Abstract
Why and how birds in colonies often breed in striking synchrony is an unsolved question. In colonies, conspecific birds often destroy eggs and kill chicks, either intentionally or not. We propose that social tranquillity at the time of laying can be achieved if a bird's stress level is partly determined by the agitation of its neighbours. Moreover, we propose that this local process, together with environmental cues, can synchronize breeding between neighbours and through a whole colony. We tested our hypotheses using a generic individual-based model where the breeding predisposition of females was updated daily depending on an increase in the photoperiod (positively) and the stress level of neighbours: negatively if they were agitated, and positively otherwise. A female laid her eggs when her stress level fell to a critical value. Even giving only a low relevance to the neighbour's stress level was enough to synchronize the laying date of neighbours and also of a huge colony. Moreover, females bred in a safer environment, which is known from field studies to increase fitness. Our study highlights the power of local adaptive (individual) behaviour to create global (colony) patterns. We argue that collective patterns such as breeding synchrony in colonial birds could have simple adaptive individual-level explanations.
Keywords: colonies, emergence, individual-based model, reproduction, social behaviour, stress
1. Introduction
Breeding synchrony is a characteristic of colonial birds—female birds in a colony are often at a strikingly similar breeding stage: laying eggs; incubating; or feeding similarly aged chicks (Gochfeld 1980; Nelson 1980; Coulson 2002). Different ultimate explanations of colony synchrony have been proposed, including predation satiation, mate finding, reduction of intraspecific interactions and formation of juvenile flocks (Darling 1938; Emlen & Demong 1975; Yom-Tov 1975; Nelson 1980; Mougin et al. 2001). However, empirical tests of these hypotheses are inconclusive, suggesting that the advantages of synchronized breeding could either be case specific or occur in different combinations, or even that synchrony can be maladaptive (Gochfeld 1980; Wittenberger & Hunt 1985). Coulson (2002) suggested that these advantages are consequences rather than (adaptive) causes of breeding synchrony. In any case, these ultimate explanations cannot inform us about the proximate mechanisms leading to breeding synchrony in the first place, which are the focus of this paper.
Darling (1938) proposed a proximate mechanism for synchrony: non-breeding birds in a colony are stimulated by the cumulative value of the voice, and the presence of the breeding members of the colony to start breeding themselves. More synchronous breeding is thus expected under the stronger social stimulation occurring in larger colonies. Some empirical studies support these predictions (Waas et al. 2000, 2005; Setiawan et al. 2007) and provide evidence of the endocrine mechanisms of social stimulation (Ball & Bentley 2000; Wingfield et al. 2000). However, the effect of colony size (i.e. of the cumulative value of stimulation) on synchronization remains unclear. Darling (1938) himself presented some data confirming a positive effect of colony size on synchrony, but later reanalyses of these data revealed statistical problems, and further data from other species showed no consistent pattern (reviewed by Gochfeld 1980).
An alternative to Darling's hypothesis would be that environmental factors such as day length, temperature or food availability trigger breeding synchrony. But empirical evidence is not compatible with this hypothesis. First, even within different colonial species breeding in the same place and feeding on the same prey, the degree of breeding synchrony can be very different (Reed et al. 2006). Second, within the same population of a bird species, different colonies or sub-colonies can be strongly synchronized themselves but considerably out of phase with other nearby colonies or sub-colonies (Coulson & White 1960; Burger 1979).
Both Darling's hypothesis and environmental factors focus on the scale of the entire colony or even larger scales. However, this is difficult to reconcile with the fact that often close neighbours and sub-colonies are more synchronized than the whole colony (Burger 1979; Thomas 1986; Murphy & Schauer 1996; Mougin et al. 2001). Moreover, Coulson & White (1960) found an effect of neighbour density rather than colony size on synchrony in kittiwakes (Rissa tridactyla). In fact, they found a higher synchrony in small colonies, since large ones had both high- and low-density sub-colonies. We thus think that local neighbour–neighbour interactions occurring within colonies may be an overlooked and relevant mechanism synchronizing breeding schedules, since there are synchrony patterns found at lower scales than those at which Darling's hypothesis and the environmental hypotheses apply.
Moreover, we propose that relevant neighbour–neighbour behavioural traits are likely to be adaptive owing to their proved potential relevance for bird fitness. Bird colonies are not peaceful places (e.g. Stokes & Boersma 2000). Colonies are huge clusters of very small territories (sometimes encompassing only some centimetres around the nest). Consequently, damage or injury caused unintentionally as a result of normal behaviour in the colony and also intentional fights (Thomas 1986; Stokes & Boersma 2000), and stealing of nest material (Wittenberger & Hunt 1985) are very frequent. These interactions occur so often, that they may cause more damage to fitness through loss of eggs and chicks than other factors, including external predation (Nelson 1980). Interactions are so relevant that even killing of adults can occur (Schüz 1944).
Thus, it seems logical that natural selection will act on behaviour to reduce these costs. In fact, Murphy & Schauer (1996) reported a higher breeding success of common guillemots (Uria aalge) that were more synchronous with their neighbours. Interestingly, a recent study demonstrates the adaptive value of one of these behaviours: common guillemots show appeasement displays towards neighbours (e.g. parasite removal), and those birds doing so lower the chances of having their eggs or chicks killed by neighbours, thus increasing their fitness (Lewis et al. 2007). Moreover, Kober & Gaston (2003) found a decrease in allopreening between neighbours and an increase in aggressive acts after hatching in Brünnich's guillemots (Uria lomvia), mirroring the higher probability of egg than chick dislodgement during adult aggression.
Accordingly, we can assume it is adaptive for birds to have some degree of certainty that their eggs will not be destroyed (deliberately or not) by very agitated neighbours. This aversion to uncertainty when laying eggs should be an ancient behaviour shared with non-colonial bird species. In the case of colonial species, a major source of uncertainty comes from interactions with close neighbours. Therefore, it may be necessary to be sensitive to the agitation of neighbours, becoming stressed and agitated in an agitated neighbourhood and calming down in a more peaceful social environment. If egg laying depends on a bird's stress level falling below a certain threshold, the sensitivity to the agitation or stress level of the neighbours will delay egg laying until a socially safe local neighbourhood is achieved.
The hypothesis that we explore in this paper is that this behaviour synchronizes neighbourhoods and also entire colonies, which can comprise thousands of birds. From the verbal formulation of our hypothesis, however, it is not clear whether local and global synchrony can be achieved by the proposed mechanism; how strong neighbour–neighbour interactions should be to produce synchronization; what are the individual adaptive consequences of modulating stress according to that of neighbours and how robust the proposed mechanism is regarding the dynamics of colony formation, colony size, habitat heterogeneity within the colony or other stressors that could introduce noise and thus counteract synchronization.
These questions are almost impossible to answer through empirical observations and field experiments. Thus, we tried to answer them by developing and analysing a simple and generic individual-based model (DeAngelis & Mooij 2005; Grimm & Railsback 2005). We show that reciprocal stress modulation between neighbours at the beginning of the breeding season is a powerful explanation of both local and whole-colony breeding synchrony in colonial birds, and that the individual behaviour we analyse may be adaptive whether or not whole-colony synchrony confers some advantage to individuals.
2. Material and methods
(a) Basic model description
The model description follows the ODD (overview, design concepts and details) protocol for describing individual- and agent-based models (Grimm & Railsback 2005; Grimm et al. 2006). The model is implemented in NetLogo v. 3.1.4 (Wilensky 1999; freely downloadable from http://ccl.northwestern.edu/netlogo/download.shtml) and available in the electronic supplementary material (basic model).
Purpose. The purpose of the model is to explore how the reciprocal modulation of stress levels between neighbouring birds within colonies can give rise to local and global breeding synchrony in a colony.
State variables and scales. The entities of the model are female birds breeding in immobile nests on a colony. A female is characterized by its own stress level (OSL) and the coordinates of its nest site. Nest sites are homogeneous and arranged on a 100×100 square grid, which represents the colony. Edge effects are avoided by applying periodic boundary conditions, i.e. the grid is a torus. One time step of the model corresponds to 1 day; simulations are usually run for 200 days or until all birds start breeding (however, breeding often occurred within 30 days of simulation). Simulations are run with a fully occupied colony, i.e. with 10 000 birds.
Process overview and scheduling. Every time step, the stress level of each bird is updated according to its OSL and that of its eight neighbours. If a female's stress level falls below a certain threshold (arbitrarily fixed at 10, which is 1/10 of the minimum initial OSL), she lays eggs and her stress level is set to zero until the end of the simulation, mirroring the typical tranquil behaviour of incubating birds (Birkhead 1978). Updating is synchronous: the calculation of the new OSL is based on the stress levels of the previous time step (asynchronous updating in a random sequence was also tested with no detectable change in the results).
Design concepts. Breeding synchrony at the local level, and in particular at the colony level, emerges from the interaction of neighbouring birds. Birds adapt their stress level to that of their neighbours: if all neighbours are stressed and show stressful behaviour, a bird's stress level might increase and her laying day therefore be delayed, thereby avoiding laying in a stressful neighbourhood. It is assumed that birds can sense the stress level of their direct neighbours (owing to their more aggressive interacting behaviour), but not of other birds. Stochasticity is assumed in the initial distribution of stress levels, where we assume a threefold difference between the least and most stressed individuals in order to test the synchronizing potential of the proposed stress-mediated mechanism. To observe the model output, we look at the distribution of laying dates at the end of simulations (range and standard deviation of laying dates, which are much used statistics in breeding synchrony studies; Gochfeld 1980) and also consider the spatial distribution of laying dates, i.e. clusters of contiguous birds that start breeding the same day. We also check the stress-level dynamics of individual birds.
Initialization. Simulations are initialized with 10 000 birds whose OSL is uniformly distributed between 100 and 300 (in arb. units).
Input. The model does not have any external input of driving environmental variables.
- Submodels. The model has only one submodel describing the stress-level dynamics of individual birds: each day, each bird's stress level (OSL) is updated according to its OSL the day before and the mean OSL of its eight neighbours (meanNSL)
where neighbourhood relevance (NR, from 0 to 1) is the relevance given to meanNSL. If NR=0, there is no interaction between neighbours at all. If NR=1, the stress level of the individual becomes equal to the mean stress level perceived from its neighbourhood. SD is the stress decay that is due to increasing day lengths. The linear decrease in OSL by SD in every time step is based on the following consideration: in temperate regions the main factor turning on the necessary endocrine pathways to start breeding is the elongation of day length (Ball & Bentley 2000; Wingfield et al. 2000). Moreover, there is evidence that it can be an advantage to breed early (Daan et al. 1989), presumably because there is limited time for breeding.(2.1)
For short time periods, the daily increase in day length is roughly constant, which is modelled by the linear decrease by SD. Thus, SD mirrors the increasing confidence of birds that breeding conditions are getting more favourable (e.g. owing to lower risk of adult and nestling starvation and by suffering adverse cold weather) and the increasing need of breeding as soon as possible. In this way, the updated stress level of each female each day is a weighted average (according to NR) of its own previous stress level and the social stress induced by close neighbours, and the elongation of the photoperiod.
(b) Robustness tests of the model
Other mechanisms than those proposed here could enhance colony breeding synchrony. Examples include the active clumping of individuals with similar breeding predisposition during the formation of colonies (something observed in several species; Kharitonov & Siegel-Causey 1988); stochasticity affecting a group (e.g. a sub-colony) delaying their reproduction, thus enhancing their relative synchrony when compared with the rest of the colony. These mechanisms really occur in nature, and introducing them into the model would only strengthen the synchronization effect of the reciprocity of stress proposed here. However, there are other factors often observed in bird colonies that could counteract the relevance of the mechanism proposed here. Moreover, colony size may also play a role in breeding synchrony.
We therefore analysed four modifications of our model in order to test the robustness of the stress-mediated mechanism in generating breeding synchrony (robustness tests in the electronic supplementary material). The modifications were: (i) a proportion p (up to 25%) of the birds, chosen randomly every day, has a stochastic component of its stress-level dynamics, which means that on those days no decay occurs due to SD, but instead a random value between 0 and SD is added to the stress level, (ii) a proportion of up to 50% of all nest sites is unavailable for breeding, simulating the potential barriers of (stress) information created by fine-grained habitat heterogeneity within colonies, (iii) not all birds are present at the colony on day 0, but a proportion of up to 50% arrives later, and (iv) different colony sizes from 9 to 1024 females. In all four modifications, we chose a conservative scenario that would be the strongest challenge of the stress-mediated synchronizing mechanism.
3. Results
(a) Individual stress-level dynamics
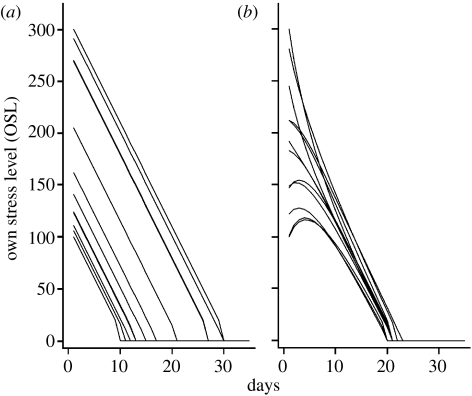
The decay of individual stress levels was identical (equal to SD) for all individuals when individuals did not modulate their stress level according to that of neighbours (i.e. for NR=0 in equation (2.1); figure 1a). However, for larger NR values, those birds with initially high stress levels ‘relaxed’ faster than a linear progress, and birds that were initially more relaxed had an initial increase in their stress levels (figure 1b). This is due to the random spatial location of individuals relative to their initial stress levels: an individual with a very low stress level is likely to have a neighbourhood with larger mean stress level, and the reverse happens for initially highly stressed individuals. After some time, however, stress-level dynamics follows the linear decrease determined by SD, but trajectories are closer to each other than for NR=0, i.e. the stress-level dynamics synchronize (figure 1b).
Figure 1.
Individual stress-level (OSL) dynamics. (a) No neighbour interaction (neighbourhood relevance NR=0); (b) NR=0.2. The upper and lower curves present the dynamics of the individual with the highest (300) and lowest (100) initial stress levels, respectively. The other 10 trajectories shown are from randomly selected birds. Note that when females achieve a stress level of 10 or less they start incubating (at the end of each individual trajectory), and thus the stress level becomes 0 the following day.
(b) Colony patterns
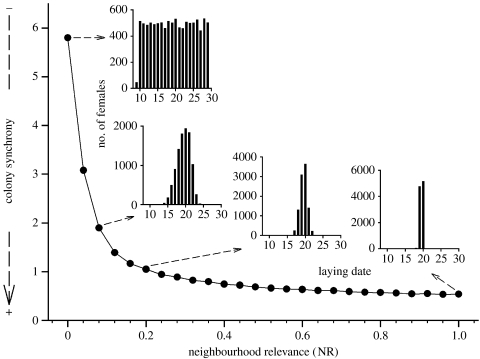
These individual dynamics have relevant colony consequences. For NR=0, owing to the parallel individual trajectories (figure 1a), the resulting histogram of laying dates follows a uniform distribution (figure 2) mirroring the same distribution in initial stress levels. This changes rapidly even if NR is only slightly increased (from 0 to 0.2), leading to a much higher breeding synchrony (lower standard deviation of laying dates and a peaked Gaussian-like distribution of laying dates; figure 2). Thus, giving even a small relevance to the stress of neighbours is enough to synchronize the whole colony.
Figure 2.
Relationship between the relevance (NR) given to the stress of neighbours (meanNSL) and the breeding synchrony of the colony. Synchrony is measured by the standard deviation of the laying dates of all the females of the colony (less standard deviation means more synchrony). Ten simulations were run for each NR; only the mean standard deviation of each of the 10 simulations is shown. Error bars of this mean are not plotted because they fall within the size of the dots. The insets show examples of the histograms found for single simulations run at different values of NR.
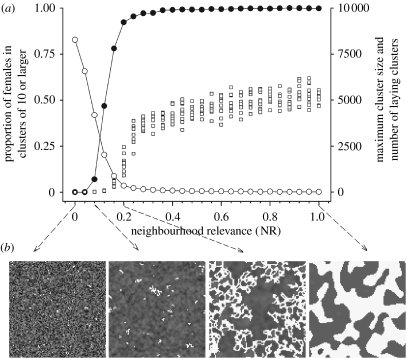
Moreover, laying synchrony has a spatial component. With NR=0, the spatial distribution of laying dates is random (figure 3). However, for NR>0, the spatial distribution of birds laying on the same day (i.e. laying clusters) shows characteristic reticulated patterns (figure 3). The number of laying clusters decreases and cluster size increases with increasing NR (figure 3). The distribution of cluster sizes is highly skewed with more small clusters than large ones. However, these few large ones group together the majority of females and extend through the colony even for low NR values (e.g. NR=0.2; figure 3).
Figure 3.
The size and shape of synchronization clusters. (a) Number of laying clusters (open circles) of contiguous females laying on the same day, for different values of NR. Also, the proportion of females laying in cluster sizes of 10 or larger (filled circles) and the maximum cluster size (open squares) out of 10 simulations run are shown. (b) Maps of the colony (100×100 females; each small square representing a female). Laying date coded by grey shades (darker shades representing earlier laying dates). Some sample clusters of females with the same laying date are shown in white (from left to right: 100, 50, 1 and 1 clusters, comprising a total of 130, 211, 3176 and 4892 females, respectively).
(c) Individual consequences
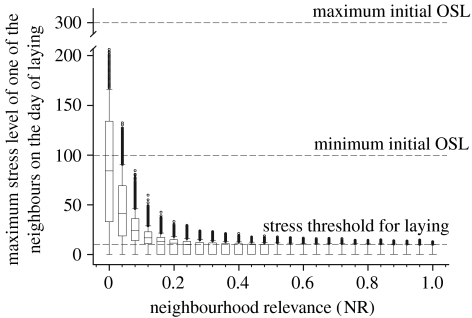
Giving some relevance to the stress level of neighbours (for NR>0) had important consequences for the social environment faced by females on their egg laying day. For NR=0, some females (by chance) experience a peaceful environment (the maximum stress level of their eight neighbours is very low), but typically the stress level of neighbours is very high. However, giving some relevance to neighbours rapidly lowers the social stress faced by females on their laying day (figure 4), i.e. even for relatively small values of NR, the mechanism described in equation (2.1) ensures that females will lay their eggs in a tranquil neighbourhood with other females already incubating or ready to do so.
Figure 4.
Individual fitness consequences of giving different relevance (NR) to the stress of neighbours (meanNSL). Box plot of the maximum stress levels perceived by each female on the day of laying. Dots show all the extreme values.
(d) Model robustness
In all four scenarios examined in our robustness tests, the stress-mediated mechanism proved to be very powerful in generating breeding synchrony at the local and colony levels, and no NR stronger than NR=0.2 had to be invoked (see the electronic supplementary material for details). Moreover, using a normal distribution of initial stress levels (instead of the uniform distribution used; results not shown), the same qualitative results were found, only differing in that a normal distribution of laying dates was also found for NR=0, although with a higher standard deviation than with NR>0.
4. Discussion
Our model shows that even a low degree of reciprocal stress modulation between neighbouring females can synchronize their breeding schedules. In these conditions, females finally breed in a socially safer environment. Thus, our model strongly predicts that giving some relevance to the stress level of neighbours is potentially adaptive and synchronizes breeding. This is in accordance with a recent study where birds showing appeasing behaviour towards neighbours increased their breeding success (Lewis et al. 2007). Moreover, an experimental study by Fetterolf (1984) showed that artificially increasing breeding synchrony (by placing eggs of similar age in experimental nests) reduced the strength of agonistic interactions among neighbours and increased reproductive success.
This adaptive behaviour performed at the scale of the neighbourhood (in the model, females were only affected by the stress level shown by the eight surrounding females) had colony-level consequences: the synchronization of breeding of a whole colony comprising 10 000 nests. Synchrony is commonly viewed as arising from some sort of cooperation, and competition is thought to destroy synchrony (reviewed by Helm et al. 2006). Note, however, that synchrony arises in the model not owing to females aiming to breed simultaneously, but because they try to avoid laying eggs with agitated females around.
The model was robust against modifications that take into account mechanisms potentially counteracting synchrony. The stress-mediated mechanism is thus also plausible in realistic scenarios, not only in idealized situations. Nevertheless, although our model reproduced important natural patterns of breeding synchrony in colonial birds, it failed to reproduce two further patterns. First, our model predicts a similar laying timing under similar environmental conditions, thus not explaining why in reality even neighbouring colonies can start breeding at different times (Emlen & Demong 1975). To explain this, we have to invoke colony-specific factors such as occasional perturbations by predators during colony formation. Second, our model produced symmetric laying date histograms (figure 2), but in nature these histograms often have a long tail on the right-hand side, i.e. some individuals breed much later than the bulk of the colony (reviewed by Gochfeld 1980). Interestingly, these long tails were reproduced when we introduced different arrival dates for different individuals, suggesting that some degree of individual heterogeneity needs to be added to our basic hypothesis to be able to reproduce some fine-grained patterns found in nature.
Our model provides a new perspective on breeding synchrony and could stimulate reanalyses of existing data and new field studies and experiments. First, we have found that for some of the outputs of the model, such as the spatial patterns shown in figure 3, there is no similar information in the literature. However, data are very likely to exist that would allow similar useful maps to be drawn. For instance, Mougin et al. (2001) reported that starting from a given nest, laying date differences with neighbours first increased and then decreased again at larger distances from the focal nest. This is precisely the expected pattern if there is laying contagion between neighbours and multiple focuses of early breeding as shown in figure 3. Ironically, however, Catry et al. (2006) argued that the results of Mougin et al. do not suggest any kind of local synchronization, but our results show that this conclusion might not be valid.
Second, our results suggest focusing more on local processes occurring within colonies to understand colony patterns. Currently, studies are either directed towards describing and explaining synchrony at the colony level (Gochfeld 1980) or focused on understanding local processes (Lewis et al. 2007). Our study strongly suggests that local interactions and colony-level patterns are inseparably linked to each other. This is supported by current models and empirical data on complex systems (as a bird colony certainly is), which show that even very local processes can lead to global patterns (Tilman et al. 1997; Camazine et al. 2001; Strogatz 2003; Solé & Bascompte 2006). Thus, further work is needed to understand the scaling up from the local processes to the global patterns found in bird colonies.
Work of this kind could help resolve other mysterious collective patterns found in colonial birds. One example is the strange pulses of colony attendance found in seabirds during colony formation (Harris 1984; Wilhelm & Storey 2002). Currently, only hypotheses have been posed to explain the adaptive reason for this collective pattern, but the issue is still open (Harris 1984; Wilhelm & Storey 2002). The solution could come from understanding how many individuals showing one or several types of key (social) behaviour can lead to these cycles that are very difficult to explain.
We showed how sensitive synchrony is to the level of NR. At the same time, NR is significant with regard to natural selection, because the higher the NR, the higher is the individual bird's fitness (figure 4). Thus, we have a solid framework (natural selection) for deriving predictions about global patterns according to scenarios potentially shaping NR both intra- and interspecifically. For instance, NR should be larger at large densities. This is because at higher densities the chances of conspecific aggressions and egg–chick losses are higher (e.g. Birkhead 1978). Accordingly, Reed et al. (2006) reported that 75% of approximately 2800 breeding pairs of a high-density breeder, the common guillemot, breed within a week while shags (Phalacrocorax aristotelis), which have colonies with lower nest densities, showed a much more extended breeding season. This was although both species shared the same island and fed on the same food. Moreover, we predict that denser, rather than larger, colonies should be more synchronous (see robustness tests in the electronic supplementary material). Accordingly, Coulson & White (1960) reported an effect of density rather than size on colony synchrony in kittiwakes, supporting our prediction. In fact, they found a higher synchrony in small colonies, since large ones had both high- and low-density sub-colonies. A positive effect of density on synchrony was also found by Birkhead (1977).
Our focus was on proximate mechanisms of breeding synchrony in colonial birds. We have deliberately ignored the question about the ultimate explanation of breeding synchrony: why do birds synchronize breeding in colonies? That is, what is the ultimate (adaptive) reason for breeding synchrony? Our study shows that this is a much more difficult question than previously thought. Several ultimate explanations have been suggested, including predation risk reduction through predator satiation (Darling 1938), finding a close mate in a similar breeding stage in case of divorce (Mougin et al. 2001), allowing fledglings to create flocks thus reducing predation risk and enhancing foraging success (Emlen & Demong 1975) and creating a secure place to breed because all birds are in a similar breeding stage (Yom-Tov 1975; Nelson 1980).
The hypothesis proposed by Yom-Tov (1975) is close to ours and illustrates the problem with current approaches to the subject. Note that this hypothesis is an ultimate explanation that did not explain how breeding synchrony emerges. By contrast, our hypothesis is a proximal one that does not aim at understanding the adaptive value of breeding synchrony at the colony level per se, but how it arises from individual-level adaptive behaviour. In fact, fitness consequences due to effects of synchrony at the colony level might be positive, negative or irrelevant. For instance, many predators can be attracted owing to the huge wave of chicks that are easy to catch. A more subtle consequence of breeding synchrony is reported by Reed et al. (2006) who studied the plasticity of individual laying dates in response to prevailing weather conditions in common guillemots. Contrary to previous studies in non-colonial birds (e.g. great tits Parus major; Nussey et al. 2005), they found a very low individual variability in their reaction to a large-scale environmental cue, the North Atlantic Oscillation (NAO). Moreover, they found a stabilizing selection against individuals departing from the populational mean response to NAO. This means that the whole population can be unable to react to changing climatic conditions because the proximate mechanisms underlying breeding synchrony are dominant.
We suggest that although breeding synchrony at the colony level could be irrelevant or even maladaptive (Wittenberger & Hunt 1985), it occurs as a by-product of the adaptive individual-level behaviour of giving some relevance to the stress level of neighbours. We propose this because neighbour–neighbour interactions are unavoidable in a colonial context, while the effects of colony-level synchrony for individuals could be more changeable according to external factors such as position within colony or abundance of predators. For instance, synchrony could be good against a satiable territorial predator occurring one year but disastrous against a gregarious predator that is attracted to the colony owing to the wave of chicks that are easy to prey upon. Thus, colony synchrony per se could be either positive, neutral or negative in different years, producing a less directional selection than the always occurring neighbour–neighbour interactions in a colonial context.
This leads to questions related to the levels of natural selection when self-organization processes are involved (Kitchen & Packer 1999), and maybe breeding synchrony could be an appropriate subject for exploring this field. However, we have shown that shifting our attention to the adaptive value of individual behaviour rather than that of collective (colony) patterns per se could be a promising avenue of research where unrelated individuals are involved, as is the case in complex vertebrate groups such as bird colonies.
Acknowledgments
We thank Fernando Hiraldo, Fabriccio Sergio, David Serrano, Julio Blas, José Luis Tella, Ainara Cortés-Avizanda, Carlos Rodríguez, Manuela G. Forero, Esperanza Ursúa and Martina Carrete, who gave valuable suggestions for this work. We would also like to thank three anonymous reviewers for their helpful comments.
Supplementary Material
Details of robustness tests and source code
NetLogo individual-based-model of the basic model studied in the ms.
NetLogo individual-based-model with the robustness tests applied to the basic model of the ms.
References
- Ball, G. F. & Bentley, G. E. 2000 Neuroendocrine mechanisms mediating the photoperiodic and social regulation of seasonal reproduction in birds. In Reproduction in context: social and environmental influences on reproduction (eds K. Wallen & J. E. Schneider), pp. 129–158. Cambridge, MA: MIT Press.
- Birkhead T.R. The effect of habitat and density on breeding success in the common guillemot (Uria aalge) J. Anim. Ecol. 1977;46:751–764. doi:10.2307/3638 [Google Scholar]
- Birkhead T.R. Behavioural adaptations to high density nesting in the common guillemot Uria aalge. Anim. Behav. 1978;26:321–331. doi:10.1016/0003-3472(78)90050-7 [Google Scholar]
- Burger J. Colony size: a test for breeding synchrony in herring gull (Larus argentatus) colonies. Auk. 1979;96:694–703. [Google Scholar]
- Camazine S, Deneubourg J.-L, Franks N.R, Sneyd J, Theraulaz G, Bonabeau E. Princeton University Press; Princeton, NJ: 2001. Self-organization in biological systems. [Google Scholar]
- Catry P, Granadeiro J.P, Oliveira P. Do Cory's shearwaters Calonectris diomedea synchronize laying among close neighborhoods? A reappraisal using data from artificial nest sites. Acta Ethol. 2006;9:87–90. doi:10.1007/s10211-006-0022-6 [Google Scholar]
- Coulson J.C. Colonial breeding in seabirds. In: Schreiber E.A, Burger J, editors. Biology of marine birds. CRC Press; Boca Raton, FL: 2002. pp. 87–113. [Google Scholar]
- Coulson J.C, White E. The effect of age and density of breeding birds on the time of breeding of the kittiwake Rissa tridactyla. Ibis. 1960;102:71–86. [Google Scholar]
- Daan S, Dijkstra C, Drent R.H, Meijer T. Food supply and the annual timing of avian reproduction. In: Oulet H, editor. Acta XIX Int. Congress of Ornithology. University of Ottawa Press; Ottawa, Canada: 1989. pp. 392–407. [Google Scholar]
- Darling F.F. Cambridge University Press; Cambridge, UK: 1938. Bird flocks and the breeding cycle. [Google Scholar]
- DeAngelis D.L, Mooij W.M. Individual-based modeling of ecological and evolutionary processes. Annu. Rev. Ecol. Syst. 2005;36:147–168. doi:10.1146/annurev.ecolsys.36.102003.152644 [Google Scholar]
- Emlen S.T, Demong N.J. Adaptive significance of synchronized breeding in a colonial bird: a new hypothesis. Science. 1975;188:1029–1031. doi: 10.1126/science.1145188. doi:10.1126/science.1145188 [DOI] [PubMed] [Google Scholar]
- Fetterolf P.M. Aggression, nesting synchrony, and reproductive fitness in ring-billed gulls. Anim. Behav. 1984;32:1004–1010. doi:10.1016/S0003-3472(84)80213-4 [Google Scholar]
- Gochfeld M. Mechanisms and adaptive value of reproductive synchrony in colonial seabirds. In: Burger J, Olla B.L, editors. Behavior of marine animals. Plenum Publishing Corporation; New York, NY: 1980. pp. 207–270. [Google Scholar]
- Grimm V, Railsback S.F. Princeton University Press; Princeton, NJ: 2005. Individual-based modeling and ecology. [Google Scholar]
- Grimm V, et al. A standard protocol for describing individual-based and agent-based models. Ecol. Modell. 2006;198:115–126. doi:10.1016/j.ecolmodel.2006.04.023 [Google Scholar]
- Harris M.P. T & AD Poiser; Staffordshire, UK: 1984. The puffin. [Google Scholar]
- Helm B, Piersma T, van der Jeugd H. Sociable schedules: interplay between avian seasonal and social behaviour. Anim. Behav. 2006;72:245–262. doi:10.1016/j.anbehav.2005.12.007 [Google Scholar]
- Kharitonov S.P, Siegel-Causey D. Colony formation in seabirds. Curr. Ornithol. 1988;5:223–272. [Google Scholar]
- Kitchen, D. M. & Packer, C. 1999 Complexity in vertebrate societies. In Levels of selection in evolution (ed. L. Keller). Monographs in Behavior and Ecology, pp. 176–196. Princeton, NJ: Princeton University Press.
- Kober K, Gaston A.J. Social interactions among breeding Brünnich's guillemots Uria lomvia suggest constraints in relation to offspring vulnerability. Ibis. 2003;145:413–418. doi:10.1046/j.1474-919X.2003.00179.x [Google Scholar]
- Lewis S, Roberts G, Harris M.P, Prigmore C, Wanless S. Fitness increases with partner and neighbour allopreening. Biol. Lett. 2007;3:386–389. doi: 10.1098/rsbl.2007.0258. doi:10.1098/rsbl.2007.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougin J.-L, Jouanin C, Mougin M.-C, Roux F. The influence of neighbours on breeding synchrony in Cory's shearwater Calonectris diomedea. Mar. Ornithol. 2001;29:51–56. [Google Scholar]
- Murphy E.C, Schauer J.H. Synchrony in egg-laying and reproductive success of neighboring common murres, Uria aalge. Behav. Ecol. Sociobiol. 1996;39:245–258. doi:10.1007/s002650050287 [Google Scholar]
- Nelson B. Hamlyn; London, UK: 1980. Seabirds. Their biology and ecology. [Google Scholar]
- Nussey D.H, Postma E, Gienapp P, Visser M.E. Selection on heritable phenotypic plasticity in a wild bird population. Science. 2005;310:304–306. doi: 10.1126/science.1117004. doi:10.1126/science.1117004 [DOI] [PubMed] [Google Scholar]
- Reed T.E, Wanless S, Harris M.P, Frederiksen M, Kruuk L.E.B, Cunningham E.J.A. Responding to environmental change: plastic responses vary little in a synchronous breeder. Proc. R. Soc. B. 2006;273:2713–2719. doi: 10.1098/rspb.2006.3631. doi:10.1098/rspb.2006.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüz E. Nest-erwerb und nest-besitz beim Weissen storch. Z. Tierpsychol. 1944;6:1–25. [Google Scholar]
- Setiawan A.N, Davis L.S, Darby J.T, Lokman P.M, Young G, Blackberry M.A, Cannell B.L, Martin G.B. Effects of artificial social stimuli on the reproductive schedule and hormone levels of yellow-eyed penguins (Megadyptes antipodes) Horm. Behav. 2007;51:46–53. doi: 10.1016/j.yhbeh.2006.08.002. doi:10.1016/j.yhbeh.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Solé R.V, Bascompte J. Princeton University Press; Princeton, NJ: 2006. Self-organization in complex ecosystems. [Google Scholar]
- Stokes D.L, Boersma P.D. Nesting density and reproductive success in a colonial seabird, the magellanic penguin. Ecology. 2000;81:2878–2891. doi:10.2307/177348 [Google Scholar]
- Strogatz, S. H. 2003 SYNC The emerging science of spontaneous order New York, NY: Hyperion.
- Thomas B.T. The behavior and breeding of adult maguari storks. Condor. 1986;88:26–34. doi:10.2307/1367749 [Google Scholar]
- Tilman D, Lehman C.L, Kareiva P. Population dynamics in spatial habitats. In: Tilman D, Kareiva P, editors. Spatial ecology. The role of space in population dynamics and interspecific interactions. Princeton University Press; Princeton, NJ: 1997. pp. 3–20. [Google Scholar]
- Waas J.R, Caulfield M, Colgan P.W, Boag P.T. Colony sound facilitates sexual and agonistic activities in royal penguins. Anim. Behav. 2000;60:77–84. doi: 10.1006/anbe.2000.1415. doi:10.1006/anbe.2000.1415 [DOI] [PubMed] [Google Scholar]
- Waas J.R, Colgan P.W, Boag P.T. Playback of colony sound alters the breeding schedule and clutch size in zebra finch (Taeniopygia guttata) colonies. Proc. R. Soc. B. 2005;272:383–388. doi: 10.1098/rspb.2004.2949. doi:10.1098/rspb.2004.2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky, U. 1999 NetLogo Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL. See http://ccl.northwestern.edu/netlogo
- Wilhelm S.I, Storey A.E. Influence of cyclic pre-lay attendance on synchronous breeding in common murres. Waterbirds. 2002;25:156–163. doi:10.1675/1524-4695(2002)025[0156:IOCPAO]2.0.CO;2 [Google Scholar]
- Wingfield J.C, Jacobs J.D, Tramontin A.D, Perfito N, Meddle S.L, Maney D.L, Soma K. Toward an ecological basis of hormone–behavior interactions in reproduction of birds. In: Wallen K, Schneider J, editors. Reproduction in context: social and environmental influences on reproduction. MIT Press; Cambridge, MA: 2000. pp. 85–128. [Google Scholar]
- Wittenberger, J. F. & Hunt, G. L. 1985 The adaptive significance of coloniality in birds. In Avian biology, vol. 8 (eds D. S. Farner & J. R. King), pp. 1–78. San Diego, CA: Academic Press.
- Yom-Tov Y. Synchronization of breeding and intraspecific interference in carrion-crow. Auk. 1975;92:778–785. [Google Scholar]
Notice of correction
Figure 4 is now presented in the correct form. 16 May 2008
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of robustness tests and source code
NetLogo individual-based-model of the basic model studied in the ms.
NetLogo individual-based-model with the robustness tests applied to the basic model of the ms.