0.020 (0.01) |
| F | 0.072 | 525.6±16.4 (p*) | 1.625±1.138 (0.16) | −0.030±0.017 (0.08) | |
| M&F | 0.084 | 515.5±12.2 (p*) | 2.438±0.870 (0.006) | −0.040±0.013 (0.002) | |
| M vs. F (p) | 0.33 | 0.27 | 0.35 | ||
| CC3 | M | 0.123 | 373.1±17.1 (p*) | 3.260±1.263 (0.01) | −0.044±0.019 (0.03) |
| F | 0.095 | 380.7±13.8 (p*) | 2.430±0.952 (0.01) | −0.033±0.014 (0.02) | |
| M&F | 0.105 | 377.9±10.6 (p*) | 2.760±0.759 (0.0001) | −0.037±0.011 (0.001) | |
| M vs. F | 0.73 | 0.60 | 0.65 | ||
| CC4 | M | 0.156 | 344.0±19.2 (p*) | 3.934±1.420 (0.008) | −0.050±0.021 (0.02) |
| F | 0.105 | 361.2±16.8 (p*) | 2.966±1.164 (0.01) | −0.038±0.017 (0.03) | |
| M&F | 0.128 | 353.3±12.5 (p*) | 3.388±0.890 (0.0001) | −0.043±0.013 (0.001) | |
| M vs. F | 0.50 | 0.60 | 0.65 | ||
| CC5 | M | 0.303 | 323.7±20.7 (p*) | 5.768±1.531 (0.0001) | −0.068±0.023 (0.004) |
| F | 0.333 | 316.5±17.5 (p*) | 5.598±1.211 (0.0001) | −0.065±0.018 (0.0001) | |
| M&F | 0.312 | 320.8±13.2 (p*) | 5.626±0.944 (0.0001) | −0.066±0.014 (0.0001) | |
| M vs. F | 0.79 | 0.93 | 0.93 | ||
| CC6 | M | 0.165 | 388.1±26.3 (p*) | 2.555±1.950 (0.20) | −0.017±0.029 (0.57) |
| F | 0.220 | 344.1±20.9 (p*) | 4.936±1.447 (0.001) | −0.057±0.021 (0.009) | |
| M&F | 0.168 | 364.4±16.6 (p*) | 3.917±1.186 (0.001) | −0.041±0.017 (0.02) | |
| M vs. F | 0.19 | 0.33 | 0.27 | ||
| CC7 | M | 0.068 | 563.6±16.5 (p*) | 1.499±1.224 (0.23) | −0.015±0.018 (0.41) |
| F | 0.192 | 537.0±15.0 (p*) | 3.097±1.035 (0.004) | −0.035±0.015 (0.03) | |
| M&F | 0.130 | 549.0±11.0 (p*) | 2.406±0.783 (0.003) | −0.026±0.012 (0.02) | |
| M vs. F | 0.24 | 0.32 | 0.41 | ||
| Weighted Average @ Midsagittal Whole CC | M | 0.197 | 450.5±12.9 (p*) | 3.037±0.958 (0.003) | −0.038±0.014 (0.01) |
| F | 0.253 | 446.1±10.2 (p*) | 2.992±0.705 (0.0001) | −0.038±0.010 (0.001) | |
| M&F | 0.220 | 448.6±8.0 (p*) | 2.988±0.569 (0.0001) | −0.038±0.008 (0.0001) | |
| M vs. F | 0.79 | 0.97 | 0.96 | ||
p*<0.000001, M=Males, F=Females.
Figure 3.
Scatter plot of the measured and fitted data of the midsagittal callosal subdivisions fractional anisotropy (1000 × FA) as function of age for the (a) gCC (b) bCC , (c) sCC and (d) the entire CC (Note the inverted-U quadratic dependence of FA vs. age; see also Table 3).
2.4 Regional Callosal Radial and Mean Diffusivities Heterogeneity, Age and Sex Effects
Callosal radial and mean diffusivities of the midsagittal areas and the entire CC are depicted in Table 4–Table 5. Figure 4–Figure 5 (a, b, c, and d) show the radial and mean diffusivities of the gCC, bCC, sCC and eCC age trajectories, respectively. The average radial and mean diffusivity callosal trajectories followed a U curve for both males and females.
3. Discussion
The corpus callosum offers one of the largest and most studied compact white matter systems to model using noninvasive MRI methods (Bartzokis et al., 2004; Caviness et al., 1996; Hasan et al., 2008; Peters and Sethares, 2002). The MRI literature on the CC is extremely discordant in regards to age-related growth rates, sex and lateralization effects (Bishop and Wahlsten, 1997; Durston et al., 2001; Clarke et al., 1989). In this study, we focused on right-handed healthy controls to avoid possible confounding effects of handedness (Westerhausen et al., 2004; Witelson and Goldsmith, 1991).
This is the first report using an entirely DTI-based semiautomated and validated approach to segment the CC using DTI-derived and co-registered scalar and vector metrics on a cohort of healthy controls aged 6–68 years. The CC segmentation approach implemented in this work extends our previous DTI-based tissue segmentation approach in which scalar and rotation-invariant DTI-derived metrics have been used to partition the brain into white matter, gray matter and cerebrospinal fluid (Hasan et al., 2007a; Hasan et al., 2008).
In this work, the diffusion anisotropy combined with the excellent orientation contrast of the CC has been used to isolate the CC upon careful identification of the midsagittal section (Hasan et al., 2005; Hasan et al., 2008; Kanabar et al., 2005). Our DTI data were acquired using high signal-to-noise ratios and high spatial resolution that minimized diffusion tensor estimation biases (Hasan, 2007; Pierpaoli et al., 1996) and partial volume averaging (Alexander et al., 2001; Pfefferbaum et al., 2003). Due to its potential relation with regional functional specificity (Aboitiz et al., 1992; Highley et al., 1999; Witelson, 1985), the callosal subdivision paradigm implemented in this work has been adopted by several conventional MRI (Rajapakse et al., 1996; Levin et al., 2000) and DTI studies (Cascio et al., 2007; Moeller et al., 2005; Hasan et al., 2005).
3.1 Regional Midsagittal Corpus Callosum Anisotropy Heterogeneity
Our DTI results reproduce commonly reported significant findings, including that the human corpus callosum diffusion anisotropy is higher in the posterior CC than in other brain regions, and that anisotropy is greater in posterior than anterior CC regions in both males and females at all ages (see Figure 3). These trends have been reported by several previous DTI reports on healthy children (Alexander et al., 2007; Snook et al., 2005) and adults (Abe et al., 2002; Chepuri et al., 2002; Hasan et al., 2005; Head et al., 2004; Madden et al., 2004; Ota et al., 2006; Peled et al., 1998; Pfefferbaum et al., 2000; Salat et al., 2005; Sullivan et al., 2006). It is noteworthy that some studies did not report this trend on healthy controls (Foong et al., 2000; Plessen et al., 2006; Schulte et al., 2005). The explanation of the diffusion anisotropy heterogeneity trend FA(CC7) > FA(CC2) in terms of axonal packing, axonal microstructure, geometry and myelination is an important, but unresolved challenging problem in MRI that enquires more modeling and histological correlations (Aboitiz et al., 1992; Beaulieu, 2002; Hasan et al., 2005; Hasan et al., 2008; Highley et al., 1999; Pierpaoli et al., 1996; Vorisek and Sykova, 1997).
3.2 Age Effects
Our results (see summary in Figure 1; Table 2–Table 5; Figure 2–Figure 5) show that the growth trajectories of CC subdivisions are nonlinear and vary with region at the macrostructural (CCA) and microstructural (FA, radial diffusivity) levels. The nonlinear growth rates of CCA confirm earlier reports predicting nonlinear growth curves of the CC (Allen et al., 1991; Cowell et al., 1992; Hayakawa et al., 1989; Pujol et al., 1993; Rauch and Jinkins, 1994). The quadratic trajectories obtained across the lifespan provide more complete information than linear curves obtained in samples with restricted age ranges that predict constant growth rates of the corpus callosum areas as has been described on studies using children (De Bellis et al., 2001; Lenroot et al., 2007; Rajapakse et al., 1996), young adults (Keshavan et al., 2002) and older adults (Salat et al., 1997; Sullivan et al., 2001).
Our DTI results on both children and adults agree well with recent DTI publications that show increasing CC anisotropy starting in utero (Bui et al., 2006), and extending to preterm neonates (Partridge et al. 2004), infants (Dubois et al., 2006), and children (Mukherjee et al, 2001; Snook et al., 2005; Alexander et al., 2007) and decreasing trends in adults (Abe et al., 2002; McLaughlin et al., 2007; Ota et al., 2006; Pfefferbaum and Sullivan, 2003; Salat et al., 2005; Sullivan et al., 2006). Some contradictions in published MRI reports on the rate of growth of the CC regions, in particular the splenium (Abe et al., 2002; Bonekamp et al., 2007; Pfefferbaum et al., 2000; compare Pfefferbaum et al., 2000; 2003; 2007) may be attributed to the use of different acquisition paradigms, the size and composition of samples, and the adoption of different CC quantification methods that did not account for the CC regional heterogeneity.
The correspondence between the CCA and its corresponding anisotropy for both males and females at all ages (compare Fig. 2 with Fig. 3) may offer an important surrogate marker for tissue maturation, development and natural aging (Hasan et al., 2008). Such strong positive correlations between callosal anisotropy and its volume attributes have been reported on healthy controls (Alexander et al., 2007; Hasan et al., 2008; Rotarska-Jagiela et al., 2008). The regional and entire CC trajectories (areas and FA) resemble those published on whole brain white matter (Courchesne et al., 2000; Hasan et al., 2007b; Sowell et al., 2003). The DTI-related metrics (FA, eigenvalues) provide complementary information about the microstructural substrates of the contributors to callosal regional maturation rates (Caviness et al., 1996; Lamantia and Rakic, 1990; Mukherjee et al., 2001). In particular, the decrease in the transverse eigenvalues during childhood and increase during adulthood with advancing age may reflect the regional dynamics of myelination and demyleination (Bartzokis et al., 2004; Beaulieu, 2002; Drobyshevsky et al., 2005; Hasan and Narayana, 2006; Rakic and Yakovlev 1968; Song et al., 2005; Vorisek and Sykova, 1997).
3.3 Sex Effects
In addition to age dependence, the CC area and anisotropy have been reported to vary with other factors such as sex and handedness. In the current study, we did not find significant differences in the rates of growth characterizing the macro and microstructural attributes of the CC in our age-matched population of boys/girls, men/women, and males/females (see Table 1–Table 4 and Figure 1–Figure 4). The callosal area growth rates have been reported to be similar in age-matched developing boys and girls (Lenroot et al., 2007; Rajapakse et al., 1996), while other studies reported that the CCA growth rates are larger in boys than girls (De Bellis et al., 2001). In adults, Johnson et al. (1994) showed the CCA rate decreases faster in men than women, while Salat et al. (1997) showed CCA decreases faster in older women than age-matched men.
As noted above, our DTI results indicate a sex-independent statistically significant trend FA(CC7) > FA(CC2) which has been also reported in both children (Snook et. al. 2005) and adults (Abe et al., 2002; Hasan et al., 2005; Ota et al., 2006; Peled et al., 1998; Sullivan et al., 2001; Sullivan et al., 2006). The available DTI reports on sex-based anisotropy differences concluded that FA(males) > FA(females) (Shin et al., 2005; Westerhausen et al., 2004), while a study by Price et al. (2005) has reported that FA(women) > FA(men) in the genu of the CC and another study by Szesko et al. (2003) has reported FA(women) > FA (men) in frontal areas that cross the genu of the CC.
Studies examining effects of handedness on CC area and anisotropy are inconsistent. For example, Witelson (1985) reported that the CCA is larger in consistent left-handers compared to consistent right-handers, while Westerhausen et al. (2004) data indicate that CCA of consistent left-handers is significantly smaller than CCA of right-handers. The CC anisotropy may vary with musical training (Bengtsson et al., 2005), age (Ota et al., 2006), pathology (Alexander et al., 2007) and other confounders. The hypothesis of sex-based axonal geometry has been discussed by Allen et al. (2001) who argued against sex-based differences in axonal microstructure. A comprehensive account of all possible contributors to these findings is beyond the scope of this work and may require a larger population.
3.4 Limitations and Concluding Remarks
Our normative database has been formed by pooling cross-sectional data collected on healthy children and adults using the same DTI protocol to help in the interpretation of data collected from patients. Due to advancing DTI technology longitudinal DTI studies are expected to be more challenging than cross-sectional studies. The primary goal of this work was to validate a DTI-based method for simultaneous measurement of both macro and microstructural attributes of the CC. We have validated the DTI method using a cross-sectional cohort and a lifespan experimental design that accounted for the confounding and nonlinear age effects.
In clinical applications (Biegon et al., 1994; Schulte et al., 2004), the midsagittal corpus callosum area and corresponding MRI-derived measures are commonly used to provide noninvasive biomarkers of central white matter atrophy which may be caused by demyelination, impaired remyelination and axonal loss due to lesions, infarcts and Wallerian degeneration (Gupta et al., 2006; Evangelou et al., 2000; Hasan et al., 2005; Highley et al., 1999; Moeller et al. 2005; Wilde et al. 2006). The CC midsagittal area, by a commonly held conjecture in neuroscience and clinical psychology, may reflect the number of axons involved in interhemispheric communication (Aboitiz et al., 1992; Highley et al., 1999; Ringo et al., 1994). A measurable loss of callosal axons, coherence and myelination impairment may result in cognitive dysfunction affecting interhemispheric communication by visual, somesthetic, auditory, and motor systems as well as complex cognitive processes involving language, attention, and spatial processing (Alexander et al., 2007; Dougherty et al., 2007; Ewing-Cobbs et al., 2006; Hasan et al., 2005; Schulte et al., 2004; Wilde et al., 2006).
These preliminary results are being investigated further using larger cohorts to incorporate age, handedness, sex and psychometric-cognitive scores in addition to DTI simulations and modeling of the relations between the macro and microstructural attributes (Hasan et al., 2007a; Hasan et al., 2008). The application of the current validated methods for callosal quantification to longitudinal studies is warranted.
Future extensions of the current studies include (I) the inclusion of more participants per decade for both males and females, (II) the modeling of the regional relations between the micro and macro structural organization, (III) the comparison of the midsagittal CC results with different approaches for CC subdivision and fiber tracking, (IV) the investigation of covariates such as volume of white matter connected through the CC to the cortex (Janicke et al., 1999; Sullivan et al., 2001; Zarei et al., 2006), and (V) the study of the interplay between CCA regional trends, cortical gray and white matter integrity (Hasan et al., 2007b; Hasan et al., 2008; Sowell et al., 2003; Shaw et al., 2008).
4. Experimental Procedure
4.1 Participants
This study included 23 boys (age mean ± SD = 11.7 ± 3.1 years), 20 girls (age mean ± SD = 10.3 ± 2.9 years), 32 men (age mean ± SD = 36.7 ± 13.5 years) and 46 women (age mean ± SD = 37.8 ± 13.4 years). Table 6 summarizes the age distribution for both males and females. The children and adolescents (N = 43; age mean ± SD = 11.0 ± 3.0 years), adults (N = 78; age mean ± SD = 37.3 ± 13.4 years), and male (N = 55; age mean ± SD = 26.2 ± 16.2 years) and female (N = 66; age mean ± SD = 29.9 ± 16.7 years) groups did not differ in age (p > 0.3). All participants (N = 121; age mean ± SD = 28.0 ± 16.7 years; range = 6–68 years) were primarily English-speaking, identified as neurologically normal by review of medical history, and were healthy at the time of the assessments. All healthy subjects were screened for history of trauma, surgery, chronic illness, alcohol and/or drug abuse, neurological illness, and current pregnancy. Controls in this study were recruited through local advertisements and did not report any neurological conditions. The MRI scans were read as “normal” by a board certified radiologist (L.A.K.). Written informed consent from the adults, guardians and adolescents, and assent from the children participating in these studies was obtained per the University of Texas Health Science Center at Houston institutional review board regulations for the protection of human subjects.
Table 6.
The distribution of males (N = 55) and females (N = 66) in the cohort of children and adults grouped by age.
| Age Group (years) | Number of Males | Number of Females |
|---|---|---|
| 6.7–12 | 14 | 17 |
| 13–19 | 11 | 3 |
| 20–29 | 9 | 17 |
| 30–39 | 9 | 9 |
| 40–49 | 3 | 11 |
| 50–59 | 8 | 6 |
| 60–68.3 | 1 | 3 |
| Total | ||
| 6.7–68.3 | 55 | 66 |
4.2 MRI and DTI Data Acquisition and Processing
We acquired whole-brain data using a Philips 3.0 T Intera system with a SENSE parallel imaging receive head coil (Philips Medical Systems, Best, Netherlands). The MRI protocol included (a) conventional MRI (3D spoiled gradient-echo (SPGR), field-of-view=240×240 mm2 (isotropic voxel size = 0.9375 mm), (b) 2D dual spin-echo images with echo/repetition times of TE1/TE2/TR=10/90/5000 ms, in the axial plane (44 axial slices, 3mm thickness, 0 gap covering the entire brain from foramen magnum to vertex) (c) and a phase-sensitive MRI in the sagittal and axial planes, in addition to a matching volume of diffusion-encoded data as described below.
The diffusion-weighted data were acquired using a single-shot spin echo diffusion sensitized echo-planar imaging (EPI) sequence with the balanced Icosa21 encoding scheme (Hasan and Narayana, 2003), a diffusion sensitization or b-factor of 1000 sec.mm−2, a repetition and echo times of TR=6100 ms, TE= 84 ms, respectively. EPI distortion artifacts were reduced by using a SENSE acceleration factor or k-space undersampling R=2 (Hasan et al., 2008). Spatial coverage matched the conventional MRI sequences described above (e.g., 44 axial sections, 3mm slice thickness and 0 mm gap with identical field-of-view). DTI acquisition time was approximately 7 minutes and resulted in SNR-independent DTI-metric estimation (Hasan, 2007).
In this work, the DTI-derived rotationally-invariant metrics included the fractional anisotropy (FA), radial and mean diffusivity. The radial diffusivity is defined as the average of the second and third eigenvalues (λ⊥ = (λ2+ λ3)/2) and has been shown by several researchers to be a marker of myelination (Beaulieu, 2002; Drobyshesvsky et al., 2005; Hasan and Narayana, 2006; Song et al., 2005). The mean diffusivity is the average of the three eigenvalues (Dav = (λ1 + λ2+ λ3)/3). The details of the DTI image processing (Hasan et al., 2007a) and DTI quality control measures (Hasan, 2007) are found elsewhere (Hasan et al., 2005; Hasan et al., 2008).
4.3 DTI based Segmentation of the Corpus Callosum and Validation
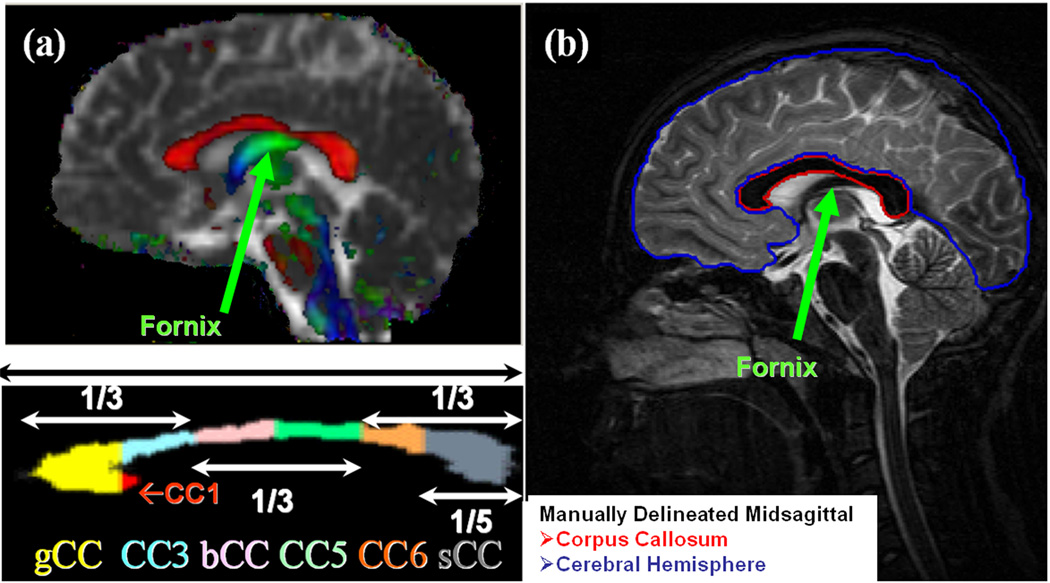
The midsagittal CC identification procedure was assisted by an experienced neurosurgeon based on the appearance of the interthalamic mass and the fornix on the isotropically interpolated DTI maps as described elsewhere (Hasan et al., 2005). The CC was then segmented on the midsagittal slice using mean diffusivity (Dav), fractional anisotropy (Hasan et al., 2007a) and the principal eigenvector (Kanabar et al., 2005; Hasan et al., 2008). To standardize the orientation for all subjects, an orientation angle γ is calculated at which the line joining the maximum anterior–posterior points of the corpus callosum is oriented with the horizontal or long axis (Figure 6a). The midsagittal CC is then rotated by - γ so that the midsagittal CC can be brought into a standard frame of reference for making the subdivisions (Hasan et al., 2008; Kanabar et al., 2005). We optimized the thresholds by comparing the segmented CC areas on a cohort of healthy control subjects (children and adults) with the respective areas obtained by manual boundary selection of the CC on corresponding mid-sagittal phase sensitive T1-weighted images which were acquired in the same scan session (see Figure 6). The procedure outlined provides the CC seven segments: CC1-rostrum, CC2-genu (or gCC), CC3-rostral midbody, CC4-anterior midbody (or bCC), CC5-posterior midbody, CC6-isthmus and CC7-splenium (or sCC; see Fig. 6a) along with the mean, standard deviation (SD) of the b=0, and DTI metrics (FA, eigenvalues, coherence etc.). Because CC1 measures were less reliable due to significant anatomic variation, we report findings for CC2 through CC7 and the entire midsagittal corpus callosum (eCC) which is defined as the sum of all callosal midsagittal sections. The DTI metric mean value of the midsagittal eCC was taken as the CC subregional area-weighted average. A detailed description of the validation of the DTI-based CC segmentation using manually delineated high resolution anatomical MRI data (Figure 6b) is provided in Hasan et al. (2008).
Figure 6.
Illustration of (a) the DTI-based segmentation of the 7 subregions of the human corpus callosum based on the Witelson (1989) seven segments geometric approach. (CC1-CC7: CC1 = rostrum; CC2 = genu = gCC; CC3 = rostral body; CC4 = anterior midbody = bCC; CC5 = posterior midbody; CC6 = isthmus = iCC; CC7 = splenium = sCC), and (b) the manual delineation of the entire midsagittal callosal area (eCC) on conventional MRI acquired in sagittal sections. The upper panel in (a) depicts a fusion of the mean diffusivity map with the principal eigenvector modulated by fractional anisotropy. Note that red color depicts compact fibers oriented in the right-left (e.g. corpus callosum), fibers oriented in the anterior-posterior are shown as green (e.g. fornix), while fibers oriented superior-inferior are shown in blue. The lower panel in (a) shows the magnified seven segments of the midsagittal CC based on the semiautomated DTI implementation of the Witelson (1989) geometric CC subdivisions.
4.4 Statistical analysis
All analyses of corpus callosum midsagittal cross-sectional areas and the corresponding DTI metrics variation were conducted using a generalized linear model with effects of both age and sex. Given previous reports (Allen et al., 1991; Bartzokis et al., 2004; Courchesne et al., 2000; Hasan et al., 2004; Hasan et al., 2007a,b; Hasan et al., 2008; McLaughlin et al., 2007; Pujol et. al., 1993; Rauch and Jinkins, 1994), both linear and quadratic age terms were included. The DTI metrics (e.g., FA,λ⊥) were modeled (fitted) for both males and females as yf=β0+β1*age+β2*age2, then the general least-squares methods were used to compute the coefficients, standard errors and their significance using analysis-of-variance (ANOVA) methods (Hasan et al., 2007b; Hasan et al., 2008). For comparison of two fit parameters between males and females, we used a two-tailed t-test of the difference (βiM-βiF) divided by the root of the pooled variance σ(βiM)2+σ(βiF)2 at the corresponding degrees of freedom (Glantz, 2002). All statistical analyses were conducted using MATLAB R12.1 Statistical Toolbox v 3.0 (The Mathworks Inc, Natick, MA).
Acknowledgements
This work is funded by NIH R01 NS052505-03 awarded to KMH, NINDS R01 NS046308 awarded to LEC, NICHD, P01 HD35946 awarded to JMF and 1 P01 NS46588 awarded to ACP. The authors wish to thank Vipul Kumar Patel, Ambika Sankar, and Christopher Halphen for helping in data acquisition, management and literature review, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol. Aging. 2002;23:433–441. doi: 10.1016/s0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- 2.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 3.Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med. 2001;45:770–780. doi: 10.1002/mrm.1105. [DOI] [PubMed] [Google Scholar]
- 4.Alexander AL, Lee JE, Lazar M, Boudos R, Dubray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. NeuroImage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Allen LS, Richey MF, Chai YM, Gorski RA. Sex differences in the corpus callosum of the living human being. J Neurosci. 1991;11:933–942. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical "disconnection" in aging and Alzheimer's disease. Neurobiol Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 8.Biegon A, Eberling JL, Richardson BC, Roos MS, Wong ST, Reed BR, Jagust WJ. Human corpus callosum in aging and Alzheimer's disease: a magnetic resonance imaging study. Neurobiol Aging. 1994;15:393–397. doi: 10.1016/0197-4580(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 9.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 10.Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neurosci Biobehav Rev. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- 11.Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horská A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M. Callosal contributions to simultaneous bimanual finger movements. J Neurosci. 2008;28:3227–3233. doi: 10.1523/JNEUROSCI.4076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bui T, Daire JL, Chalard F, Zaccaria I, Alberti C, Elmaleh M, Garel C, Luton D, Blanc N, Sebag G. Microstructural development of human brain assessed in utero by diffusion tensor imaging. Pediatr Radiol. 2006;36:1133–1140. doi: 10.1007/s00247-006-0266-3. [DOI] [PubMed] [Google Scholar]
- 14.Cader S, Johansen-Berg H, Wylezinska M, Palace J, Behrens TE, Smith S, Matthews PM. Discordant white matter N-acetylasparate and diffusion MRI measures suggest that chronic metabolic dysfunction contributes to axonal pathology in multiple sclerosis. NeuroImage. 2007;36:19–27. doi: 10.1016/j.neuroimage.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 15.Cascio C, Styner M, Smith RG, Poe MD, Gerig G, Hazlett HC, Jomier M, Bammer R, Piven J. Reduced relationship to cortical white matter volume revealed by tractography-based segmentation of the corpus callosum in young children with developmental delay. Am J Psychiatry. 2006;163:2157–2163. doi: 10.1176/ajp.2006.163.12.2157. [DOI] [PubMed] [Google Scholar]
- 16.Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cerebr Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 17.Chepuri NB, Yen YF, Burdette JH, Li H, Moody DM, Maldjian JA. Diffusion anisotropy in the corpus callosum. Am. J. Neuroradiol. 2002;23:803–808. [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke S, Kraftsik R, Van der Loos H, Innocenti G. Forms and measures of the adult and developing corpus callosum: Is there sexual dimorphism? J Comp Neurol. 1989;280:213–230. doi: 10.1002/cne.902800205. [DOI] [PubMed] [Google Scholar]
- 19.Cook ND. The Brain Code: mechanisms of information transfer and the role of the corpus callosum. London: Cook, N.D.; 1986. [Google Scholar]
- 20.Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 21.Cowell PE, Allen LS, Zalatimo NS, Denenberg VH. A developmental study of sex and age interactions in the human corpus callosum. Brain Res Dev Brain Res. 1992;66:187–192. doi: 10.1016/0165-3806(92)90079-c. [DOI] [PubMed] [Google Scholar]
- 22.Davatzikos C, Resnick SM. Sex differences in anatomic measures of interhemispheric connectivity: correlations with cognition in women but not men. Cereb Cortex. 1998;8:635–640. doi: 10.1093/cercor/8.7.635. [DOI] [PubMed] [Google Scholar]
- 23.De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 24.De Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J. Neuropathol. Exp. Neurol. 1985;44:578–591. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Doraiswamy PM, Figiel GS, Husain MM, McDonald WM, Shah SA, Boyko OB, Ellinwood EH, Krishnan KR. Aging of the human corpus callosum: magnetic resonance imaging in normal volunteers. J Neuropsychiatry Clin Neurosci. 1991;3:392–397. doi: 10.1176/jnp.3.4.392. [DOI] [PubMed] [Google Scholar]
- 26.Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci U S A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubb A, Gur R, Avants B, Gee J. Characterization of sexual dimorphism in the human corpus callosum. NeuroImage. 2003;20:512–519. doi: 10.1016/s1053-8119(03)00313-6. [DOI] [PubMed] [Google Scholar]
- 29.Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants' cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. NeuroImage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Durston S, Hulshoff, Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Estruch R, Nicolás JM, Salamero M, Aragón C, Sacanella E, Fernández-Solà J, Urbano-Márquez A. Atrophy of the corpus callosum in chronic alcoholism. J Neurol Sci. 1997;146:145–151. doi: 10.1016/s0022-510x(96)00298-5. [DOI] [PubMed] [Google Scholar]
- 32.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain. 2000;123:1845–1849. doi: 10.1093/brain/123.9.1845. [DOI] [PubMed] [Google Scholar]
- 33.Ewing-Cobbs L, Hasan KM, Prasad MR, Kramer L, Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR Am J Neuroradiol. 2006;27:879–881. [PMC free article] [PubMed] [Google Scholar]
- 34.Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert SF. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cogn. 2008 doi: 10.1016/j.bandc.2008.01.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. Review. [DOI] [PubMed] [Google Scholar]
- 37.Georgy BA, Hesselink JR, Jernigan TL. MR imaging of the corpus callosum. AJR Am J Roentgenol. 1993;160:949–955. doi: 10.2214/ajr.160.5.8470609. [DOI] [PubMed] [Google Scholar]
- 38.Giedd JN, Castellanos FX, Casey BJ, Kozuch P, King AC, Hamburger SD, Rapoport JL. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:665–669. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- 39.Glantz SA. Primer of biostatistics. 5th ed. New York: McGraw-Hill; 2002. [Google Scholar]
- 40.Gupta RK, Saksena S, Hasan KM, Agarwal A, Haris M, Pandey CM, Narayana PA. Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: serial diffusion tensor imaging. J Magn Reson Imaging. 2006;24:549–555. doi: 10.1002/jmri.20677. [DOI] [PubMed] [Google Scholar]
- 41.Hampel H, Teipel SJ, Alexander GE, Horwitz B, Teichberg D, Schapiro MB, Rapoport SI. Corpus callosum atrophy is a possible indicator of region and cell type-specific neuronal degeneration in Alzheimer disease: a magnetic resonance imaging analysis. Arch Neurol. 1998;55:193–198. doi: 10.1001/archneur.55.2.193. [DOI] [PubMed] [Google Scholar]
- 42.Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: Theoretical analysis and validation. Magn Reson Med. 2003;50:589–598. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- 43.Hasan KM, Kanabar BP, Santos RM, Ewing-Cobbs L, Narayana PA. Age dependence of the fractional anisotropy of genu and splenium of human corpus callosum using optimized DT-MRI. Proc. 12th International Society of Magnetic Resonance in Medicine; Kyoto, Japan. 2004. p. 338. [Google Scholar]
- 44.Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Fractional Diffusion Tensor Anisotropy of the Seven Segments of the Normal-Appearing White Matter of the Corpus Callosum in Healthy Adults and Relapsing Remitting Multiple Sclerosis. J Magn Reson Imaging. 2005;21:735–743. doi: 10.1002/jmri.20296. [DOI] [PubMed] [Google Scholar]
- 45.Hasan KM, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn Reson Med. 2006;56:130–137. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- 46.Hasan KM, Halphen C, Sankar A, Eluvathingal TJ, Kramer L, Stuebing KK, Ewing-Cobbs L, Fletcher JM. Diffusion tensor imaging-based tissue segmentation: validation and application to the developing child and adolescent brain. Neuroimage. 2007a;34:1497–1505. doi: 10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasan KM, Sankar A, Halphen C, Kramer LA, Brandt ME, Juranek J, Cirino PT, Fletcher JM, Papanicolaou AC, Ewing-Cobbs L. Development and Organization of Human Brain Tissue Compartments across Lifespan using Diffusion Tensor Imaging. Neuroreport. 2007b;18:1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- 48.Hasan KM. A framework for quality control and parameter optimization in diffusion tensor imaging: theoretical analysis and validation. Magn Reson Imaging. 2007;25:1196–1202. doi: 10.1016/j.mri.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasan KM, Ewing-Cobbs L, Kramer LA, Fletcher JM, Narayana PA. Diffusion Tensor Quantification of the Macro and Microstructure of the Human Midsagittal Corpus Callosum across the Lifespan. NMR in Biomedicine. 2008 doi: 10.1002/nbm.1286. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayakawa K, Konishi Y, Matsuda T, Kuriyama M, Konishi K, Yamashita K, Okumura R, Hamanaka D. Development and aging of brain midline structures: assessment with MR imaging. Radiology. 1989;172:171–177. doi: 10.1148/radiology.172.1.2740500. [DOI] [PubMed] [Google Scholar]
- 51.Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- 52.Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, van Zijl PC, Mori S. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. NeuroImage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain. 1999;122:99–110. doi: 10.1093/brain/122.1.99. [DOI] [PubMed] [Google Scholar]
- 54.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 55.Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PCM, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: application of the mid-sagittal morphology of corpus callosum. NeuroImage. 2005;26:295–305. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 56.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, Krystal JH, Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Res. 2008;162:256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jancke L, Preis S, Steinmetz H. The relation between forebrain volume and midsagittal size of the corpus callosum in children. Neuroreport. 1999;10:2981–2985. doi: 10.1097/00001756-199909290-00020. [DOI] [PubMed] [Google Scholar]
- 58.Johnson SC, Farnworth T, Pinkston JB, Bigler ED, Blatter DD. Corpus callosum surface area across the human adult life span: effect of age and gender. Brain Research Bulletin. 1994;35:373–377. doi: 10.1016/0361-9230(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 59.Kanabar BP, Hasan KM, Sajja BR, Narayana PA. A Diffusion Tensor Imaging based semi-automated segmentation and subdivision of the human corpus callosum: correlation of anisotropy and callosal area and application to gender based differences. Proceedings of the 13th International Society of Magnetic Resonance in Medicine; Miami, Florida. 2005. p. 1347. [Google Scholar]
- 60.Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, Sweeney JA, Minshew N, Pettegrew JW. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sci. 2002;70:1909–1922. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
- 61.Klaas PA, Hannay JH, Caroselli JS, Fletcher JM. Interhemispheric transfer of visual, auditory, tactile, and visuomotor information in children with hydrocephalus and partial agenesis of the corpus callosum. J Clin Exp Neuropsychol. 1999;21:837–850. doi: 10.1076/jcen.21.6.837.851. [DOI] [PubMed] [Google Scholar]
- 62.LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 64.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levin HS, Benavidez DA, Verger-Maestre K, Perachio N, Song J, Mendelsohn DB, Fletcher JM. Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology. 2000;54:647–653. doi: 10.1212/wnl.54.3.647. [DOI] [PubMed] [Google Scholar]
- 66.Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. Positive correlations between corpus callosum thickness and intelligence. NeuroImage. 2007;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Machado AM, Simon TJ, Nguyen V, McDonald-McGinn DM, Zackai EH, Gee JC. Corpus callosum morphology and ventricular size in chromosome 22q11.2 deletion syndrome. Brain Res. 2007;1131:197–210. doi: 10.1016/j.brainres.2006.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. NeuroImage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 69.McLaughlin NC, Paul RH, Grieve SM, Williams LM, Laidlaw D, Dicarlo M, Clark CR, Whelihan W, Cohen RA, Whitford TJ, Gordon E. Diffusion tensor imaging of the corpus callosum: a cross-sectional study across the lifespan. Int J Dev Neurosci. 2007;25:215–221. doi: 10.1016/j.ijdevneu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Mitchell TN, Free SL, Merschhemke M, Lemieux L, Sisodiya SM, Shorvon SD. Reliable callosal measurement: population normative data confirm sex-related differences. AJNR Am J Neuroradiol. 2003;24:410–418. [PMC free article] [PubMed] [Google Scholar]
- 71.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 72.Muetzel RL, Collins PF, Mueller BA, Schissel A, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. NeuroImage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 74.Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Overmeyer S, Simmons A, Santosh J, Andrew C, Williams SC, Taylor A, Chen W, Taylor E. Corpus callosum may be similar in children with ADHD and siblings of children with ADHD. Dev Med Child Neurol. 2000;42:8–13. doi: 10.1017/s0012162200000037. [DOI] [PubMed] [Google Scholar]
- 76.Pandya DN, Karol EA, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Res. 1971;32:31–43. doi: 10.1016/0006-8993(71)90153-3. [DOI] [PubMed] [Google Scholar]
- 77.Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22:1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 78.Peled S, Gudbjartsson H, Westin CF, Kikinis R, Jolesz FA. Magnetic resonance imaging shows orientation and asymmetry of white matter fiber tracts. Brain Res. 1998;780:27–33. doi: 10.1016/s0006-8993(97)00635-5. [DOI] [PubMed] [Google Scholar]
- 79.Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 80.Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- 81.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 82.Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- 83.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 84.Plessen KJ, Gruner R, Lundervold A, Hirsch JG, Xu D, Bansal R, Hammar A, Lundervold AJ, Wentzel-Larsen T, Lie SA, Gass A, Peterson BS, Hugdahl K. Reduced white matter connectivity in the corpus callosum of children with Tourette syndrome. J Child Psychol Psychiatry. 2006;47:1013–1022. doi: 10.1111/j.1469-7610.2006.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA. The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2005;76:585–587. doi: 10.1136/jnnp.2004.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- 87.Putnam MC, Wig GS, Grafton ST, Kelley WM, Gazzaniga MS. Structural organization of the corpus callosum predicts the extent and impact of cortical activity in the nondominant hemisphere. J Neurosci. 2008;28:2912–2918. doi: 10.1523/JNEUROSCI.2295-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rajapakse JC, Giedd JN, Rumsey JM, Vaituzis AC, Hamburger SD, Rapoport JL. Regional MRI measurements of the corpus callosum: a methodological and developmental study. Brain Dev. 1996;18:379–388. doi: 10.1016/0387-7604(96)00034-4. [DOI] [PubMed] [Google Scholar]
- 89.Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. J Comp Neurol. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- 90.Rauch RA, Jinkins JR. Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behav Brain Res. 1994;64:65–78. doi: 10.1016/0166-4328(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 91.Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. Review. [DOI] [PubMed] [Google Scholar]
- 92.Rotarska-Jagiela A, Schönmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. NeuroImage. 2008;39:1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 93.Salat D, Ward A, Kaye JA, Janowsky JS. Sex differences in the corpus callosum with aging. Neurobiol. Aging. 1997;18:191–197. doi: 10.1016/s0197-4580(97)00014-6. [DOI] [PubMed] [Google Scholar]
- 94.Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alteration in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 95.Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb. Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- 96.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shin YW, Kim DJ, Ha TH, Park HJ, Moon WJ, Chung EC, Lee JM, Kim IY, Kim SI, Kwon JS. Sex differences in the human corpus callosum: diffusion tensor imaging study. Neuroreport. 2005;16:795–798. doi: 10.1097/00001756-200505310-00003. [DOI] [PubMed] [Google Scholar]
- 98.Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. NeuroImage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 99.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 100.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 101.Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Age-related degradation in the central nervous system: assessment with diffusion-tensor imaging and quantitative fiber tracking. Radiology. 2008;247:179–188. doi: 10.1148/radiol.2471070707. [DOI] [PubMed] [Google Scholar]
- 102.Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiol Aging. 2001;22:603–611. doi: 10.1016/s0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- 104.Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- 105.Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, Bilder RM, Frevert T, Lim K. Sex differences in frontal lobe white matter microstructure: A DTI study. Neuroreport. 2003;14:2469–2473. doi: 10.1097/00001756-200312190-00035. [DOI] [PubMed] [Google Scholar]
- 106.Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. Anat Rec. 1954;119:119–135. doi: 10.1002/ar.1091190109. [DOI] [PubMed] [Google Scholar]
- 107.Vorisek I, Sykova E. Evolution of anisotropic diffusion in the developing rat corpus callosum. J Neurophysiol. 1997;78:912–919. doi: 10.1152/jn.1997.78.2.912. [DOI] [PubMed] [Google Scholar]
- 108.von Plessen K, Lundervold A, Duta N, Heiervang E, Klauschen F, Smievoll AI, Ersland L, Hugdahl K. Less developed corpus callosum in dyslexic subjects--a structural MRI study. Neuropsychologia. 2002;40:1035–1044. doi: 10.1016/s0028-3932(01)00143-9. [DOI] [PubMed] [Google Scholar]
- 109.Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res Cogn Brain Res. 2004;21:418–426. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 111.Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. 2006;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- 112.Witelson SF. The brain connection: the corpus callosum is larger in left-handers. Science. 1985;229:665–668. doi: 10.1126/science.4023705. [DOI] [PubMed] [Google Scholar]
- 113.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 114.Witelson SF, Goldsmith CH. The relationship of hand preference to anatomy of the corpus callosum in men. Brain Res. 1991;545:175–182. doi: 10.1016/0006-8993(91)91284-8. [DOI] [PubMed] [Google Scholar]
- 115.Xu D, Mori S, Solaiyappan M, van Zijl PC, Davatzikos C. A framework for callosal fiber distribution analysis. NeuroImage. 2002;17:1131–1143. doi: 10.1006/nimg.2002.1285. [DOI] [PubMed] [Google Scholar]
- 116.Yu C, Li J, Liu Y, Qin W, Li Y, Shu N, Jiang T, Li K. White matter tract integrity and intelligence in patients with mental retardation and healthy adults. NeuroImage. 2008b;40:1533–1541. doi: 10.1016/j.neuroimage.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 117.Zaidel E, Iacoboni M. The parallel brain: The Cognitive Neuroscience of the Corpus Callosum. Cambridge: MIT press; 2003. [Google Scholar]
- 118.Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]