Abstract
Many prey species have evolved defensive colour patterns to avoid attacks. One type of camouflage, disruptive coloration, relies on contrasting patterns that hinder predators' ability to recognize an object. While high contrasts are used to facilitate detection in many visual communication systems, they are thought to provide misleading information about prey appearance in disruptive patterns. A fundamental tenet in disruptive coloration theory is the principle of ‘maximum disruptive contrast’, i.e. disruptive patterns are more effective when higher contrasts are involved. We tested this principle in highly contrasting stripes that have often been described as disruptive patterns. Varying the strength of chromatic contrast between stripes and adjacent pattern elements in artificial butterflies, we found a strong negative correlation between survival probability and chromatic contrast strength. We conclude that too high a contrast leads to increased conspicuousness rather than to effective camouflage. However, artificial butterflies that sported contrasts similar to those of the model species Limenitis camilla survived equally well as background-matching butterflies without these stripes. Contrasting stripes do thus not necessarily increase predation rates. This result may provide new insights into the design and characteristics of a range of colour patterns such as sexual, mimetic and aposematic signals.
Keywords: chromatic contrast, predator–prey, colour signals, colour vision, disruptive coloration
1. Introduction
Animals are thought to have developed various adaptations to avoid the attacks of predators (Ruxton et al. 2004; Caro 2005). Many species protect themselves by means of camouflage, thus hindering predators' ability to either detect or recognize the prey. A well-known form of camouflage is background matching where animals disguise themselves by strongly resembling their background. However, disruptive coloration relies on contrasting patterns that impede recognition of animals as potential prey objects. Although researchers have made progress in demonstrating the effectiveness of disruptive coloration (Merilaita 1998; Cuthill et al. 2005; Merilaita & Lind 2005; Schaefer & Stobbe 2006; Stevens & Cuthill 2006; Stevens et al. 2006; Fraser et al. 2007), many aspects of how disruptive patterns deceive the visual and cognitive abilities of predators remain as yet unclear (Sherratt et al. 2005; Endler 2006).
Disruptive patterns consist of a combination of highly contrasting elements. In general, signal theory predicts that the more a visual signal contrasts to its background or to other parts of the body, the more conspicuous it is. However, contrasting elements may draw predators' attention away from the true outline of an animal, thus providing misleading information about prey appearance. In the influential work by Thayer (1909) and Cott (1940), it has been suggested that the highest contrasts yield the strongest disruptive effect (termed ‘maximum disruptive contrast’). Although field experiments on disruptive coloration by Cuthill et al. (2005) indicated that highly contrasting patterns indeed led to more effective camouflage than low-contrasting patterns, other predation experiments showed that artificial prey items suffered from a higher predation risk when pattern elements contrasted to the background than when pattern elements matched the background (Stevens et al. 2006). The latter result indicates that very high contrasts may lead to enhanced conspicuousness, thereby eliminating the protective effect of disruptive patterns. The central tenet of maximum disruptive contrast thus remains contentious, although it is clearly critical to understanding how disruptive coloration operates.
Visual contrast can be caused by differences in both achromatic and chromatic characteristics of adjacent pattern elements. Studies on foraging behaviour suggest that various animals use both achromatic and chromatic cues to detect prey and other food sources (e.g. Giurfa et al. 1997; Kelber 2005). Studies on predator–prey relationships indicate that prey species as diverse as reptiles, birds and insects are often more camouflaged with respect to chromatic cues than to achromatic cues (Hâstad et al. 2005; Théry et al. 2005; Stuart-Fox et al. 2006). Field experiments on disruptive coloration of artificial moths also suggest that colour contrasts may play a crucial role in achieving protection (Schaefer & Stobbe 2006). However, the definite relevance of chromatic cues for the effectiveness of disruptive coloration is yet to be investigated (Endler 2006; Stevens et al. 2006).
A number of recent studies supported the protective function of contrasting colour patterns if they are situated at the body margins, effectively breaking up the outline of objects (reviewed by Stevens et al. 2006). Contrasting patterns on the body outline are thus presumably a key phenomenon of disruptive coloration, providing more effective camouflage than the same patterns inside an object (Cuthill et al. 2005). Contrasting stripes running across the body have been interpreted as a form of disruptive coloration in a variety of animals (e.g. Brattstrom 1955; Oxford & Gillespie 1998; Messenger 2001). Stripes might prevent prey recognition by either segmenting the body into several apparently unconnected parts or fooling predators' visual edge detection mechanism when stripes touch the body outline (Osorio & Srinivasan 1991). Among the most commonly cited examples are wing patterns with strongly contrasting stripes in butterflies (Higgins & Riley 1970; Edmunds 1974; Brakefield et al. 1992; Fric et al. 2004). However, owing to the paucity of experimental support for these assumptions, a recent review concludes that evidence for stripes serving as disruptive coloration is weak (Stevens 2007).
To test whether contrasting stripes can provide a protective effect, we used a well-established method to evaluate avian predation rates on artificial butterflies in a deciduous forest (see Cuthill et al. 2005; Schaefer & Stobbe 2006). In our experiments, we used the wing pattern of Limenitis camilla, bearing a bright stripe running across the brown wings. By creating butterflies that differed only in the chromatic but not in the achromatic contrast of their potentially disruptive white stripes to the background as well as to the adjacent brown coloration of the wings, we tested the fundamental premise of maximum disruptive contrast. This assumption predicts that enhanced colour contrast of disruptive patterns leads to increased survival benefits of artificial butterflies. Furthermore, we tested whether predation rates differ depending on which parts of the wings contrast to the background and which parts blend into it.
2. Material and methods
(a) Models
We used the white admiral (L. camilla) as a model species. Limenitis camilla (Lepidoptera: Nymphalidae) is a common diurnal butterfly in central Europe, which sunbathes with outspread wings on small forest paths and oak trees (Lederer 1960; Steiner 2004). Most Palaearctic Limenitis have been considered to be disruptively coloured (Higgins & Riley 1970; Edmunds 1974; Brakefield et al. 1992), and predation experiments with jays revealed no apparent distastefulness of several Limenitis species (Platt et al. 1971). Limenitis camilla is monomorphic; all individuals wear a broad white stripe on fore- and hindwings.
Artificial butterflies were printed with a Xerox Phaser 8200DX printer (1200 dpi) on different kinds of paper, but in each case on the same day to avoid differences in colour quality due to changes in the printer toner. The models matched L. camilla in size (wingspan 50 mm) and pattern. As in the sole experiment on the potentially disruptive effect of wing stripes (Silberglied et al. 1980), we additionally created a butterfly whose bright stripes were covered by the brown coloration of the rest of the wing. Obliterating the stripes resulted in a uniform brown model with no obvious resemblance to an indigenous unpalatable species, making a mimetic relationship to other butterflies unlikely. To evaluate whether the entirely brown models are protected by background matching, we compared the survival probabilities of the entirely brown model against that of an artificial moth demonstrated to be cryptic which we used in previous experiments (Schaefer & Stobbe 2006). The latter model resembled the prime example of background matching, the white form of the peppered moth (Biston betularia), in colour contrasts against white birch trunks. The moth models showed lower predation rates on birch trees than on other backgrounds (oak bark and moss) and had a significantly better survival probability than the models that contrasted strongly to the background of birch trees. Predation experiments were carried out in 2006 and 2007 in the same forest patch, and we found no differences in predation pressure between years. We assumed in this comparison that the artificial moth and the butterfly model did not differ strongly in post-detection attractiveness to predators.
The manipulation of the models was carried out using PhotoShop v. 8. To evaluate the general relevance of our results, we additionally produced models with a reversed pattern, i.e. white artificial butterflies with brown stripes and, for an experiment with a paired design, entirely white models.
(b) Colour measurements
To assess colour objectively, we measured the reflectance spectra of 10 artificial butterflies of each type, 10 oak bark samples as the natural background against which artificial butterflies were displayed and took 10 measurements of two L. camilla specimens. Colour measurements were carried out using an Ocean Optics USB2000 spectrometer and a Top Sensor System Deuterium–Halogen DH-2000 (both Ocean Optics) as a standardized light source. Reflectance was measured as the proportion of a standard white reference tile (diffuse PTFE, WS-2). Measurements were taken using a coaxial fibre cable (QR400-7, Ocean Optics) that was mounted inside a black plastic tube to exclude ambient light. The angle of illumination and reflection was fixed at 45°. The ambient light was measured 2 m above ground with a cosine-corrected probe, which measured the incoming light over an angle of 180°. Before measuring the ambient light, the spectrometer was calibrated with a calibration lamp of known energy output (Ocean Optics LS-1-CAL).
To analyse contrasts between butterflies and background, we first modelled the probability of photon catches according to the model of Vorobyev & Osorio (1998) and Vorobyev et al. (2001) using the spectral sensitivities of blue tits (Cyanistes caeruleus; Hart et al. 2000) and the prevailing ambient light. We used the cone sensitivities of blue tits because they were the most common insectivorous birds in the study plot. Based on the photon catches of the four cones used for colour vision, we calculated chromatic contrasts (ΔS), while achromatic contrasts (ΔL) were calculated based on the photon catch of the avian double cone following Siddiqi et al. (2004). The units for ΔS and ΔL are jnds (just noticeable differences), where 1 jnd is at the discrimination threshold for birds. This threshold is set by noise originating in the cones and validated by comparison with behavioural detection thresholds (Maier & Bowmaker 1993). Values less than 1 jnd indicate that two colours are indistinguishable and values greater than 1 jnd indicate increasing contrasts (Cazetta et al. 2008). To assess whether butterfly models vary in their contrasts to the background, we tested for differences in contrasts based upon cone catches using one-way ANOVA and post hoc Scheffé tests.
We tested the concept of maximum disruptive contrast providing maximal camouflage for chromatic contrasts. The achromatic contrasts between the stripes and the remaining wing coloration or the background did not differ between the models (all mean values lay between 34.5 and 44.1 jnds; both ANOVA: F4,49<2.52, p>0.05). This is explicable because oil droplets in the double cone that presumably function in achromatic tasks absorb light below 430 nm (Hart 2001).
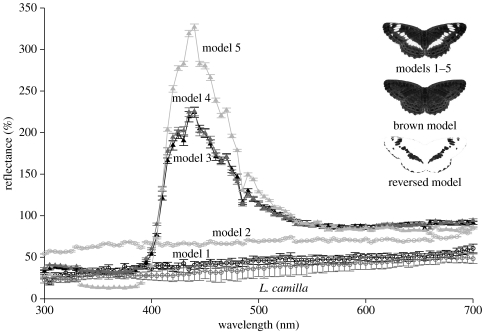
We printed butterfly models on different kinds of paper (producer: Soennecken, Streit, elite) that varied in reflectance. The stripes remained unprinted and thus only varied owing to variation in the reflectance of the paper type and not owing to the printer toner. For a bird's eye, not all models had white stripes (note the peak in reflectance at values approx. 430 nm for models 3–5 in figure 1). Since the stripes were always brighter than brown hues, we refer to the stripes as being ‘bright’ in the following. The brown parts of the wings of all models showed equal chromatic and achromatic contrasts to the background of oak bark (both ANOVA: F4,49<1.08, p>0.05).
Figure 1.
Reflectance spectra of the stripes of all the five models and L. camilla specimen. Shown are the mean values and standard errors (each n=10). The design of other model types is also illustrated; the reflectance spectra of the reversed model are identical to that of model 4.
The bright stripes varied in their chromatic contrast to the background and to the brown parts of the wings (both ANOVA: F4,49>17.1, p<0.001). Even though some of our models show spectra that are unlikely to occur in nature, the chromatic contrast values of all models fall well within the range of naturally occurring prey items (Schaefer et al. 2007). The contrast values between the background and the stripes of models 1 and 2 were lowest, while model 5 had the highest values (model 1: 15.4 jnds (mean) ±0.9 (s.e.), model 2: 18.1 jnds ±1.0, model 3: 27.7 jnds ±0.9, model 4: 29.1 jnds ±0.7, model 5: 36.4 jnds ±0.5; post hoc Scheffé test: models 1 and 2<models 3 and 4 with all p>0.001, model 5>models 1–4 with all p<0.001; see also figure 1). Models 1 and 2 with low-contrast values were very similar to each other and showed no differences in chromatic contrasts (both post hoc Scheffé test p>0.05). Both models 3 and 4 had intermediate values of similar contrasts as they were printed on the same kind of paper but in different years (both post hoc Scheffé test p>0.05).
Chromatic contrast values of the stripes to the brown parts of the wings showed a similar pattern, except that model 3 did not differ from models 1 and 2 (post hoc Scheffé test: models 1 and 2<model 4 with all p<0.001, model 5>models 1–4 with all p<0.001, model 1=model 2=model 3 with all p>0.05). Model 3 was printed with a toner different from that used for model 4, so that the brown parts were very similar but not exactly the same.
The comparison of the stripes of artificial models with the stripes of L. camilla specimen revealed that the stripes of models 1 and 2 showed the highest similarity to the stripes of that species (with chromatic contrast values between 3 and 7 jnds), whereas the stripes of models 3–5 showed significantly higher contrast values between 21 and 31 jnds.
(c) Survival experiments
We tested the survival probabilities of all models, including the model with the reversed pattern, in a mixed deciduous forest of 39 ha (Mooswald, Freiburg, Germany; 48° N, 8° E). For this purpose, each artificial butterfly was provided with a piece of brown Plasticine underneath the paper wings, which matched the brown parts of the wings and resembled the body of a butterfly. Plasticine bodies were approximately 2.5 cm long and projected underneath the wings approximately 1 cm. They were used instead of mealworms because these were often consumed by insects. The models were positioned on the trunks of oak trees (Quercus rubra) at a height of approximately 1.5 m, and trees were at least 5 m apart from each other. The survival of the artificial butterflies was checked after 24, 48, 72 and 96 hours. If birds attacked the butterflies, beak marks could be detected in the Plasticine bodies of the prey items (see also Brodie 1993). If insects attacked the models, feeding marks were much smaller and did not show the typical shape of a beak mark. Thirteen trials were run once a week from June to August 2006 and in June 2007. One of the models was tested in 2006 (‘model 4’) as well as in 2007 (‘model 3’). We found no effect of the year on the survival probability (Wald=0.001, p=0.98). Differences in the survival probabilities after 96 hours were analysed using Cox regressions.
(d) Paired experiments
Models sporting coloured stripes that differed from naturally occurring white stripes might have gained survival benefits owing to effects associated with unknown wing colours rather than owing to disruptive coloration. To test for such an effect, we compared the survival rates of the model with a reversed pattern (bright models with brown stripes) with those of entirely white models. We predicted that both butterflies should survive equally well if birds responded with neophobia. By contrast, the reversed model should survive better than the white butterfly if survival probabilities are a function of visual appearances. Both models were printed on the same paper as models 3 and 4. Differing from the other experiments, the models were glued on stones and equipped with a dead mealworm underneath the paper wings. The models were positioned in pairs (one reversed, one entirely white) with a separation of no more than 1 m in the Botanical Gardens of the University of Freiburg. The survival of the models was checked hourly. As soon as one of the models in a pair had been preyed upon, both models were removed. Observed predators were black redstarts (Phoenicurus ochruros) and house sparrows (Passer domesticus).
3. Results
To evaluate the camouflage effects of our models, we first tested whether the resemblance to the background of oak bark protected the entirely brown models against visual hunting predators. The comparison of survival rates of brown models with that of a background-matching model of B. betularia revealed that there were no differences in predation rates (Wald=0.21, p=0.65, n=90 and 47, respectively). We therefore considered the brown model as background matching and used it as a reference against which we tested the effects of contrasting stripes.
The first model we used (model 4) showed bright stripes of intermediate contrast running across the artificial wings. This model was as well protected as the brown model without stripes (Wald=2.686, p=0.1, both n=90) and the background-matching model of 2005 (Wald=2.661, p=0.1, n=90 and 47, respectively). The reversal of the pattern did not lead to a different survival probability. White models with brown stripes suffered from the same predation risk as brown models with white stripes printed on the same paper (Wald=0.12, p=0.73, both n=90).
In a paired test aimed to distinguish the effects of camouflage from that of unnatural wing coloration, we found that birds attacked entirely bright models more often than bright models with brown stripes (paired t-test, t=3.32, p<0.01, n=13).
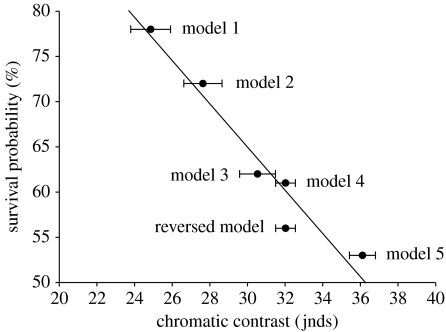
Brown models that differed in the chromatic contrast of the bright stripes against the remaining wing colour and against the background also differed in survival probabilities. Low levels of chromatic contrast (‘model 1’ and ‘model 2’) led to increased survival resulting in values equivalent to the survival probabilities of the cryptic model (both Wald<0.73, p>0.39, all n=90) and an even lower predation risk than the model with stripes of intermediate contrast (both Wald>4, p<0.05, all n=90). Increased chromatic contrast of the white stripes (‘model 5’) resulted in high predation rates. Model 5 suffered from a higher predation rate than the entirely brown model and models 1 and 2 (both Wald>5.25, p<0.02, all n=90). Consequently, and in contrast to the prediction of maximum disruptive contrast, we found a strong negative correlation between the survival probability and the chromatic contrast strength of the stripes to both the background and the brown parts of the wings (both r2>0.97, p<0.01; figure 2).
Figure 2.
Linear regression between the chromatic contrast strength of adjacent wing colours and the probability of surviving after 96 hours. Shown are the mean values and standard errors (each n=10).
4. Discussion
Our study is the first to show a negative relationship between the magnitude of chromatic contrast and the survival probability of disruptively coloured artificial butterflies. At the same time, however, our results document that contrasting stripes do not necessarily result in lower survival probabilities than those of monochrome brown butterflies. Because striped butterflies sporting stripes with low or intermediate contrasts survived equally well as monochrome butterflies and as a background-matching moth used in previous experiments, we conclude that contrasting stripes can, in spite of their conspicuousness, have a protective effect comparable with that of background-matching patterns.
In contrast to the predictions of maximum disruptive contrast, our experiments with models having different chromatic contrasts of the stripes demonstrate that chromatic contrast strength is negatively correlated with survival probability. Enhanced chromatic contrasts of stripes increase conspicuousness and thereby decrease survival probabilities rather than providing effective camouflage. This finding is concordant with the results of Stevens et al. (2006), which showed that heightened achromatic contrasts of marginal patterns do not increase the disruptive effect. Increased contrast strength seems to lead to improved camouflage only if all pattern elements match the background (Cuthill et al. 2005). Thus, we propose that the term maximum disruptive contrast is misleading as contrast above a critical threshold can only be maximized at the cost of increased conspicuousness. However, the generality of this conjecture should be tested with a range of different contrasts and patterns in future studies.
Conspicuousness can be achieved through differences in chromatic and achromatic characteristics of patterns. Because achromatic cues are used in tasks such as textural and edge discrimination (Osorio et al. 1999; Jones & Osorio 2004), they have attracted much attention. Several studies provided evidence for achromatic cues being used in foraging (e.g. Giurfa et al. 1997; Spaethe et al. 2001), particularly in the detection of small or distant objects. However, because the intensity of illumination can vary drastically, achromatic contrasts are thought to be less important in object identification (Kelber et al. 2003), and some insects and birds base their foraging decisions primarily on chromatic contrasts (Kelber 2005; Schaefer et al. 2006). Colour vision is therefore likely to be important for object detection or classification. Since we kept the achromatic contrast of stripes constant, our experiments show that chromatic contrasts determine the survival rates of our models and that chromatic cues alone are able to fool predators' perception.
Our experiments are the first to show that contrasting stripes running across the whole body of an animal do not necessarily increase conspicuousness, but can have a protective effect comparable with that of background matching. The only study to date that investigated the protective function of disruptive stripes in butterflies did not find any survival benefits of the striped morph compared with that of morphs with obliterated stripes (Silberglied et al. 1980). This result, however, does not necessarily exclude any protective effects of contrasting stripes as the obliteration of the stripes might have changed the appearance of the butterflies into either cryptic or mimetic coloration (Waldbauer & Sternburg 1983; Endler 1984). In our study, we showed that the brown reference model is protected by background matching. All models bearing stripes were a priori expected to suffer from higher predation rates than the brown background-matching model, as the stripes are high-contrast signals that should result in increased conspicuousness to predators (Lehrer & Bischof 1995; Giurfa et al. 1996; Ne'eman & Kevan 2001; Stuart-Fox et al. 2003). However, our results show that, depending on the contrast strength, stripes on the body surface can lead to a camouflage effect comparable with that of background-matching coloration. This finding indicates that many other species with striped coloration may be protected against visually hunting predators through disruptive camouflage.
Our results indicate that stripes influence survival rates if they contrast with the rest of the body, while contrasts with the background are less important. This is because reversed models with brown stripes that have low contrasts to the background survived equally well as Limenitis models printed on the same paper. Camouflage in Limenitis relies on strong contrasts of the bright stripe, both to the typical background and to the rest of the wings, while the brown parts of the wings blend into the brown background of bark and soil. Thus, bright stripes might draw birds' attention away from recognizing the butterflies as prey objects. This result corresponds to well-known theories about disruptive coloration stating that highly contrasting markings crossing the body distract predators' attention away from the real shape (Thayer 1909; Cott 1940). The novel part of our experiments is the insight that it is not relevant which parts of the body contrast to the background. Even if the stripes blend into the background of oak bark and the rest contrasts strongly to it, with the outline of the wings being clearly visible against the background, the artificial models survive as well as their brown counterparts with bright stripes.
We hypothesize that stripes have a protective function because predators perceive the stripes as independent objects that divide the whole wing surface into several apparently unconnected fragments. The visual mechanism underlying such an optical separation of wings might be similar to that of contrasting marginal patterns. Stripes might fool the edge detection mechanisms of predators (Osorio & Srinivasan 1991; Stevens & Cuthill 2006) as they touch the wing outline at both the top and the bottom of the wing. Marginal contrasting blotches can disguise the body outline and have been demonstrated to be more effective than ‘internal’ disruptive patterns (Cuthill et al. 2005). The points where stripes touch the outline are, however, relatively small compared with the proportion of bright colour inside the wing. At present, it is not clear whether the marginal areas of the stripes are indeed large enough to break up the whole outline of the wing.
The reflectance spectra of L. camilla specimen resemble the models with low chromatic contrasts and low predation risk (models 1 and 2). Although stripes in butterflies might be involved in and formed by sexual selection (Kronforst et al. 2006), sexual selection is unlikely to explain the white stripes in L. camilla, as this species is monomorphic in shape and colour of stripes; males readily mistake other males for females when confronted with a dead specimen (Lederer 1960). Our experiments with artificial models suggest that the coloration of L. camilla has most probably evolved as protective coloration against visually hunting predators.
In summary, our experiments show that although the colours of an animal need to be contrasting to achieve a disruptive effect, the protective value of disruptive coloration is abolished by strong contrasts owing to enhanced conspicuousness.
Acknowledgments
We are grateful to Karin Brandt and Frank Scherag for their help in conducting the experiments and to Graeme Ruxton, Sami Merilaita and an anonymous reviewer for their helpful comments on earlier versions of the manuscript. N.S. was sponsored by a PhD grant from the Cusanuswerk and H.M.S. by a grant from the German Science Foundation (Scha 1008/4-1).
References
- Brakefield P.M, Shreeve T.G, Thomas J.A. Avoidance, concealment, and defence. In: Dennis R.L.H, editor. The ecology of butterflies in Britain. Oxford University Press; Oxford, UK: 1992. pp. 93–119. [Google Scholar]
- Brattstrom B.H. The coral snake ‘mimic’ problem and protective coloration. Evolution. 1955;9:217–219. doi:10.2307/2405591 [Google Scholar]
- Brodie E.D., III Differential avoidance of coral snake banded patterns by free-ranging avian predators in Costa Rica. Evolution. 1993;47:227–235. doi: 10.1111/j.1558-5646.1993.tb01212.x. doi:10.2307/2410131 [DOI] [PubMed] [Google Scholar]
- Caro T. The adaptive significance of coloration in mammals. Bioscience. 2005;55:125–136. doi:10.1641/0006-3568(2005)055[0125:TASOCI]2.0.CO;2 [Google Scholar]
- Cazetta, E., Schaefer, H. M. & Galetti, M. In press. Why are fruits so colourful? The relative importance of achromatic and chromatic contrasts for detection by birds. Evol. Ecol (doi:10.1007/s10682-007-9217-1)
- Cott H. Methuen; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Cuthill I.C, Stevens M, Sheppard J, Maddocks T, Párraga C.A, Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Edmunds M. Longman; New York, NY: 1974. Defence in animals. [Google Scholar]
- Endler J.A. Progressive background matching in moths, and a quantitative measure of crypsis. Biol. J. Linn. Soc. 1984;22:187–231. doi:10.1111/j.1095-8312.1984.tb01677.x [Google Scholar]
- Endler J.A. Disruptive and cryptic coloration. Proc. R. Soc. B. 2006;273:2425–2426. doi: 10.1098/rspb.2006.3650. doi:10.1098/rspb.2006.3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S, Callahan A, Klassen D, Sherratt T.N. Empirical test of the role of disruptive coloration in reducing detectability. Proc. R. Soc. B. 2007;274:1325–1331. doi: 10.1098/rspb.2007.0153. doi:10.1098/rspb.2007.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fric Z, Konvicka M, Zrzavy J. Red & black or black & white? Phylogeny of the Araschnia butterflies (Lepidoptera: Nymphalidae) and evolution of seasonal polyphenism. J. Evol. Biol. 2004;17:265–278. doi: 10.1111/j.1420-9101.2003.00681.x. doi:10.1111/j.1420-9101.2003.00681.x [DOI] [PubMed] [Google Scholar]
- Giurfa M, Vorobyev M, Kevan P, Menzel R. Detection of coloured stimuli by honeybees: minimum visual angles and receptor specific contrasts. J. Comp. Physiol. A. 1996;178:699–709. doi:10.1007/BF00227381 [Google Scholar]
- Giurfa M, Vorobyev M, Brandt R, Posner B, Menzel R. Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic signals. J. Comp. Physiol. A. 1997;180:235–243. doi:10.1007/s003590050044 [Google Scholar]
- Hart N.S. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 2001;20:675–703. doi: 10.1016/s1350-9462(01)00009-x. doi:10.1016/S1350-9462(01)00009-X [DOI] [PubMed] [Google Scholar]
- Hart N.S, Partridge J.C, Cuthill I.C. Retinal asymmetry in birds. Curr. Biol. 2000;10:115–117. doi: 10.1016/s0960-9822(00)00297-9. doi:10.1016/S0960-9822(00)00297-9 [DOI] [PubMed] [Google Scholar]
- Hâstad O, Victorsson J, Ödeen A. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc. Natl Acad. Sci. USA. 2005;102:6391–6394. doi: 10.1073/pnas.0409228102. doi:10.1073/pnas.0409228102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins L.G, Riley N.D. Houghton Mifflin Co; Boston, MA: 1970. A field guide to the butterflies of Britain and Europe. [Google Scholar]
- Jones C.D, Osorio D. Discrimination of oriented visual textures by poultry chicks. Vision Res. 2004;44:83–89. doi: 10.1016/j.visres.2003.08.014. doi:10.1016/j.visres.2003.08.014 [DOI] [PubMed] [Google Scholar]
- Kelber A. Alternative use of chromatic and achromatic cues in a hawkmoth. Proc. R. Soc. B. 2005;272:2143–2147. doi: 10.1098/rspb.2005.3207. doi:10.1098/rspb.2005.3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelber A, Vorobyev M, Osorio D. Animal colour vision: behavioural tests and physiological concepts. Biol. Rev. 2003;78:81–118. doi: 10.1017/s1464793102005985. doi:10.1017/S1464793102005985 [DOI] [PubMed] [Google Scholar]
- Kronforst M.R, Young L.G, Kapan D.D, McNeely C, O'Neill R.J, Gilbert L.E. Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc. Natl Acad. Sci. USA. 2006;103:6576–6580. doi: 10.1073/pnas.0509685103. doi:10.1073/pnas.0509685103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer G. Verhaltensweisen der Imagines und der Entwicklungsstadien von Limenitis camilla camilla L. (Lep. Nymphalidae) Z. Tierpsychol. 1960;1715:521–546. [Google Scholar]
- Lehrer M, Bischof S. Detection of model flowers by honeybees: the role of chromatic and achromatic contrast. Naturwissenschaften. 1995;82:145–147. doi:10.1007/BF01177278 [Google Scholar]
- Maier E.J, Bowmaker J.K. Colour vision in the passeriform bird. Leiothrix lutea: correlation of visual pigment absorbance and oil droplet transmission with spectral sensitivity. J. Comp. Physiol. A. 1993;172:295–301. doi:10.1007/BF00216611 [Google Scholar]
- Merilaita S. Crypsis through disruptive coloration in an isopod. Proc. R. Soc. B. 1998;265:1059–1064. doi:10.1098/rspb.1998.0399 [Google Scholar]
- Merilaita S, Lind J. Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proc. R. Soc. B. 2005;272:665–670. doi: 10.1098/rspb.2004.3000. doi:10.1098/rspb.2004.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger J.B. Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 2001;76:473–528. doi: 10.1017/s1464793101005772. doi:10.1017/S1464793101005772 [DOI] [PubMed] [Google Scholar]
- Ne'eman G, Kevan P.G. The effect of shape parameters on maximal detection distance of model targets by honeybee workers. J. Comp. Physiol. A. 2001;187:653–660. doi: 10.1007/s003590100237. doi:10.1007/s003590100237 [DOI] [PubMed] [Google Scholar]
- Osorio D, Srinivasan M.V. Camouflage by edge enhancement in animal coloration patterns and its implications for visual mechanisms. Proc. R. Soc. B. 1991;244:81–85. doi: 10.1098/rspb.1991.0054. doi:10.1098/rspb.1991.0054 [DOI] [PubMed] [Google Scholar]
- Osorio D, Miklósi A, Gonda Z. Visual ecology and perception of coloration patterns by domestic chicks. Evol. Ecol. 1999;13:673–689. doi:10.1023/A:1011059715610 [Google Scholar]
- Oxford G.S, Gillespie R.G. Evolution and ecology of spider coloration. Annu. Rev. Entomol. 1998;43:619–643. doi: 10.1146/annurev.ento.43.1.619. doi:10.1146/annurev.ento.43.1.619 [DOI] [PubMed] [Google Scholar]
- Platt A.P, Coppinger R.P, Brower L.P. Demonstration of the selective advantage of mimetic Limenitis butterflies presented to caged avian predators. Evolution. 1971;25:692–701. doi: 10.1111/j.1558-5646.1971.tb01927.x. doi:10.2307/2406950 [DOI] [PubMed] [Google Scholar]
- Ruxton G.D, Sherratt T.N, Speed M. Oxford University Press; Oxford, UK: 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. [Google Scholar]
- Schaefer H.M, Stobbe N. Disruptive coloration provides camouflage independent of background matching. Proc. R. Soc. B. 2006;273:2427–2432. doi: 10.1098/rspb.2006.3615. doi:10.1098/rspb.2006.3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer H.M, Levey D.J, Schaefer V, Avery M.L. The role of chromatic and achromatic signals for fruit detection by birds. Behav. Ecol. 2006;17:784–789. doi:10.1093/beheco/arl011 [Google Scholar]
- Schaefer H.M, Schaefer V, Vorobyev M. Are fruit colors adapted to consumer vision and brids equally efficient in detecting colorful signals? Am. Nat. 2007;169:S159–S169. doi: 10.1086/510097. doi:10.1086/510097 [DOI] [PubMed] [Google Scholar]
- Sherratt T.N, Rashed A, Beatty C.D. Hiding in plain sight. Trends Ecol. Evol. 2005;20:414–416. doi: 10.1016/j.tree.2005.05.010. doi:10.1016/j.tree.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Siddiqi A, Cronin T.W, Loew E.R, Vorobyev M, Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. doi:10.1242/jeb.01047 [DOI] [PubMed] [Google Scholar]
- Silberglied R.E, Aiello A, Windsor D.M. Disruptive coloration in butterflies: lack of support in Anartia fatima. Science. 1980;209:617–619. doi: 10.1126/science.209.4456.617. doi:10.1126/science.209.4456.617 [DOI] [PubMed] [Google Scholar]
- Spaethe J, Tautz J, Chittka L. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl Acad. Sci. USA. 2001;98:3898–3903. doi: 10.1073/pnas.071053098. doi:10.1073/pnas.071053098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, H. 2004 Zwischen Licht und Schatten—zur Ökologie des Kleinen Eisvogels (Limenitis camilla) in der Davert/NRW. In Institut für Landschaftsökologie, vol. Diploma. Münster, Germany: Westfälische Wilhelms-Universität.
- Stevens M. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Soc. B. 2007;274:1457–1464. doi: 10.1098/rspb.2007.0220. doi:10.1098/rspb.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Cuthill I.C. Disruptive coloration, crypsis and edge detection in early visual processing. Proc. R. Soc. B. 2006;273:2141–2147. doi: 10.1098/rspb.2006.3556. doi:10.1098/rspb.2006.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Cuthill I.C, Windsor A.M.M, Walker H.J. Disruptive contrast in animal camouflage. Proc. R. Soc. B. 2006;273:2433–2438. doi: 10.1098/rspb.2006.3614. doi:10.1098/rspb.2006.3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Fox D, Moussalli A, Marshall N.J, Owens I.P.F. Conspicuous males suffer from higher predation risk: visual modelling and experimental evidence from lizards. Anim. Behav. 2003;66:541–550. doi:10.1006/anbe.2003.2235 [Google Scholar]
- Stuart-Fox D, Whiting M.J, Moussalli A. Camouflage and colour change: antipredator responses to bird and snake predators across multiple populations in a dwarf chameleon. Biol. J. Linn. Soc. 2006;88:437–446. doi:10.1111/j.1095-8312.2006.00631.x [Google Scholar]
- Thayer G.H. MacMillan; New York, NY: 1909. Concealing-coloration in the animal kingdom: an exposition of the laws of disguise through color and pattern. [Google Scholar]
- Théry M, Debut M, Gomez D, Casas J. Specific color sensitivities of prey and predator explain camouflage in different visual systems. Behav. Ecol. 2005;16:25–29. doi:10.1093/beheco/arh130 [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. doi:10.1098/rspb.1998.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M, Brandt R, Peitsch D, Laughlin S.B, Menzel R. Colour thresholds and receptor noise: behaviour and physiology compared. Vision Res. 2001;41:639–653. doi: 10.1016/s0042-6989(00)00288-1. doi:10.1016/S0042-6989(00)00288-1 [DOI] [PubMed] [Google Scholar]
- Waldbauer G.P, Sternburg J.G. A pitfall in using painted insects in studies of protective coloration. Evolution. 1983;37:1085–1086. doi: 10.1111/j.1558-5646.1983.tb05635.x. doi:10.2307/2408421 [DOI] [PubMed] [Google Scholar]