Abstract
Evolution via sexual selection has traditionally been viewed as isolated from life-history constraints. As additionally reproductive resource allocation in males is underexplored, it is rather unclear how life-history factors have shaped lifetime investment into male sexually selected traits. Against this background, we here investigate male butterfly mating success in relation to age, nutritional status, assay condition and wing damage. As predicted, based on a low residual reproductive value, older males had a considerably higher mating success than younger males. Comparisons between virgin and once-mated males suggest that this pattern is related to age per se rather than differential ratings of the resource receptive female. We found no evidence for male body size or condition being important, supporting the notion that in weaponless animals intrinsic motivation is more important for mating success than the differences in physical properties (such as body size or condition). Flight cage experiments suggest that such differences in motivation may be masked under more natural conditions, where flight performance, having a clear impact on mating success (as evidenced by wing manipulation experiments), is likely to be crucial. We conclude that the life-history perspective is a fruitful one for gaining a better understanding of the evolution of sexually selected characters and the predictions derived from contest theory do also apply to male mating success.
Keywords: Lepidoptera, life-history theory, male reproduction, resource-holding potential, sexual selection, wars of attrition
1. Introduction
It is a fundamental assumption of conventional evolutionary theory that animal life histories are shaped by trade-offs among fitness-related traits (Stearns 1992; Roff 2002). Nevertheless, trait evolution via sexual selection has traditionally been viewed as isolated from life-history constraints (Höglund & Sheldon 1998; Kokko 1998; Candolin 2000; Kemp 2002a; Kokko et al. 2002). Recent theory, however, predicts that sexually selected characters should be subject to the same allocation trade-offs that apply to more traditional life-history traits (Enquist & Leimar 1990; Kemp 2002a, 2006). Consequently, there has been a concerted effort towards viewing the processes and outcomes of sexual selection from a life-history perspective in recent years (Höglund & Sheldon 1998; Kokko 1998; Kemp 2002a, 2006; Kokko et al. 2002).
The life-history point of view basically acknowledges that individuals have restricted lifespans and expendable resources, and that an investment allocated to a particular function cannot be used for future investment (Kemp 2006). The idea of such trade-offs between present and future reproduction (or, more generally speaking, investment) is not new, but has been rarely addressed explicitly in behavioural ecology (Kemp 2006), except in the field of female mate choice (e.g. Kokko 1997, 1998; Kokko et al. 2002; Proulx et al. 2002). Moreover, most empirical efforts to identify the trade-offs involved in shaping reproductive traits have focused on the costs of females, by concentrating on traits that are easy to measure (e.g. Chapman et al. 1998; Poizat et al. 1999; Reznick et al. 2000; Barnes & Partridge 2003; Fischer 2007). Patterns in male reproductive and especially behavioural traits, by contrast, remained largely unexplored, (but see Kemp 2002a,b, 2006). It is therefore rather unclear how life-history factors affect lifetime investment in male sexually selected traits (Höglund & Sheldon 1998; Kemp 2002a, 2006; Kotiaho & Simmons 2003; Ferkau & Fischer 2006).
Against this background and inspired by the papers of Kemp (2002a,b, 2003, 2006) and others (e.g. Hunt et al. 2004), this study explores life-history effects, namely age, on male mating strategies in the tropical butterfly Bicyclus anynana (Butler 1879). Male mating behaviour is evidently under strong sexual selection and is moreover likely to induce non-trivial costs (Enquist & Leimar 1990; Kemp & Wiklund 2001; Kemp 2002a, 2006). Throughout the animal kingdom, male mating success and settlement of intrasexual contests often depend on body size, strength, energy reserves or weaponry, i.e. on the individual's resource-holding potential (RHP; Parker 1974; e.g. Marden & Waage 1990; Kemp & Wiklund 2001; Lappin et al. 2006). In groups lacking any obvious physical means usually associated with animal aggression such as butterflies, however, simple asymmetries in RHP are unlikely to determine male mating success (Kemp & Wiklund 2001, 2004; Kemp et al. 2006; Bergman et al. 2007). Male butterfly contests, for example, usually consist of non-contact aerial disputes, closely resembling ‘wars of attrition’ (Maynard Smith 1982; Kemp & Wiklund 2001; Kemp 2002a). Here, the settlement seems to depend purely on a male's willingness to persist (e.g. Kemp & Wiklund 2001, 2004; Kemp 2000a, 2002a; Kemp et al. 2006). Likewise, butterfly mating success seems to depend on the male persistence during courtship (Fischer 2006; Geister & Fischer 2007). Yet, what determines a male's ‘motivation’ to persist is hitherto poorly understood.
Life-history theory predicts that risky behaviour such as injurious fighting should generally increase with age. This is because the residual reproductive value (RRV, Williams 1966), i.e. the opportunity for future reproduction, decreases with age; younger males generally pay greater costs than older ones when being killed or injured (Parker 1974; Enquist & Leimar 1990; Kemp 2002a, 2006). However, a clear empirical examination of this phenomenon has often been hindered by age-based changes in physical attributes or experience, factors that may well impact on male mating success (Marden & Waage 1990; Olsson & Shine 1996; Whitehouse 1997; but Kemp 2002a,b, 2006).
Taking such complications into account and elaborating on recent theoretical and empirical results on male contest behaviour (Kemp 2002a,b, 2003, 2006), we here investigate male mating success in relation to age using male competition experiments. Thus, we here focus on a parameter even more closely related to fitness than territorial success, which is not necessarily indicative of the mating success (Deinert et al. 1994). Further, we extend the previous results by directly manipulating nutritional status and wing deterioration (and thus RHP), the males' experience and assay conditions. Based on the above arguments, we generally predict older males to be more persistent/aggressive while courting females or defeating competitors, thereby gaining a higher mating success than younger males. At the same time our design enables us to explore to what extent asymmetries in RHP and a priori experience may modulate male mating success. Note, for instance, that exclusively using naive animals while investigating age effects might cause asymmetries in the rating of the resource ‘receptive female’ (as older males experienced longer periods without having access to females than younger ones). Finally, by testing male mating performance under artificial and semi-natural settings, we try to disentangle the effects of the males' willingness (eagerness/motivation) from those of their ability, the latter potentially being impaired in older males. While older males are expected to outperform younger ones in an artificial setting (small cage) based on their higher motivation, this may not necessarily hold true under more natural conditions (i.e. in a larger flight cage). Here, factors such as the males' responsiveness, manoeuvrability and flight performance (i.e. ‘ability’), potentially diminishing with age, should become more important (cf. Joron & Brakefield 2003).
2. Material and methods
(a) Study organism and experimental population
Bicyclus anynana is a tropical, fruit-feeding butterfly distributed from southern Africa to Ethiopia (Larsen 1991). This species exhibits striking phenotypic plasticity with two seasonal morphs, which functions as an adaptation to alternate wet–dry seasonal environments and the associated changes in resting background and predation (Brakefield 1997; Lyytinen et al. 2004). Reproduction is essentially confined to the warmer wet season when oviposition plants are abundantly available, during which two to three generations occur. During the colder dry season, reproduction ceases and butterflies do not mate before the first rains at the beginning of the next wet season (Windig 1994; Brakefield 1997). For mate location, male B. anynana butterflies pursue a perch-and-chase strategy. Perching males can be found in high densities, and they are frequently involved in circuit and chasing flights (Brakefield & Reitsma 1991; Joron & Brakefield 2003). Females may mate repeatedly and are able to reject courting males (Brakefield & Reitsma 1991; Brakefield et al. 2001). Thus, male persistence seems to have a large impact on male mating success (Fischer 2006; Geister & Fischer 2007).
A laboratory stock population of B. anynana was established in 2003 from several hundred eggs derived from a well-established stock population at the University of Leiden, The Netherlands. The Leiden population was founded in 1988 from over 80 gravid females collected at Nkhata Bay, Malawi. In each generation, several hundred individuals are reared, maintaining high levels of heterozygosity at neutral loci (Van't Hof et al. 2005). For this study, butterflies from the Bayreuth stock population were used.
(b) Butterfly rearing
Prior to each of the following mating experiments, two cohorts of approximately 1000 eggs each were collected from several hundred stock females, with the second cohort being collected approximately 10 days after the first one. To minimize the confounding effects of female age, eggs for the first cohort were collected before all butterflies had eclosed (thus among the females producing the second cohort there were still fresh ones), and all females were kept at a cool temperature of 20°C without oviposition substrate for the time in between. Larvae were reared in population cages on young maize plants at high relative humidity (70%) and 12 L : 12 D cycle throughout. Depending on the experiment (see below), rearing took place either partly at 20°C and partly at 27°C, or completely at 27°C (for logistic reasons), though the two cohorts belonging to one experiment were always treated identically. The temperatures chosen are similar to the daily highs during the wet and dry season, respectively, in the field (Brakefield & Reitsma 1991). All resulting butterflies had wet season phenotypes.
The resulting pupae were removed daily from the rearing cages and were sexed. Male pupae were weighed to the nearest 0.01 mg on the day after pupation (as proxy for adult body size; except for experiment 4, see below), and afterwards kept individually until adult eclosion, while female pupae were transferred group-wise to hanging cages. Following adult eclosion, all males were individually marked and kept separated by eclosion day. Unless otherwise stated, male and female butterflies were kept apart until the experiments started. The two cohorts were used to produce two age classes of male butterflies, old (first cohort) and young (second; for details see below) ones.
(c) Mating experiments
To explore the effects of male age, feeding treatment, assay condition and/or wing damage on male mating success, four different experiments were carried out as detailed below.
In experiment 1 four groups of males were used, the factors being male age (‘old’ 10(–14) (mean 10.3) days versus ‘young’ 2 days) and feeding treatment (‘control’: males were provided moist banana ad libitum versus ‘food restriction’: males had access to banana for 2 hours per 48 hours, but otherwise to water only; note that young food-restricted males were not fed prior to the mating trials on day 2). The mating trials involved the competition among four randomly chosen virgin males (one from each group) for a single, 2- to 5-day-old virgin female in a cylindrical net cage (diameter 38 cm and height 10 cm) at 27°C. Such assay conditions have been successfully used before in B. anynana (Breuker & Brakefield 2002; Joron & Brakefield 2003; Robertson & Monteiro 2005; Fischer 2006; Geister & Fischer 2007).
The four males were introduced into the cages in random order and the female approximately 10 min later to start the trial. The cages were checked for matings at least every 15 min for the following 8 hours (mating in B. anynana lasts approximately 30 min; Joron & Brakefield 2003). The first male to mate was scored as ‘winner’. The trial was terminated thereafter, and all males were frozen for later analyses (i.e. there was no reuse of any butterflies). In 88 trials, 79 matings were recorded. In addition to the pupal mass (see above), body dry mass and relative fat content were measured for all males. The latter was determined as mass difference between adult dry mass (after 48 hours at 70°C) and the remaining dry mass after two fat extractions. In each extraction, fat was extracted for 48 hours using 2 ml of dichloromethane (CH2Cl2)/methanol (CH3OH) (2 : 1) solution for each butterfly (cf. Fischer et al. 2003). Prior to extractions, legs, wings and antennae were removed. Both traits (dry mass and relative fat content) are known to decrease with age in butterflies, presumably as a consequence of resource depletion, and are thus conveniently measurable proxies of male condition (e.g. Karlsson 1994; Stjernholm et al. 2005; Stjernholm & Karlsson 2006).
Experiment 2 used the same set-up as above with only a few modifications. Here, males were 3 (young class) and 13 (old) days old. Further, males from all four groups were mated once to virgin females prior to the mating trial (on days 1 and 11, respectively, i.e. 2 days before the trial). The reasoning behind that was to account for potential differences in the rating of the resource receptive female (see above). As this rating may differ across males not having had access to a female for 3 days when compared with 13 days, we kept the time since the last mating constant across age classes. In 86 trials, 80 matings were recorded.
In experiment 3, again 3-day (young) and 13-day (old) old males were used, which were once again mated 2 days before the mating trials (see above). The factor ‘feeding treatment’ was dropped here (resulting in only two treatment groups, all fed with moist banana ad libitum) in order to reduce work load and because the prior results did not indicate any interactive effects. The principal difference between experiment 3 and the earlier ones is that here experiments were not conducted in small hanging cages, but in a large flight cage (195×115×90 cm), allowing for a more complete expression of courtship behaviour (cf. Joron & Brakefield 2003). Several plants were placed into the cage to imitate natural vegetation structures and to provide perch sites.
In this experiment, equal numbers (20–44, depending on the trial) of young and old males were introduced into the flight cage in random order. After 10 min, young virgin females (60% of the total number of males) were released into the cage to start the trial. Following the release of the last female (time 0), a stop clock was started to measure the time to mating for the successful males. Mating couples were removed from the flight cage to avoid second matings. Pooled over 4 consecutive days, 123 successful matings were observed (number of females used, 159). At the end of each experimental day, all males used were frozen for a later analysis of wing wear. The classification of wing wear followed the studies of Karlsson (1994) and Kemp (2000b, 2002a): (i) fresh, no signs of scale loss, wings undamaged; (ii) rather fresh, some wing scales lost, only minor wing damage (if any); (iii) wings moderately worn, wing scales partly lost, wings damaged at margins; (iv) wings worn and substantially damaged, substantial loss of scales; and (v) wings extremely worn, wing scales largely lost, parts of wings missing. Scoring was done by one person (T.G.) only.
The last experiment (experiment 4) explored the effect of wing damage on the mating success in hanging cages as well as in the above-mentioned flight cage. Males were randomly divided among two groups, an untreated control group and a treated one, in which the distal parts of both fore- and hindwings were cut off on day 1 after eclosion. Wings were cut off along an imaginary straight line from the hindwings' posterior eyespot to the forewings' anterior eyespot, thus removing approximately one-third of the wings. These experiments exclusively used 3-day-old virgin males. In the hanging cages, two males (a control and treated one) competed for a single virgin female (for details, cf. experiments 1 and 2), while in the flight cage 62 males per treatment and 74 females were introduced at a time. In the former case, 65 matings were recorded in 89 trials and in the latter, 57 matings (for 74 females). All butterflies were fed ad libitum.
(d) Data analysis
The frequencies of successful matings per treatment group was tested against even distributions (null hypothesis) using Χ2-tests. Additionally, to test simultaneously for the effects of age, feeding treatment and various covariates (pupal mass, body dry mass and wing wear) on the mating success of B. anynana males, we calculated generalized nonlinear models (GNLM) with a binomial error distribution and a logit link function (binary data, successful or unsuccessful). This approach is problematic because the assumption of non-independency of data is violated: if one male wins, the other(s) lose and thus their scores are not independent. Nevertheless we decided to present these models here for illustrative purposes, as they allow for the inclusion of covariates and thus give a much more complete picture. Additionally, all such results are fully supported by the above Χ2-tests.
Group differences in continuous traits (pupal mass, adult dry mass, relative fat content, wing wear and time to mating) were tested by using either two-way analyses of variance (ANOVAs) or Mann–Whitney U tests. To meet ANOVA requirements, data were transformed prior to testing as appropriate. All statistical tests were performed using Statistica v. 6.1. Throughout the paper, only significant interaction terms are presented. All means are given ±1 s.e.
3. Results
(a) Experiment 1. Male mating success in relation to age and feeding treatment
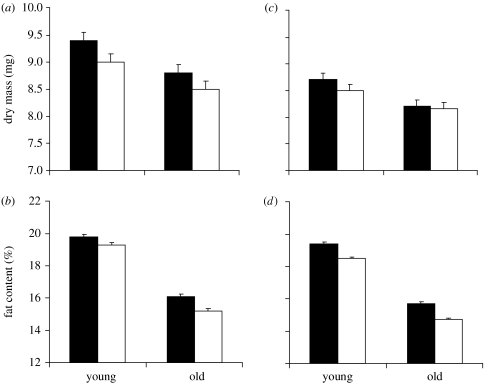
While male pupal mass did not differ across age classes (F1,312<0.1, p=0.994) and (adult) feeding treatments (F1,312=2.7, p=0.101) confirming random sampling, both factors significantly affected male adult dry mass and relative fat content (figure 1a,b). Older males showed, on average, a lighter dry mass (8.64±0.11 versus 9.20±0.11 mg; F1,312=13.2, p=0.0003) accompanied by a lower relative fat content (15.7±0.06 versus 19.5±0.06%; F1,312=2469.1, p<0.0001) than younger males. Likewise, food-stressed males were lighter (8.75±0.10 versus 9.09±0.12 mg; F1,312=4.7, p=0.0305) and had a lower relative fat content (17.28±0.16 versus 17.93±0.17%; F1,312=71.0, p<0.0001) compared with control males, although the effects were considerably smaller than those induced by age differences. A significant age×feeding treatment interaction for relative fat content (F1,312=7.1, p=0.0082) indicates that, as would be expected, the effects of food stress were more pronounced in older males (figure 1b).
Figure 1.
(a,c) Male adult dry mass and (b,d) relative fat content in relation to age and feeding treatment in the butterfly B. anynana (means +1 s.e.; (a,b) experiment 1 and (c,d) experiment 2). For details on experimental treatments see text. Sample size per group is 79 (experiment 1) and 80 (experiment 2). Filled bars, control; open bars, starved.
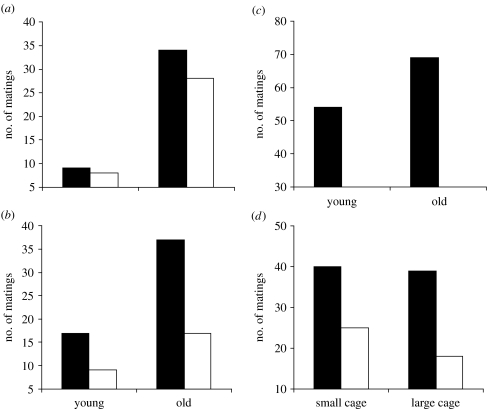
Despite their evidently poorer condition in terms of body mass and storage reserves, older males were successful in 62 out of 79 mating trials (Χ12=25.6, p<0.0001), while mating success was rather similar across feeding treatments (food-stressed males: 36; control males: 43; Χ12=0.6, p=0.43; figure 2a). These results are fully supported by the GNLM models given in table 1a for illustrative purposes, showing that mating success is only affected by age, but not by feeding treatment or pupal mass.
Figure 2.
Male B. anynana mating success in relation to (a) age and feeding treatment in virgin males, (b) age and feeding treatment in males having mated 2 days prior to the mating trials already, (c) age in a larger flight cage allowing for a more complete expression of courtship behaviour (note that the factor feeding treatment was dropped here) and (d) artificial wing damage and cage size (small versus large). (a,b) Filled bars, control; open bars, starved, (c) filled bars, control and (d) filled bars, control; open bars, damaged. For details on experimental treatments see text.
Table 1.
Effects of age, feeding treatment or wing wear (covariate), and pupal mass (covariate) on male mating success in B. anynana, tested by generalized nonlinear models (binomial error distribution, logit link function). (Note that results are presented for illustrative purposes only. Parts a, b and c refer to experiments 1, 2 and 3, respectively. d.f.=1 throughout. For a and b, results do not differ qualitatively when replacing pupal by adult dry mass; models including pupal mass as covariate are presented here as dry mass was not measured in experiment 3.)
| factor | Wald Χ2 | p |
|---|---|---|
| a | ||
| age | 30.31 | <0.0001 |
| feeding treatment | 0.60 | 0.4407 |
| age×feeding treatment | 0.10 | 0.7549 |
| pupal mass | 0.16 | 0.6867 |
| b | ||
| age | 11.36 | 0.0007 |
| feeding treatment | 11.28 | 0.0008 |
| age×feeding treatment | 0.50 | 0.4806 |
| pupal mass | 0.08 | 0.7732 |
| c | ||
| age | 8.37 | 0.0038 |
| wing wear | 5.87 | 0.0154 |
| pupal mass | 0.48 | 0.4903 |
(b) Experiment 2. Male mating success in relation to age and feeding treatment after a pre-copulation
As above, pupal mass was not significantly different between age classes (F1,316=1.8, p=0.182) or feeding treatments (F1,316<0.1, p=0.891), while older males had significantly lighter dry mass (8.18±0.08 versus 8.58±0.09 mg; F1,316=10.7, p=0.0012) and lower relative fat content (15.2±0.07 versus 18.9±0.07%; F1,316=2126.1, p<0.0001) than younger males (figure 1c,d). Food stress, by contrast, affected only relative fat content (food stressed: 16.6±0.16% versus control: 17.5±0.16%; F1,316=139.0, p<0.0001), but not dry mass (F1,316=0.8, p=0.387).
In this experiment, where all males had already mated 2 days prior to the mating trials, mating success was significantly affected by both age class and feeding treatment. Older males were more successful than younger ones (54 versus 26 matings; Χ12=9.8, p=0.0175) and control males more successful than food-stressed ones (54 versus 26; Χ12=9.8, p=0.0175; figure 2b). Again, GNLM models gave qualitatively identical results, confirming the significant effects of age and feeding treatment, but no effect of body size on mating success (table 1b).
(c) Experiment 3. Male mating success in relation to age: flight cage experiments
In the large flight cage, mating success did not differ significantly between old and young males (69 versus 54; Χ12=1.8, p=0.176; figure 2c). Again, pupal mass did not differ across age classes (old: 143.1±1.5 versus young: 147.0±1.3 mg; Z=−1.6, p=0.105). Older males (85±13 min) mated significantly sooner than younger ones (127±17 min; Z=8.9, p<0.0001), though wing wear was significantly worse in old (3.2±0.1) compared with young males (2.0±0.1; Z=8.9, p<0.0001). Accounting for the latter in a GNLM model shows that mating success depended on both male wing wear and age (table 1c). This indicates that, although the older males' numerical advantage is limited, they still perform better if variation in wing wear is accounted for, with poorer wing wear generally reducing mating success.
(d) Experiment 4. Male mating success in relation to wing damage
In the small hanging cages, control males tended to outperform wing-damaged ones (40 versus 25 matings; Χ12=3.5, p=0.0628), the difference being more pronounced in the large flight cage (39 versus 18 matings; Χ12=7.7, p=0.0054; figure 2d).
4. Discussion
In line with our a priori predictions, this study demonstrates that 8-10 days older male B. anynana had a generally higher mating success than younger ones (but also see below). This difference in lifespan is considered ecologically highly relevant. Although B. anynana may reach considerably longer lifespans under laboratory conditions (e.g. Pijpe et al. 2006), field life expectancies in butterflies typically range between 10 and 14 days (e.g. Brakefield & Reitsma 1991; Fischer & Fiedler 2001; but Molleman et al. 2007). At the same time, older males showed substantial reductions in two traits likely to be an indicative of condition (cf. Marden & Waage 1990; Kemp & Wiklund 2001; Lappin et al. 2006), body dry mass (−6%) and relative fat content (−20%). As consequently older males were more successful despite a clearly diminished condition, these findings suggest that, as expected, asymmetries in RHP are unlikely to have a larger impact on male mating success in B. anynana and other butterflies or weaponless animals (but see below; Kemp & Wiklund 2001, 2004; Kemp et al. 2006; Bergman et al. 2007). Also note in this context that, throughout all experiments, male body size (measured as pupal mass) did not affect mating success.
Rather, the better performance of older males lends strong support for the notion of intrinsic differences in aggressiveness and persistence, which are thought to have caused the patterns found. Such intrinsic differences in turn can be explained by few future reproduction opportunities for older compared with younger males (i.e. a low RRV). Consequently, males should be more willing to invest in potentially costly behaviour as they age, as older males stand to lose substantially less than younger ones (Parker 1974; Enquist & Leimar 1990; Kemp 2002a, 2006). Indeed, the behavioural differences between old and young males were very obvious to the observers, with older males being much more aggressive and persistent than younger ones when courting females (although this was not quantified, a detailed behavioural analysis is subject to work in progress). The potential costs associated with such enhanced aggressiveness during courtship (or male–male contests) include opportunity costs due to loss of time as well as energetic costs, injury (due to unintended physical contact), and predation costs (Enquist & Leimar 1990; Kemp & Wiklund 2001; Kemp 2002a, 2006).
The results on the effect of feeding treatment on male mating success are somewhat ambiguous. While feeding treatment did not affect mating success in experiment 1, food-stressed males gained less matings than control males in experiment 2. Both experiments were similar in design, the principal difference being that all males were mated once prior to the mating trials in experiment 2, while experiment 1 involved competition among virgin males. It is thus tempting to conclude that the difference is causally related to mating number. The resource expenditures associated with mating such as those for courtship and spermatophore formation are likely to be more easily bearable by control than by food-restricted males. For instance, although spermatophores in B. anynana are comparably small, later spermatophores have a decreased dry mass and a higher water content than earlier ones suggesting that spermatophore production is indeed physiologically costly (Ferkau & Fischer 2006). Nevertheless, manipulation of adult feeding regime has at best a minor impact on earlier spermatophores that are essentially larval in origin (Ferkau & Fischer 2006).
An alternative explanation would be that old males were 10 days old in experiment 1 compared with 13 days in experiment 2, potentially exaggerating any potential effects of adult food limitation. By contrast, a direct impact of fat content or body mass is unlikely. Although adult food restriction reduced or tended to reduce both proxies of male condition, the resulting variation was considerably less pronounced compared with age-related differences, where it obviously did not impede the older males' better performance. Most importantly, however, male age was still a significant predictor of male mating success, even if potential differences in the evaluation of the resource receptive female are accounted for. Thus, the enhanced aggressiveness and persistence of older males does not seem to be related to differences in the rating of the resource in question, but rather to differences in age per se.
The evidence collated thus far clearly suggests a higher mating success of older male B. anynana, based on a higher intrinsic motivation. However, these results were (deliberately) obtained in a rather artificial setting, i.e. in small hanging cages (to specifically test for differences in motivation). An important question to answer next is whether a higher motivation translates into a higher mating success under more natural conditions. In a larger flight cage mimicking those conditions, the older male advantage was considerably less pronounced, not attaining significance anymore (though the general trend of older males performing better remains; 69 versus 54 matings). This outcome was expected, as mating performance does not only depend on motivation but also on physiological items, such as energy reserves, responsiveness, flight endurance, manoeuvrability (e.g. Joron & Brakefield 2003), all of which are likely to diminish with age (Karlsson 1994; Stjernholm et al. 2005; Stjernholm & Karlsson 2006). Still, a higher motivation is empirically supported as older males took on average significantly less time to initiate mating than younger males. Thus, if older males manage to spot and approach females timely, they have a very good chance to gain a mating.
Nevertheless, the older males' higher motivation to mate seems to be at least partly impaired by physical deficiencies under more natural conditions. This finding has important implications for experimental tests of hypotheses associated with differences in the RRV, as intrinsic differences in the willingness to adopt more risky strategies may prove difficult to detect under certain experimental conditions. An important factor impairing mating success of older males seems to be flight performance, as suggested by two lines of evidence. First, if differences in male wing wear (used as a proxy of flight performance), being significantly poorer in older males, are statistically controlled for, male age is again a significant factor affecting male mating success, even under more natural conditions. Thus, poorer wing wear is generally disadvantageous in terms of mating success, but older males tend to be more successful despite much poorer wing wear, again supporting the notion of a higher intrinsic motivation. Overall, wing wear and consequently locomotor performance seem to be much more important for male B. anynana mating success than the body mass or the amount of storage reserve. Second, experimental manipulation of wings yielded evidence for a causal link between flight performance and mating success. Manipulated males with damaged wings were less successful than control males of the same age, with differences being more exaggerated under less restricted experimental conditions, thus echoing the above results.
In conclusion, we here provide compelling evidence for a mating advantage of older males, caused by a higher intrinsic motivation resulting in higher levels of aggressiveness and persistence during courtship (and possibly male–male contests). These findings are consistent with the predictions resulting from RRV theory. Importantly, older males are more successful despite diminished resources and wing wear, at least under restrictive experimental conditions focusing on intrinsic motivation. This clearly supports the notion that in weaponless animals such as butterflies, intrinsic motivation (persistence) is much more important for conflict resolution and mating success than differences in physical properties (e.g. body size, condition). Such differences in motivation, however, may be masked under more natural conditions, where physical attributes are likely to be more important. There is no way to decide in general which experimental design is most appropriate, as this will depend on the question addressed. Our findings demonstrate that the arguments for male contest competition (Kemp 2002a, 2003, 2006) can be extrapolated to other costly aspects of male behaviour, opening up a window of opportunity for future research. As has been argued before (Kemp 2002a, 2006), an explicit consideration of life-history theory in behavioural studies provides a fruitful perspective for investigating the evolution of sexually selected behaviours for some time to come.
Acknowledgments
We acknowledge financial support from the German Research Council (DFG grant no. Fi 846/1-3 and Fi 846/1-4 to K.F.).
References
- Barnes A.I, Partridge L. Costing reproduction. Anim. Behav. 2003;66:199–204. doi:10.1006/anbe.2003.2122 [Google Scholar]
- Bergman M, Gotthard K, Berger D, Olofsson M, Kemp D.J, Wiklund C. Mating success of resident versus non-resident males in a terrotorial butterfly. Proc. R. Soc. B. 2007;274:1659–1665. doi: 10.1098/rspb.2007.0311. doi:10.1098/rspb.2007.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakefield P.M. Phenotypic plasticity and fluctuating asymmetry as responses to environmental stress in the butterfly Bicyclus anynana. In: Bijlsma R.R, Loeschke V, editors. Environmental stress: adaptation and evolution. Birkhäuser; Basel, Germany: 1997. pp. 65–78. [Google Scholar]
- Brakefield P.M, Reitsma N. Phenotypic plasticity, seasonal climate and the population biology of Bicyclus butterflies (Satyridae) in Malawi. Ecol. Entomol. 1991;16:291–303. [Google Scholar]
- Brakefield P.M, El Filali E, Van der Laan R, Breuker C.J, Saccheri I.J, Zwaan B.J. Effective population size, reproductive success and sperm precedence in the butterfly, Bicyclus anynana, in captivity. J. Evol. Biol. 2001;14:148–156. doi: 10.1046/j.1420-9101.2001.00248.x. doi:10.1046/j.1420-9101.2001.00248.x [DOI] [PubMed] [Google Scholar]
- Breuker C.J, Brakefield P.M. Female choice depends on size but not symmetry of dorsal eyespots in the butterfly Bicyclus anynana. Proc. R. Soc. B. 2002;269:1233–1239. doi: 10.1098/rspb.2002.2005. doi:10.1098/rspb.2002.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolin U. Increased signalling effort when survival prospects decrease: male–male competition ensures honesty. Anim. Behav. 2000;60:417–422. doi: 10.1006/anbe.2000.1481. doi:10.1006/anbe.2000.1481 [DOI] [PubMed] [Google Scholar]
- Chapman T, Miyatake T, Smith H.K, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proc. R. Soc. B. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. doi:10.1098/rspb.1998.0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinert E.I, Longin J.T, Gilbert L.E. Mate competition in butterflies. Nature. 1994;370:23–24. doi:10.1038/370023a0 [Google Scholar]
- Enquist M, Leimar O. The evolution of fatal fighting. Anim. Behav. 1990;39:1–9. doi:10.1016/S0003-3472(05)80721-3 [Google Scholar]
- Ferkau C, Fischer K. Costs of reproduction in male Bicyclus anynana and Pieris napi butterflies: effects of mating history and food limitation. Ethology. 2006;112:1117–1127. doi:10.1111/j.1439-0310.2006.01266.x [Google Scholar]
- Fischer K. Reduced male mating vigor in selection lines of the butterfly Bicyclus anynana. J. Insect Behav. 2006;19:657–668. doi:10.1007/s10905-006-9057-9 [Google Scholar]
- Fischer K. Control of reproduction and a survival cost to mating in female Bicyclus anynana butterflies. Ecol. Entomol. 2007;32:674–681. doi:10.1111/j.1365-2311.2007.00922.x [Google Scholar]
- Fischer K, Fiedler K. Resource-based territoriality in the butterfly Lycaena hippothoe and environmentally induced behavioural shifts. Anim. Behav. 2001;61:723–732. doi:10.1006/anbe.2000.1662 [Google Scholar]
- Fischer K, Brakefield P.M, Zwaan B.J. Plasticity in butterfly egg size: why larger offspring at lower temperatures? Ecology. 2003;84:3138–3147. doi:10.1890/02-0733 [Google Scholar]
- Geister T.L, Fischer K. Testing the beneficial acclimation hypothesis: cool males—hot love? Behav. Ecol. 2007;18:658–664. doi:10.1093/beheco/arm024 [Google Scholar]
- Höglund J, Sheldon B.C. The cost of reproduction and sexual selection. Oikos. 1998;83:478–483. doi:10.2307/3546675 [Google Scholar]
- Hunt J, Brooks R, Jennions M.D, Smith M.J, Bentsen C.L, Brussiere L.F. High-quality male field crickets invest heavily in sexual display but die young. Nature. 2004;432:1024–1027. doi: 10.1038/nature03084. doi:10.1038/nature03084 [DOI] [PubMed] [Google Scholar]
- Joron M, Brakefield P.M. Captivity masks inbreeding effects on male mating success in butterflies. Nature. 2003;424:191–194. doi: 10.1038/nature01713. doi:10.1038/nature01713 [DOI] [PubMed] [Google Scholar]
- Karlsson B. Feeding habits and change of body composition with age in three nymphalid butterflies species. Oikos. 1994;69:224–230. doi:10.2307/3546142 [Google Scholar]
- Kemp D.J. Butterfly contests: neither paradoxical nor contradictory. Anim. Behav. 2000a;60:44–46. doi: 10.1006/anbe.1999.1305. [DOI] [PubMed] [Google Scholar]
- Kemp D.J. Contest behaviour in territorial male butterflies: does size matter? Behav. Ecol. 2000b;11:591–596. doi:10.1093/beheco/11.6.591 [Google Scholar]
- Kemp D.J. Sexual selection constrained by life history in a butterfly. Proc. R. Soc. B. 2002a;269:1341–1345. doi: 10.1098/rspb.2002.2000. doi:10.1098/rspb.2002.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D.J. Butterfly contests and flight physiology: why do older males fight harder? Behav. Ecol. 2002b;213:456–461. doi:10.1093/beheco/13.4.456 [Google Scholar]
- Kemp D.J. Twilight fighting in the evening brown butterfly, Melanitis leda (L.) (Nymphalidae), age and residency effects. Behav. Ecol. Sociobiol. 2003;54:7–13. doi:10.1007/s00265-003-0602-7 [Google Scholar]
- Kemp D.J, Wiklund C. Fighting without weaponry: a review of male–male contest competition in butterflies. Behav. Ecol. Sociobiol. 2001;49:429–442. doi:10.1007/s002650100318 [Google Scholar]
- Kemp D.J, Wiklund C. Residency effects in animal contests. Proc. R. Soc. B. 2004;271:1707–1711. doi: 10.1098/rspb.2004.2775. doi:10.1098/rspb.2004.2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D.J. Ageing, reproductive value, and the evolution of lifetime fighting behaviour. Biol. J. Linn Soc. 2006;88:565–578. doi:10.1111/j.1095-8312.2006.00643.x [Google Scholar]
- Kemp D.J, Wiklund C, Gotthard K. Life history effects upon contest behaviour: age as a predictor of territorial contest dynamics in two populations of the speckled wood butterfly, Pararge aegeria L. Ethology. 2006;112:471–477. doi:10.1111/j.1439-0310.2005.01173.x [Google Scholar]
- Kokko H. Evolutionary stable strategies of age-dependent sexual advertisement. Behav. Ecol. Sociobiol. 1997;41:99–107. doi:10.1007/s002650050369 [Google Scholar]
- Kokko H. Good genes, old age, and life-history trade-offs. Evol. Ecol. 1998;12:739–750. doi:10.1023/A:1006541701002 [Google Scholar]
- Kokko H, Brooks R, McNamara J.M, Houston A.I. The sexual selection continuum. Proc. R. Soc. B. 2002;269:1331–1340. doi: 10.1098/rspb.2002.2020. doi:10.1098/rspb.2002.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotiaho J.S, Simmons L.W. Longevity cost of reproduction for males but no longevity cost of mating or courtship for females in the male-dimorphic dung beetle Onthophagus binodis. J. Insect Physiol. 2003;49:817–822. doi: 10.1016/S0022-1910(03)00117-3. doi:10.1016/S0022-1910(03)00117-3 [DOI] [PubMed] [Google Scholar]
- Lappin A.K, Brandt Y, Husak J.F, Macedonia J.M, Kemp D.J. Gaping displays reveal and amplify a mechanistically based index of weapon performance. Am. Nat. 2006;168:100–113. doi: 10.1086/505161. doi:10.1086/505161 [DOI] [PubMed] [Google Scholar]
- Larsen T.B. University Press; Oxford, UK: 1991. The butterflies of Kenya. [Google Scholar]
- Lyytinen A, Brakefield P.M, Lindstrom L, Mappes J. Does predation maintain eyespot plasticity in Bicyclus anynana? Proc. R. Soc. B. 2004;271:279–283. doi: 10.1098/rspb.2003.2571. doi:10.1098/rspb.2003.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden J.H, Waage J.K. Escalated damselfly territorial contests are energetic wars of attrition. Anim. Behav. 1990;39:954–959. doi:10.1016/S0003-3472(05)80960-1 [Google Scholar]
- Maynard Smith J. University Press; Cambridge, UK: 1982. Evolution and theory of games. [Google Scholar]
- Molleman F, Zwaan B.J, Brakefield P.M, Carey J.R. Extraordinary long life spans in fruit-feeding butterflies can provide window on evolution of life span and aging. Exp. Gerontol. 2007;42:472–482. doi: 10.1016/j.exger.2007.01.008. doi:10.1016/j.exger.2007.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Shine R. Does reproductive success increase with age or size in species with indeterminate growth? A case study using sand lizards (Lacerta agilis) Oecologia. 1996;105:175–178. doi: 10.1007/BF00328543. doi:10.1007/BF00328543 [DOI] [PubMed] [Google Scholar]
- Parker G.A. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. doi:10.1016/0022-5193(74)90111-8 [DOI] [PubMed] [Google Scholar]
- Pijpe J, Fischer K, Brakefield P.M, Zwaan B.J. Consequences of divergent selection on pre-adult traits for adult lifespan under benign conditions in the butterfly Bicyclus anynana. Mech. Ageing Dev. 2006;127:802–807. doi: 10.1016/j.mad.2006.07.006. doi:10.1016/j.mad.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Poizat G, Rosecchi E, Crivelli A.J. Empirical evidence of a trade-off between reproductive effort and expectation of future reproduction in female three-spined sticklebacks. Proc. R. Soc. B. 1999;266:1543–1548. doi:10.1098/rspb.1999.0813 [Google Scholar]
- Proulx S.R, Day T, Rowe L. Older males signal more reliability. Proc. R. Soc. B. 2002;269:2291–2299. doi: 10.1098/rspb.2002.2129. doi:10.1098/rspb.2002.2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick D, Nunney L, Tessier A. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 2000;15:421–425. doi: 10.1016/s0169-5347(00)01941-8. doi:10.1016/S0169-5347(00)01941-8 [DOI] [PubMed] [Google Scholar]
- Robertson K.A, Monteiro A. Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proc. R. Soc. B. 2005;272:1541–1546. doi: 10.1098/rspb.2005.3142. doi:10.1098/rspb.2005.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Stearns S.C. University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Stjernholm F, Karlsson B. Reproductive expenditure affects utilization of thoracic and abdominal resources in male Pieris napi butterflies. Funct. Ecol. 2006;20:442–448. doi:10.1111/j.1365-2435.2006.01120.x [Google Scholar]
- Stjernholm F, Karlsson B, Boggs C.L. Age-related changes in thoracic mass: possible reallocation of resources to reproduction in butterflies. Biol. J. Linn. Soc. 2005;86:363–380. doi:10.1111/j.1095-8312.2005.00542.x [Google Scholar]
- Van't Hof A.E, Zwaan B.J, Saccheri I.J, Daly D, Bot A.N.M, Brakefield P.M. Characterization of 28 microsatellite loci for the butterfly Bicyclus anynana. Mol. Ecol. Notes. 2005;5:169–172. doi:10.1111/j.1471-8286.2004.00870.x [Google Scholar]
- Whitehouse M.E.A. Experience influences male–male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae) Anim. Behav. 1997;53:913–923. doi:10.1006/anbe.1996.0313 [Google Scholar]
- Williams G.C. Natural selection, the cost of reproduction and a refinement of Lack's priniciple. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]
- Windig J.J. Reaction norms and the genetic-basis of phenotypic plasticity in the wing pattern of the butterfly Bicyclus anynana. J. Evol. Biol. 1994;7:665–695. doi:10.1046/j.1420-9101.1994.7060665.x [Google Scholar]