Abstract
Fossils from Liang Bua (LB) on Flores, Indonesia, including a nearly complete skeleton (LB1) dated to 18 kyr BP, were assigned to a new species, Homo floresiensis. We hypothesize that these individuals are myxoedematous endemic (ME) cretins, part of an inland population of (mostly unaffected) Homo sapiens. ME cretins are born without a functioning thyroid; their congenital hypothyroidism leads to severe dwarfism and reduced brain size, but less severe mental retardation and motor disability than neurological endemic cretins. We show that the fossils display many signs of congenital hypothyroidism, including enlarged pituitary fossa, and that distinctive primitive features of LB1 such as the double rooted lower premolar and the primitive wrist morphology are consistent with the hypothesis. We find that the null hypothesis (that LB1 is not a cretin) is rejected by the pituitary fossa size of LB1, and by multivariate analyses of cranial measures. We show that critical environmental factors were potentially present on Flores, how remains of cretins but not of unaffected individuals could be preserved in caves, and that extant oral traditions may provide a record of cretinism.
Keywords: Homo floresiensis, Homo sapiens, cretinism, congenital hypothyroidism, craniometry
1. Introduction
Fossils from Liang Bua (LB) on Flores, Indonesia, including LB1 (a nearly complete skeleton dated to 18 kyr BP) and LB6 (mandible and scapula, dated to 15 kyr BP), were assigned to a new species Homo floresiensis (Brown et al. 2004; Morwood et al. 2005). This attribution has provoked controversy but analysis of the brain endocast (Falk et al. 2005, 2007) and of cranial and postcranial material (Argue et al. 2006) supported the new species. Further details and analyses of shoulder bones (Larson et al. 2007) and of carpal (wrist) bones (Tocheri et al. 2007) have provided additional evidence of primitive features. The new species concept has been challenged in favour of attribution as modern humans (that is, species Homo sapiens) displaying genetic pathology (Weber et al. 2005; Jacob et al. 2006; Martin et al. 2006; Hershkovitz et al. 2007).
New evidence of modern humans in East Timor, with similar stone tools to those found at LB at 42 kyr BP (O'Connor 2007) strongly indicates modern human presence on Timor, and therefore on neighbouring Flores, well before these key fossils. Thus, hypotheses of the form that LB1 is a pathological human (species H. sapiens) should be tested. Various genetic dwarfisms (IGF-related postnatal growth retardation, pituitary dwarfism, primordial microcephalic dwarfisms) have been suggested. The first three of these were rejected in the initial publication (Brown et al. 2004) and the third also rejected by endocast comparisons (Falk et al. 2007). Laron Syndrome, which has skeletal manifestations similar to pituitary dwarfism, has been proposed as the pathology of LB1 (Hershkovitz et al. 2007).
Myxoedematous endemic (ME) cretinism is a form of dwarfism present in Indonesia in recent times (Goslings et al. 1977) and at high prevalence rates up to 4.7% in central Africa (Bastenie et al. 1962), and is an environmental disorder with potential to be maintained within a population indefinitely, unlike genetic dwarfisms that are subject to natural selection. We hypothesize that LB1 and LB6 are ME cretins, part of an inland population of (mostly unaffected) modern humans (H. sapiens). ME cretins are born without a functioning thyroid due to environmental factors including iodine deficiency. The resulting (congenital) hypothyroidism leads to severe dwarfism and reduced brain size, but less severe mental retardation and motor disability than in neurological endemic cretinism (Boyages et al. 1988; DeLong 1993). Sporadic cretinism is congenital hypothyroidism caused by genetic factors and sporadic cretins have similar skeletal manifestations.
Here, we compare the published descriptions and data on sporadic and endemic cretins with LB1 and LB6. Two sporadic cretins have been well described, a 28-year-old European male, Dolega's case (DC; Dolega 1891; Knaggs 1928), and a 23-year-old Singhalese female we refer to as Hill's cretin (HC; Hill 1936). Summaries of further descriptive data are provided from the literature and additional skulls that have been inspected. We obtained cranial measurements parallel to those of Howells' sample of modern humans without obvious pathology (Howells 1996) for five cretins and four microcephalics (one with Down's Syndrome (DS)) and some fossil hominins for comparison. These measurements were studied using multivariate analyses and compared with LB1 in order to test statistically the hypothesis that LB1 is a cretin.
2. Material and methods
Anteroposterior length of the pituitary fossa and whole brain of LB1 were measured from expanded images of the LB1 endocast (fig. S2E in Falk et al. 2005).
Individual cranial measures of 19 available variables of 2523 individuals from 28 modern human populations, including 69 Andaman Islanders, were taken from the data collected by Howells (1996), together with the same variables for LB1 (Brown et al. 2004), HC (Hill 1936) and ‘Nariokotome’ (Homo erectus: Walker & Leakey 1993). Additional measurements were made of HC from photographic images (British Museum of Natural History, BMNH 1949.12.7.16, skull marked 335 S f 23). Skulls from the Greig collection (GC), Royal College of Surgeons (RCS) of Edinburgh Museum, including four that were clearly cretins (GC5391, GC5458, GC5459 and GC5461), two microcephalics (GC0939 and GC5397) and one microcephalic DS (GC5464) were examined and measured. A microcephalic half-skull from RCS of England, Hunterian Museum (RCSHM/Osteo 95) and Wenner-Gren casts of ‘Kabwe’ (Homo heidelbergensis) and ‘Mrs Ples’ (Australopithecus africanus) were measured. The details of the skulls are provided in the electronic supplementary material, table S1. Independent of the numerical analysis, 9 out of the 19 cranial variables were selected based on predicted sensitivity to the relatively greater reduction and delay of endochondral compared with subperiosteal bone growth of cretins. The variables and the values used for LB1 and HC are provided in the electronic supplementary material, table S2.
The cranial measures were divided by geometric means of each individual in order to examine differences in shape, but not size, between individuals. Principal components analysis (PCA) was based on the correlation matrix and was repeated for the covariance matrix (software SPSS v. 13.0). As the variables have different variances, the correlation matrix is preferred (Quinn & Keough 2002; Brown et al. 2004), and as the conclusions are the same, only results using correlation matrix are reported. Null hypotheses (that the average dissimilarity between pairs of objects in different groups is the same as the average of the dissimilarities between pairs of the same group) were tested by analysis of similarities (ANOSIM), a non-parametric analogue of analysis of variance, using Primer v. 6 (Clarke & Gorley 2006) with Euclidean distance as the measure of dissimilarity. The test statistic (R) is normally positive with maximum value 1, with R=0 for the null hypothesis of no difference between groups. The p-values were calculated for 999 random changes of the labelling of groups (Clarke & Warwick 2001; Quinn & Keough 2002; Clarke & Gorley 2006). Discriminant function analysis (DFA), including jackknifing, was done using software SPSS v. 13.0.
3. Results
(a) Stature
ME cretinism in central Africa (Bastenie et al. 1962; Delange et al. 1971; Delange 1974) reduced the mean stature of affected adult males to approximately 70% of normal (a comparative image is reproduced in the electronic supplementary material, figure S1), similar to the estimated stature of LB1 compared with statures of male central highlanders of Flores (table 1).
Table 1.
Mean heights (m) of male and female adult ME cretins and male reference populations. (Cretins are Uele adult (more than 18 years) cretins with absent or doubtful thyroid (Dumont et al. 1963), Idjwi (Delange 1974) and Xinjiang myxoedematous cretins (Ma et al. 1982). Normals are reference populations as follows: Uele, Bantus of western shore of Lake Kivu; and Idjwi Island, Bantu/Nilotics of the eastern shore of Lake Kivu (Vis 1969). LB1 is included as Flores male cretin (Brown et al. 2004), but may be female, and Flores normals are Lio highlanders (Keers 1948).)
| male | female | total | |||
|---|---|---|---|---|---|
| population | ME cretins (m) | normals (m) | ratio | ME cretins (m) | ME cretins (m) |
| Uele | 1.04±0.15 (n=6) | 1.62 | 0.67 | 1.11±0.08 (n=9) | 1.08 |
| Idjwi | 1.20±0.04 (n=3) | 1.72 | 0.70 | 1.16±0.04 (n=3) | 1.18 |
| Xinjiang | — | — | — | — | 1.22 |
| Flores | 1.06 (est.) | 1.59 | (0.67) | — | — |
(b) Craniofacial features
Reduced cartilaginous skull growth produces depressed nasal bridge (Boyages et al. 1988), obvious in HC, DC and seems to be present in LB1, although there is some damage in this region. Furthermore, adult African (Uele) ME cretins (Melot et al. 1962) have open anterior fontanelles, also evident in DC, HC and possibly replicated in the damaged LB1. The vault is thick in DC and LB1. The Uele cretins, HC and DC have reduced cranial indices (73 or more), as does LB1. DC and LB1 have similar foramen magnum lengths (28, 31 mm), breadths (21, 24 mm) and breadth/length ratios (0.75, 0.77). Skull asymmetry is present in HC, GC cretins and LB1 (Falk et al. 2005; Jacob et al. 2006). Bony styloid, vaginal and anterior and posterior clinoid processes are absent in DC, GC cretins and LB1. Frontal sinuses are absent in European sporadic cretins (6–35 years old; Dreyfus et al. 1950), African ME cretins (Melot et al. 1962), DC and LB1. Forward projecting jaws and teeth are evident in DC and HC, as well as in ME cretins (Melot et al. 1962; Delange 1974), sporadic cretins (Engel et al. 1941; Bellini & Neves 1956) and LB1 and LB6.
Chins are variable and often much undeveloped in European cretins, e.g. flat symphysis in DC and male athyrotic cretin (Kranz 1912; electronic supplementary material, figures S2 and S3), and poorly developed chins are shown in adult athyrotic cretins (Ortner & Hotz 2005). Uele (Bastenie et al. 1962) and Idjwi (Delange et al. 1972) ME cretins display mandibular hypoplasia (evident in the electronic supplementary material, figure S4), and HC has a negative chin (positive incurvatio and negative prominence). Lack of chin development can be seen in sagittal radiographs of sporadic cretins (Silverman & Currarino 1960; König 1968), although radiographs including the chin are not available for Uele or Idjwi cretins. The lack of chin in LB1 and LB6 mandibles is therefore consistent with cretinism, but many contemporary humans on Flores have receding symphyses (Keers 1948; Jacob et al. 2006) that may point to a contribution of racial factors.
The teeth of sporadic cretins are normal size, with mean diameter of permanent molars within 1% of LB1 (electronic supplementary material, table S3), but their reduced body size makes them megadont, as is noted for LB1 (Brown et al. 2004). In cretinism, delayed and missing teeth lead to adult mixed dentition (Bellini & Neves 1956; Andersen 1961) as in DC and HC, and a sporadic cretin aged 20 years with stature 0.94 m had four deciduous molars and a deciduous canine with 21 permanent teeth (Taylor & Appleton 1929).
LB1's lower first premolars (P3s) are reported to have bifurcated roots, unlike H. sapiens, but these also show mesiodistal crown elongation relative to H. sapiens, unlike the other mandibular teeth (Brown et al. 2004, fig. 5). Captured images from X-ray scans presented in The mystery of the human hobbit (BBC Horizon, 2005) clearly show a buccogingival ridge sloping to the buccal surface of the mesial root, as in human lower first deciduous molars (dm1s) and unlike P3s that are symmetrical (Zeisz & Nuckolls 1949). Crown diameters (mesiodistal) and crown indices are consistent with Australian Aboriginal dm1s but not their P3s (p<0.01 for both male and female samples; electronic supplementary material, table S4). Occlusal surfaces of these putative P3s have distinctive angled ridges from the mesial to buccal border (approx. 45° to the mesiodistal axis) in both LB1 (evident on left only) and LB6 (evident on both). These are similar to the triangular occlusal surface described as a variant of the normal, more rectangular morphology of dm1s (Joergensen 1956; Butler & Hughes 1984; Kitagawa et al. 1996), and which have been reported in Australian Aborigines and Melanesians (Butler & Hughes 1984). The bifurcated roots are also consistent with dm1s, having divergent mesial and distal branches with some apical convergence, and the mesial root longer than the distal root. However, significant taurodontism, that is the presence of a substantial neck due to an apical displacement of the bifurcation of the roots, is evident in these and other LB1 teeth. Taurodontism is more pronounced in Australo-Melanesian populations in both deciduous and permanent molars (Rao & Arathi 2006). Therefore, the putative P3s of LB1 and LB6 appear to be deciduous molars (dm1s) that are retained due to the absence of the (permanent) first premolars that would normally displace them. When this occurs, the deciduous teeth can remain in good condition for extended periods and may show no root resorption (Ith-Hansen & Kjaer 2000).
(c) Postcranial features
Experimental thyroidectomy reduces growth in limb bone lengths (endochondral) more than widths (subperiosteal), thus simulating increased robusticity that was increased by a factor of 1.3 in experimental dog cretins (Dye & Maugham 1929). Both DC and LB1 show this (table 2) and have long bones that are smooth with poorly marked muscle scars inconsistent with true robusticity. Human cretins have long arms relative to legs, and the humerofemoral index variable and up to 78 (Schinz et al. 1952) probably due to greater length reduction in weight-bearing legs than in non-weight-bearing arms. In LB1, this figure is estimated to be 87 (normal European males, 71). Further data on long bone indices are presented in the electronic supplementary material, table S5, but the significance of differences between the athyroid cretins and LB1 is unclear owing to uncertain measurement conventions.
Table 2.
Relative robusticity (RR) of long bones of a sporadic cretin (DC) and LB1. (DC, Europeans (Dolega 1891) radius and tibia lengths are for diaphysis only. LB1, pygmy (Brown et al. 2004; Morwood et al. 2005) with pygmy means estimated from regression lines. RR is calculated from ratios of length (L) and breadth (W, for DC, Europeans) or circumference (C, for LB1, pygmy) as either ((W1/W2)/(L1/L2)) or ((C1/C2)/(L1/L2)). Absolute values in normal and italic rows are not directly comparable.)
| humerus | radius | femur | tibia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | W or C | RR | L | W | RR | L | W or C | RR | L | W | RR | |
| DC | 12.1 | 4.4 | 8.8 | 2.3 | 16.5 | 4.4 | 12.0 | 4.2 | ||||
| Europeans | 29.5 | 6.8 | 20.0 | 3.1 | 40.0 | 9.0 | 29 | 8.9 | ||||
| DC/Europeans | 0.41 | 0.65 | 1.56 | 0.44 | 0.74 | 1.68 | 0.41 | 0.49 | 1.20 | 0.41 | 0.47 | 1.14 |
| LB1 | 24.3 | 7.1 | 19.0 | — | 28.0 | 6.6 | 23.5 | — | ||||
| Pygmy | 26.3 | 4.9 | — | — | 35.8 | 6.8 | — | — | ||||
| LB1/pygmy | 0.93 | 1.45 | 1.57 | — | — | — | 0.78 | 0.98 | 1.25 | — | — | — |
Humeral torsion (Larson 1988) is reduced by an average of 20° in smaller Swiss adult endemic cretins (data provided in the electronic supplementary material, table S6 from Bircher 1908) and is also reduced in DC. LB1 humeral torsion was estimated to be reduced by 50° (Brown et al. 2004), but a later estimate of torsion is 120° compared with modern human mean of 142° (Larson et al. 2007). The reduction of 22° is therefore consistent with cretinism.
The right clavicle (LB1/5) is missing the medial (sternal) end, but the predicted length (L) is 91 mm (87.5–94.6; Larson et al. 2007) and the greatest width (W) of the acromial end is 16 mm (W/L=0.176 (0.169–0.183)). The clavicles of HC are 83 mm in length (but breadth unknown), each with no secondary centre at the sternal end, incomplete ossification at both the acromial and sternal ends, and prominent conoid processes consistent with that of the LB1 clavicle. Clavicles of DC are 78 mm in length and the greatest width of acromial ends 15 mm (W/L=0.192). Thus, DC and LB1 have greater relative acromial breadth (W/L) than African-American males (mean 0.150) and females (mean 0.142; Terry 1959).
A scapula assigned to LB6 (LB6/4) has a broad and square inferior end (Larson et al. 2007), consistent with unfused and still cartilaginous and therefore lost secondary centres for the inferior angle and the vertebral border (a useful comparative image is fig. 206 of Gray 1918). These centres are unfused in both HC and DC and as they normally fuse late, from ca 20 years (Miles & Bulman 1995) LB6 could be a young adult or older cretin with characteristic delayed fusion.
Medial femoral rotation is noted in two (out of four) cretins (Ortner & Hotz 2005) and femoral head inclination is reduced in cretins (Schinz et al. 1952), with further data being provided in the electronic supplementary material, table S6 from (Bircher 1908), and in LB1. The same applies to cretin humeri (Schinz et al. 1952) but the state of the LB1 humerus is not clear due to damage. The feet of DC are relatively large (10.5 cm, 64% of femur) compared with normal (20.4 cm, 51% of femur), as is the foot of LB1 (Jacob et al. 2006).
The posterior height of adult cretin lumbar vertebral bodies are much more reduced than the width because the epiphyses are not ossified, with a height-to-width ratio of 0.58 (calculated from an X-ray image, Borg et al. 1975). This is similar to the reduction in LB1, with a posterior height of 17 mm and a width of 32 mm (ratio 0.53), compared with normal European height of 22 mm and width of 32 mm (ratio 0.69; Jacob et al. 2006).
Recently, three carpal bones (left trapezoid, capitate and scaphoid) attributed to LB1 were reported to have primitive morphologies similar to Pan and Pongo (Tocheri et al. 2007). Carpal bones of adult cretins have not been described systematically, but trapezoid and scaphoid (also trapezium and lunate) of HC (at 22 years of age) are largely cartilaginous, DC carpals (at 28 years of age) are not fully ossified, and carpal bone ossification is greatly delayed (Frank & Brüll 1980). The most significant reported difference (Tocheri et al. 2007) between the carpals of LB1 and modern humans is that the palmar view of the trapezoid shows large articular surfaces and a very small non-articular surface, thus similar to the wedge-shaped trapezoids of the great apes. However, unlike, for example, chimpanzees and humans (Lewis 1989:101), the LB1 trapezoid appears to have a much smaller (anteroposterior) height than the scaphoid and capitate. To see this, the images of the LB1 scaphoid and capitate must be expanded by 125% and compared with the trapezoid (Tocheri et al. 2007; M. W. Tocheri 2007, unpublished datum). The known radiographs of adult cretins (Jacobs 1973; Borg et al. 1975; Thijn 1986) are posteroanterior radiographs and do not readily show the morphology of the palmar side nor the height of the carpal bones.
(d) Brain comparison and measurement of pituitary fossa (length of sella turcica)
HC and DC brains lacked obvious pathology at autopsy, consistent with the endocast of LB1 (Falk et al. 2005). An enlarged pituitary is noted for African ME cretins of Uele (Melot et al. 1962) and Idjwi Island (Delange 1974), HC and others (Bellini & Neves 1956; Andersen 1961).
The pituitary fossa (sella turcica) length of LB1 was measured to be 12.9 mm, within the range of Chinese ME cretins (14.0±3.1 mm, n=58) and outside normals (8.6±1.2 mm, n=5; He 1984), a highly significant (p<0.001, two tailed) result.
Congenital hypothyroidism can reduce brain size by approximately 50%. Brains of European adult male endemic cretins scaled with height to 735 g (700 cm3) at 1 m, based on the data presented in the electronic supplementary material, table S7 (De Quervain & Wegelin 1936). An adult female cretin's brain weighed 900 g (857 cm3; DeLong 1993), and an 18-month infant cretin had brain weight of 570 g (55% of normal for that age) with reduced gyri (Adams & Rosman 1971). In a severely iodine-deficient area of China, two 8-month foetus brains were 52.5% less than normal for age (De Quervain & Wegelin 1936; Liu et al. 1989) and the rate of microcephaly (less than 3 s.d. below USA norms) decreased from 27 to 11% following iodine administration (Cao et al. 1994).
(e) Morphometric analysis
The plot of the first three PCs on 19 cranial variables showed LB1 and the cretins located within the normal humans (electronic supplementary material, figure S7). However, for the two groups (i) LB1 and cretins and (ii) modern humans, the similarity is significantly greater within than between these groups (p<0.001, ANOSIM R=0.998), confirming them as separate groups. Similarly, the group of LB1 and cretins differed from the group of microcephalics (p=0.012, ANOSIM R=0.685).
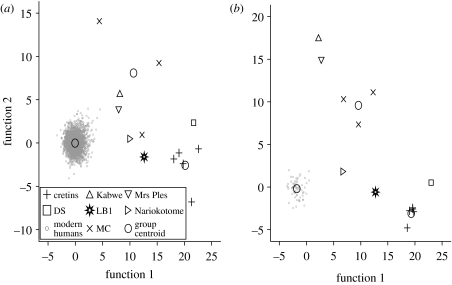
The first three PCs of the nine cranial variables showed greater separation of LB1 from modern humans, as shown in figure 1 for the calculation including all modern humans, and in figure 2 with only Andaman Islanders included. In these calculations, the two groups (i) LB1 and cretins and (ii) modern humans also have significantly greater similarity within than between groups for all modern humans (p<0.001, ANOSIM R=0.992) and for Andaman Islanders only (p<0.001, ANOSIM R=0.977). In addition, the group of LB1 and the cretins is significantly different from the microcephalics (p=0.012, ANOSIM R=0.914) and the group of LB1 and cretins is significantly different from Andaman Islanders and from the microcephalics (p=0.012, ANOSIM R=0.827).
Figure 1.
(a–c) Plots of the first three PCs from nine cranial measures, size adjusted, show LB1 grouping with cretins (n=5) and not with microcephalics (n=3) or normal modern humans. All are H. sapiens except Kabwe (H. heidelbergensis), Mrs Ples (A. africanus) and Nariokotome (Homo erectus (sensu lato)). These PCs explain 64.8% of the variance (37.2, 15.8 and 11.8%). Residual correlation with size is in the electronic supplementary material, figure S8 and the component matrix is in the electronic supplementary material, table S8.
Figure 2.
(a–c) Plots of the first three PCs from nine cranial measures, size adjusted, show LB1 grouping with cretins (n=5) and not with microcephalics (n=3) or normal modern humans. All are H. sapiens except Kabwe (H. heidelbergensis), Mrs Ples (A. africanus) and Nariokotome (Homo erectus (sensu lato)). These PCs explain 76.0% of the variance (40.1, 24.3 and 11.6%). The component matrix is in the electronic supplementary material, table S9.
DFAs using 19 cranial variables and 3 groups (cretins, microcephalics and modern humans) as well as unclassified individuals (LB1, DS and fossil hominins) generated two functions that, with jackknifing, correctly classified all modern humans (n=2523) and all cretins (n=5), and assigned LB1 to the cretin group with posterior probability p>0.999 (figure 3a). The fossil hominins were all assigned to the microcephalic group (p>0.999). When repeated with Andaman Islanders only (n=69), the same assignments resulted and the grouping of LB1 with cretins was even more apparent in the DFA plot (figure 3b) with the fossil hominins again assigned to the microcephalic group p>0.949.
Figure 3.
Plots of the discriminant functions 1 and 2 for three groups (modern humans, microcephalics and cretins) and unassigned individuals. (a) Modern human group is from 28 populations including Andaman Islanders (n=2523). (b) Modern human group is Andaman islanders only (n=69). In each case, these functions correctly classify all modern humans, and all cretins (n=5) but not all microcephalics (n=3), and assign LB1 to the group of cretins (posterior probability p>0.999). Numerical results are in the electronic supplementary material, tables S10 and S11.
4. Discussion
Many of the similarities between LB1 and patients with Laron Syndrome (Hershkovitz et al. 2007) also apply to other small human specimens including cretins, but there are important differences. In particular, growth hormone-insensitive dwarfs have delicate faces, marked chins, protruding foreheads, thin vaults and diploes, normal or even small pituitary fossa, and delicate long bones, hands and feet, all the reverse of conditions in athyrotic cretins and LB1.
We have described numerous anatomical features that are held in common between LB1 and modern human cretins. In cretins, most of these features are directly attributable to the special interferences in growth, which characterize cretinism, differ from the growth reductions of other dwarfing syndromes and are not present at all in non-cretinous specimens of other hominin species.
The pituitary fossa of LB1 is enlarged, despite the small size of the cranium, and a clear sign of pathology not noted previously. This increase in cretins is due to negative feedback control, with low thyroxine increasing pituitary size and therefore thyroid-stimulating hormone concentration (Yamada et al. 1976). Other conditions that may give rise to pituitary enlargement are rare (von Werder 1996), are due to local factors (e.g. tumours) and are not associated with the growth disturbances of cretinism. Laron Syndrome is not associated with increased pituitary fossa (Kornreich et al. 2003).
The craniometric PCA with all 19 variables (size standardized) showed that, for the first three PCs, the morphology of LB1 is within the variation present in modern humans, a result consistent with its attribution as H. sapiens. The ANOSIM, however, showed greater similarity within the groups of LB1 plus cretins and modern humans than between these groups. The PCA on the nine variables more especially sensitive to the growth disturbance of cretinism, however, not only separated LB1 from the full set of modern humans but also clearly grouped LB1 with the cretins and apart from the microcephalics. This result was even more apparent when only Andaman Islanders, a small-bodied population probably related to the aboriginal population of Flores but not the cretins were included. The significance of the grouping of LB1 with cretins and not microcephalics was confirmed by ANOSIM and further confirmed by DFA, and in each case the result was obtained with the full set of modern humans as well as a smaller set of Andaman Islanders. These results reject the null hypotheses of no differences between groups in favour of the alternative hypothesis that LB1 is a cretin, distinct from normal humans and genetic microcephalics. This does not mean that LB1 is proved to be a cretin, but that the hypothesis has been tested and has survived this test.
The unusual morphology of the LB1 trapezoid, considered to be evidence that LB1 represents a truly ancient lineage (Tocheri et al. 2007) appears to be consistent with, and may be indicative of, cretinism. The greatly reduced anteroposterior height of the trapezoid is consistent with loss of a palmar section of a normal, but bipartite, human trapezoid. The extended articular surface on the scaphoid for the trapezoid in the published images and measurements is consistent with such loss. A bipartite trapezoid divided into a palmar part and a dorsal part twice as large, with both parts articulating with the second metacarpal, scaphoid, capitate and trapezium as well as with each other has been described (Turner 1883; Gruber 1887 (see electronic supplementary material)). The dorsal parts of these trapezoids seem fully consistent in size and morphology with the LB1 trapezoid. Bipartite trapezoids are very rare (Gruber 1887 (see electronic supplementary material)), but in cretins carpal ossification centres remain separate (Wilkins 1941) and unfused well into adulthood, as the bipartite scaphoids of a 29-year-old cretin (O'Rahilly 1953). Inspection of the actual carpal specimens of LB1, particularly the palmar surface of the trapezoid, and its articulations onto the scaphoid and capitate would test the presence of bipartite trapezoid.
The endocranial volume (ECV) of LB1 is only 417 cm3, although this may have been reduced by post-mortem deformation. Synchondroses in the basicranium (Madeline & Elster 1995), including the paired posterior intraoccipital, anterior intraoccipital and petro-occipital synchondroses, and the single spheno-occipital synchondrosis tend to remain open (cartilaginous) in adult cretins (Dolega 1891; Knaggs 1928). Widths of 2–4 mm are reported (Stoccada 1915; Schinz et al. 1952) and a 13-year-old cretin skull displayed a very wide petro-occipital gap (MacCallum & Fabyan 1907). When discovered, the LB1 material was soft and deformable, and if LB1 were a cretin, the lack of preservation of the cartilaginous synchondroses would have led to post-mortem reduction of the ECV.
An early Flores ‘pygmoid’ skull had ECV of 1204 cm3 (Martin et al. 2006), but the source population of ‘Negrito’ pygmies probably included individuals of 1000 cm3 or less, as Andamanese females averaged 1128 cm3 (smallest 1025 cm3), one of four females, San, was 1075 cm3 and a single normal female, Veddah, was 950 cm3 (Flower 1889). The average ECV of New Ireland female skulls was 1176 cm3 with 5% below 1000 cm3, and the smallest, otherwise normal, adult was only 790 cm3 and another similar was 900 cm3 (Schlaginhaufen 1954; Dart 1956). Therefore, approximately 5% of female ME cretins arising within a Negrito pygmy population could have ECVs less than 500 cm3. In hunter-gatherer populations, young cretins may experience undernutrition arising from lack of mobility or estrangement, further decreasing their brain growth (as low protein isocaloric diet reduced brain weights of monkeys by 24%; Dodge et al. 1975) and shifting the distribution of ECVs.
Could the environmental conditions on Flores give rise to this pathology in hunter-gatherers? The combination of low iodine with high thiocyanate and low selenium induces thyroid necrosis in rats, a model for ME cretinism (Contempré et al. 2004). Endemic goitre has been recorded throughout the Indonesian archipelago from Sumatra to Timor, and iodized salt was introduced into selected areas from 1927 (Kelly & Snedden 1960) and has been mandated by the law since 1994. The Province of Nusa Tenggara Timur (NTT), which includes Flores, Sumba and West Timor, had the highest total goitre rate (TGR) of all provinces in the national surveys of 1996/1998 at 38.1% of school children surveyed, and in Manggarai District (western Flores) TGR was 41.5% (Indonesia, Ministry of Health 1998) and classified as a severe endemic area; a later survey found TGR of 51.5% (World Bank 2004). These rates of goitre in NTT and Manggarai, high compared with the rest of present-day Indonesia, are not high enough to predict substantial current cretinism. However, they do indicate that hunter-gatherer populations would have been severely iodine deficient without access to iodine-rich coastal resources. The LB site is inland, with the closest coasts to north and south of average straight-line distance of 24 km but substantially increased near to the glacial maximum (National Geographic, April 2005, p. 7, 8 maps but these are without stated bathymetric basis). Freshwater fish (found at LB) if consumed, as on Idjwi Island (Delange 1974), would not correct iodine deficiency.
In Malang District of East Java, with similar geology to western Flores (Whitten et al. 1996; Monk et al. 1997), average selenium concentrations in eggs collected from free-living chickens in 11 villages with volcanic and limestone soils were 0.15–0.24 μg g−1 (dry weight yolk) and 0.18–0.28 μg g−1 (dry weight albumen) and indicative of environmental selenium deficiency (Untoro et al. 1999). Low serum selenium (29.6 μg l−1; Soekirman et al. 2003) in school children with palpable goitre in Cimanggu sub-District, Central Java, was similar to that in school children (31 μg l−1) in Idjwi Island cretinism area.
Increased serum thiocyanate, arising from cyanogenic glucosides in cassava food was associated with African ME cretinism on Idjwi Island (Delange 1974), and appears to be a necessary third factor (Contempré et al. 2004). On Flores, cyanogenic plant foods would be available to hunter-gatherers, including native bamboos Gigantochloa spp., Bambusa spp. (Dransfield & Widjaja 1995), which are forest foods of Nuaulu sago cultivators and swiddeners on Seram, Indonesia (R. Ellen 2006, unpublished data, 2007) and are highly cyanogenic (Chang & Hwang 1993). Dioscorea (Wiriadinata 1998) and harvesting of Dioscorea tubers, probably Dioscorea hispida (Seibert 1992), are reported at Ruteng Park (approx. 20 km from LB), this species in Nepal containing 3–6 ppm hydrogen cyanide equivalent (Bhandari & Kawabata 2005). Many Acacia species are cyanogenic, and their seeds widely consumed by Australian hunter-gatherers (Maslin et al. 1988), but the cyanogenic potential of related species on Flores is not known.
We are unaware of any recent ME cretinism on Flores, although 126 cases were on record in Central Java in 1932 (Eerland 1933) and additional reports are from Sumatra (van Bommel 1930), Borneo (Clarke 1951), and Central Java (Goslings et al. 1977), all associated with agricultural populations consuming cassava (electronic supplementary material, figures S5 and S6). No information on Flores is included in the reviews of goitre and cretinism in Indonesia (Wilken 1890; van Bommel 1930; Kelly & Snedden 1960), but direct Dutch control of Flores was only assumed in 1907, and missionaries first entered western Flores only from 1921, after the aboriginal hunter-gatherers were assimilated into the agricultural population (Erb 1997).
ME endemic cretins escape the severe neurological deficits of neurological endemic cretins (DeLong 1993) having milder mental deficiency, greater self-reliance and a general lack of mobility deficits (Wang et al. 1982). In agricultural populations, ME cretins are well cared for, but in seasonally mobile hunter-gatherer populations, the limited mobility of cretins could lead to separation, particularly of adult cretins. Use of caves by adult cretins and lack of burial would explain the cretin remains at LB, while seasonal mobility, alternative shelters and systematic burial would explain the absence of the remains of normal individuals. A population (n=25–100) with 1% cretinism is calculated to produce 4–15 deaths of adult cretins per kiloyear, which is enough to explain the discovered remains at LB.
Stories of ebu gogo (greedy ancestors) among the Nage of central Flores, who are largely of aboriginal descent (Keers 1948), seem to have an empirical basis (Forth 1988) and are a possible record of H. floresiensis (Forth 2005) but provide striking parallels with ME cretinism. Unlike ‘spirits’, ebu gogo are described as mortal, without supernatural powers and no longer present. They lived in caves (lia ula, children's cave), were short, ‘roughly built’, hairy, ‘pot bellied’, stupid, the females had ‘pendulous breasts’, they stole food, could not cook and had imperfect language (Forth 1988). These characteristics are all consistent with ME cretinism, including the retention of lanugo hair in sporadic cretins (Perloff 1955), the ‘pot belly’ or distended abdomen of ME cretins as noted at Idjwi and Borneo (Clarke 1951) and the pendulous breasts of sporadic (Jackson 1952) and ME cretins (Clarke 1951). In addition, an enigmatic story of a mother's grief following the killing of her ebu gogo child becomes intelligible if the mother was a normal member of the social group but the child was a cretin. Thus, these stories may be a record of cretinism, and if so would be consistent with its persistence until the (fairly recent) assimilation of the hunter-gatherer populations.
5. Conclusion
The hypothesis that LB1 is a cretin is supported by the presence of numerous skeletal features characteristic of congenital hypothyroidism, and has survived statistical testing on the significance of the pituitary fossa enlargement and the similarity of cranial morphology. Primitive dental and wrist morphology seem explicable by cretinism but further work is required to confirm this. The reported brain size of LB1 (sample size of 1) is smaller than would be expected of a cretin but consistent with brain sizes reported in small brained populations and undernutrition of cretins in hunter-gatherer societies as well as post-mortem reductions of cretin skull volumes. The critical environmental factors (low iodine and selenium and the use of cyanogenic foods) for ME cretinism are potentially present on Flores. Furthermore, ME cretinism can occur at high prevalence and for extended periods consistent with multiple individuals at LB. Other proposed pathologies are not consistent with the skeletal remains, and as rare genetic conditions with low evolutionary fitness seem inconsistent with the preservation of multiple individuals. The hypothesis proposed by the discoverers that LB1 represents a separate species long separated from our lineage is not required by the skeletal data does not explain the extremely small brain size of LB1, and seems inconsistent with the much earlier presence of modern humans making similar stone tools on nearby Timor. However, until the original fossils are examined in relation to the cretinism hypothesis, and until more cretin skeletons can be examined, the cretin hypothesis must continue to be tentative.
Acknowledgments
We thank A. Connell and the RCS, Edinburgh; M. Cooke and RCS, England; D. Bulbeck, M. Oxenham and R. Kruszinski; J. Vukovic, A. Stoll, T. Hagen and L. Zhang for translations; J. K. Dennison for the Supplementary Appendix; R. Ellen for data from the Nuaulu Ethnobotanical Database and M. Anderson for help with statistics; Australian Research Council Discovery grants (to C.E.O.) and a Leverhulme Trust grant (to C.E.O.) have assisted this work.
Supplementary Material
Supplementary figures S1–S8 and references
Supplementary tables S1–S12 and references
Bipartition of the trapezoid bone into a dorsal and palmar secondary trapezoid (translation)
References
- Adams R.D, Rosman N.P. Neuromuscular system. In: Werner S.C, Ingbar S.H, editors. The thyroid. Harper & Row; New York, NY: 1971. pp. 1168–1180. [Google Scholar]
- Andersen H.J. Studies in hypothyroidism in children. Acta Paediatr. Scand. 1961;50(Suppl. 125):1–150. [PubMed] [Google Scholar]
- Argue D, Donlon D, Groves C, Wright R. Homo floresiensis: microcephalic, pygmoid, Australopithecus or Homo? J. Hum. Evol. 2006;51:360–374. doi: 10.1016/j.jhevol.2006.04.013. doi:10.1016/j.jhevol.2006.04.013 [DOI] [PubMed] [Google Scholar]
- Bastenie P.A, Ermans A.M, Thys O, Beckers C, Van den Schriek H.G, De Visscher M. Endemic goiter in the Uele region. III. Endemic cretinism. J. Clin. Endocrinol. Metab. 1962;22:187–193. doi: 10.1210/jcem-22-2-187. [DOI] [PubMed] [Google Scholar]
- Bellini M.A, Neves I. The skull in childhood myxedema; its roentgen appearance. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1956;76:495–498. [PubMed] [Google Scholar]
- Bhandari M.R, Kawabata J. Bitterness and toxicity in wild yam (Dioscorea spp.) tubers of Nepal. Plant Foods Hum. Nutr. 2005;60:129–135. doi: 10.1007/s11130-005-6841-1. doi:10.1007/s11130-005-6841-1 [DOI] [PubMed] [Google Scholar]
- Bircher E. Über eine Coxa vara entsprechende Difformität der Schulter (Humerus varus) bei Kretinen. Deutsche Zeitschrift f. Chirurgie. 1908;96:598–617. [Google Scholar]
- Borg S.A, Fitzer P.M, Young L.W. Roentgenologic aspects of adult cretinism: two case reports and review of the literature. Am. J. Roentgenol. 1975;123:820–828. doi: 10.2214/ajr.123.4.820. [DOI] [PubMed] [Google Scholar]
- Boyages S.C, et al. A comparative study of neurological and myxedematous endemic cretinism in western China. J. Clin. Endocrinol. Metab. 1988;67:1262–1271. doi: 10.1210/jcem-67-6-1262. [DOI] [PubMed] [Google Scholar]
- Brown P, Sutikna T, Morwood M.J, Soejono R.P, Jatmiko, Saptomo E.W, Due R.A. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature. 2004;431:1055–1061. doi: 10.1038/nature02999. doi:10.1038/nature02999 [DOI] [PubMed] [Google Scholar]
- Butler P.M, Hughes S.G. The delta variation of the human first lower deciduous molar: a problem of morphogenesis. Les Colloques de L'INSERM, Morphogenèse et différenciation dentaires, INSERM. 1984;125:57–72. [Google Scholar]
- Cao X.Y, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N. Engl. J. Med. 1994;331:1739–1744. doi: 10.1056/NEJM199412293312603. doi:10.1056/NEJM199412293312603 [DOI] [PubMed] [Google Scholar]
- Chang J.Y.C, Hwang L.S. Study on quantitative determination of taxiphyllin in bamboo shoots. J. Chin. Agric. Chem. Soc. 1993;31:506–517. [Google Scholar]
- Clarke M.C. Some impressions of the Muruts of North Borneo. Trans. R. Soc. Trop. Med. Hyg. 1951;44:453–464. doi: 10.1016/s0035-9203(51)80022-1. doi:10.1016/S0035-9203(51)80022-1 [DOI] [PubMed] [Google Scholar]
- Clarke K.R, Gorley R.N. Primer-E Ltd; Plymouth, UK: 2006. Primer v6: user manual/tutorial. [Google Scholar]
- Clarke K.R, Warwick R.M. Primer-E Ltd; Plymouth, UK: 2001. Change in marine communities: an approach to statistical analysis and interpretation. [Google Scholar]
- Contempré B, Morreale de Escobar G, Denef J, Dumont J.E, Many M. Thiocyanate induces cell necrosis and fibrosis in selenium- and iodine-deficient rat thyroids: a potential experimental model for myxedematous endemic cretinism in central Africa. Endocrinology. 2004;145:994–1002. doi: 10.1210/en.2003-0886. doi:10.1210/en.2003-0886 [DOI] [PubMed] [Google Scholar]
- Dart R.A. The relationship of brain size and brain pattern to human status. S. Afr. J. Med. Sci. 1956;21:23–45. [PubMed] [Google Scholar]
- Delange, F. 1974 Endemic goitre and thyroid function in central Africa Monographs in Paediatrics, vol. 2. Basel, Switzerland: Karger. [PubMed]
- Delange F, Ermans A.M, Vis H.L, Stanbury J.B. Endemic cretinism in Idjwi Island (Kivu Lake, Republic of the Congo) In: Hetzel B.S, Pharoah P.O.D, editors. Endemic Cretinism. Proc. Symp. held at Institute of Human Biology, Goroka, TPNG, January, 1971. Institute of Human Biology; Goroka, Papua New Guinea: 1971. pp. 33–54. [Google Scholar]
- Delange F, Ermans A.M, Vis H.L, Stanbury J.B. Endemic cretinism in Idjwi Island (Kivu Lake, Republic of the Congo) J. Clin. Endocrinol. Metab. 1972;34:1059–1066. doi: 10.1210/jcem-34-6-1059. [DOI] [PubMed] [Google Scholar]
- DeLong G.R. Effects of nutrition on brain development in humans. Am. J. Clin. Nutr. Suppl. 1993;57:286S–290S. doi: 10.1093/ajcn/57.2.286S. [DOI] [PubMed] [Google Scholar]
- De Quervain F, Wegelin C. Pathologie und Klinik in Einzeldarstellungen. Julius Springer; Berlin, Germany; Vienna, Austria: 1936. Der Endemische Kretinismus. [Google Scholar]
- Dodge P.R, Prensky A.L, Feigin R.D. Mosby; St Louis, MO: 1975. Nutrition and the developing nervous system. [Google Scholar]
- Dolega D. Ein Fall von Cretinismus beruhend auf einer primären Hemmung des Knochenwachsthums. Beit. z. Pathol. Anat. 1891;9:488–509. [Google Scholar]
- Dransfield, S. & Widjaja, E. A. (eds) 1995 Plant resources of south-east Asia: bamboos, no 7. Leiden, The Netherlands: Backhuys.
- Dreyfus G, Fischgold H, Zara M, Frank L.J. Absence des sinus crâniens dans le myxoedème congénital. Ann. Endocrinol. (Par.) 1950;11:423–426. [PubMed] [Google Scholar]
- Dumont J.E, Ermans A.M, Bastenie P.A. Thyroidal function in a goiter endemic. IV. Hypothyroidism and endemic cretinism. J. Clin. Endocrinol. Metab. 1963;23:325–335. doi: 10.1210/jcem-23-9-847. [DOI] [PubMed] [Google Scholar]
- Dye J.A, Maugham G.H. The thyroid gland as a growth-promoting and form-determining factor in the development of the animal body. Am. J. Anat. 1929;44:331–368. doi:10.1002/aja.1000440302 [Google Scholar]
- Eerland, L. D. 1933 Kropf in Niederlandisch Ostindien. In Zweite Internationale Kropfkonferenz (ed. O. Stiner), pp. 469–481. Bern, Switzerland: Verlag Hans Huber.
- Ellen R. Modern crises and traditional strategies: local ecological knowledge in island southeast Asia. Berghahn; New York, NY: 2007. Introduction; pp. 1–45. [Google Scholar]
- Engel M.B, Bronstein I.P, Brodie A.G, Wesoke P. A roentgenographic cephalometric appraisal of untreated and treated hypothyroidism. Am. J. Dis. Child. 1941;61:1193–1214. [Google Scholar]
- Erb M. Contested time and place: constructions of history in Todo, Manggarai (western Flores, Indonesia) J. Southeast Asian Stud. 1997;28:47–77. [Google Scholar]
- Falk D, et al. The brain of Homo floresiensis. Science. 2005;308:242–245. doi: 10.1126/science.1109727. doi:10.1126/science.1109727 [DOI] [PubMed] [Google Scholar]
- Falk D, et al. Brain shape in human microcephalics and Homo floresiensis. Proc. Natl Acad. Sci. USA. 2007;104:2513–2518. doi: 10.1073/pnas.0609185104. doi:10.1073/pnas.0609185104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower W.H. Description of two skeletons of Akkas, a pygmy race from central Africa. J. R. Anthropol. Inst. 1889;18:3–19. [Google Scholar]
- Forth G. KITLV Press; Leiden, The Netherlands: 1988. Beneath the volcano: religion, cosmology and spirit classification among the Nage of eastern Indonesia. [Google Scholar]
- Forth G. Hominids, hairy hominoids and the science of humanity. Anthropol. Today. 2005;21:13–17. doi:10.1111/j.0268-540X.2005.00353.x [Google Scholar]
- Frank M, Brüll D. Handskelett-Entwicklung bei Kindern mit primärer Hypothyreose. Fortschritte der Medizin. 1980;44:1713–1716. [PubMed] [Google Scholar]
- Goslings B.M, Djokomoeljanto R, Docter R, van Hardeveld C, Hennemann G, Smeenk D, Querido A. Hypothyroidism in an area of endemic goiter and cretinism in Central Java, Indonesia. J. Clin. Endocrinol. Metab. 1977;44:481–490. doi: 10.1210/jcem-44-3-481. [DOI] [PubMed] [Google Scholar]
- Gray, H. 1918 Anatomy of the human body Lea & Febiger: Philadelphia, PA. See http://bartleby.com/107/50.html
- Gruber W. Bipartition des Os multangulum minus in ein M.secundarium dorsale et volare. Virchows Arch. 1887;110:551–554. doi:10.1007/BF01928546 [Google Scholar]
- He T.Y. X-ray manifestations of bone in myxedematous endemic cretinism. Zhonghua Fang She Xue Za Zhi. 1984;18:279–282. [PubMed] [Google Scholar]
- Hershkovitz I, Kornreich L, Laron Z. Comparative skeletal features between Homo floresiensis and patients with primary growth hormone insensitivity (Laron syndrome) Am. J. Phys. Anthropol. 2007;134:198–208. doi: 10.1002/ajpa.20655. doi:10.1002/ajpa.20655 [DOI] [PubMed] [Google Scholar]
- Hill W.C.O. Two examples of infantilism. Ceylon J. Sci. (D) 1936;IV:71–118. [Google Scholar]
- Howells W.W. Howells' craniometric data on the Internet. Am. J. Phys. Anthropol. 1996;101:441–442. doi: 10.1002/ajpa.1331010302. doi:10.1002/ajpa.1331010302 [DOI] [PubMed] [Google Scholar]
- Indonesia, Ministry of Health. Pusal Penilitian dan Pengembangan Gizi and Direktorat Bina Gizi Masyarakat; Jakarta, Indonesia: 1998. Survei Nasional Pemetaan Gangguan Akibat Kekurangan Yodium. [Google Scholar]
- Ith-Hansen K, Kjaer I. Persistence of deciduous molars in subjects with agenesis of the second premolars. Eur. J. Orthod. 2000;22:239–243. doi: 10.1093/ejo/22.3.239. doi:10.1093/ejo/22.3.239 [DOI] [PubMed] [Google Scholar]
- Jackson W.P.U. Studies of adult cretins. S. Afr. Med. J. 1952;26:605–607. See also pp. 631–633, 645–647. [PubMed] [Google Scholar]
- Jacob T, Indriati E, Soejono R.P, Hsü K, Frayer D.W, Eckhardt R.B, Kuperavage A.J, Thorne A, Henneberg M. Pygmoid Australomelanesian Homo sapiens skeletal remains from Liang Bua, Flores: population affinities and pathological abnormalities. Proc. Natl Acad. Sci. USA. 2006;103:13 421–13 426. doi: 10.1073/pnas.0605563103. doi:10.1073/pnas.0605563103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P. Harvey, Miller & Metcalf; London, UK: 1973. Atlas of hand radiographs. [Google Scholar]
- Joergensen K.D. Bianco Lunos Bogtrykkeri; Copenhagen, Sweden: 1956. The deciduous dentition. [Google Scholar]
- Keers W. Indisch Instituut; Amsterdam, The Netherlands: 1948. An anthropological survey of the eastern Little Sunda Islands. The Negritos of the eastern Little Sunda Islands. The Proto-Malays of The Netherlands East-Indies. [Google Scholar]
- Kelly, W. C. & Snedden, W. W. 1960 Prevalence and geographical distribution of endemic goitre. In Endemic goitre, pp. 27–233. Geneva, Switzerland: World Health Organization. [PubMed]
- Kitagawa Y, Manabe Y, Oyamada J, Rokutanda A. Morphological and anthropological aspects of human triangular deciduous lower first molar teeth. Arch. Oral Biol. 1996;41:387–391. doi: 10.1016/0003-9969(95)00131-x. doi:10.1016/0003-9969(95)00131-X [DOI] [PubMed] [Google Scholar]
- Knaggs R.L. Cretinism. Br. J. Surg. 1928;16:370–400. doi:10.1002/bjs.1800166304 [Google Scholar]
- König M.P. Springer; Berlin, Germany: 1968. Die Kongenitale Hypothyreose und der Endemische Kretinismus. [Google Scholar]
- Kornreich L, Horev G, Schwarz M, Karmazyn B, Laron Z. Pituitary size in patients with Laron syndrome (primary GH insensitivity) Eur. J. Endocrinol. 2003;148:339–341. doi: 10.1530/eje.0.1480339. doi:10.1530/eje.0.1480339 [DOI] [PubMed] [Google Scholar]
- Kranz P. Schilddrüse und Zähne. Deutsche Monatschr. f. Zahnheilk. 1912;30:1–35. [Google Scholar]
- Larson S.G. Subscapularis function in gibbons and chimpanzees: implications for interpretation of humeral head torsion in hominoids. Am. J. Phys. Anthropol. 1988;76:449–462. doi:10.1002/ajpa.1330760405 [Google Scholar]
- Larson S.G, Jungers W.L, Morwood M.J, Sutkina T, Jatmiko, Saptomo E.W, Due R.A, Djubiantono T. Homo floresiensis and the evolution of the human shoulder. J. Hum. Evol. 2007;53:718–731. doi: 10.1016/j.jhevol.2007.06.003. doi:10.1016/j.jhevol.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Lewis O.J. Clarendon Press; Oxford, UK: 1989. Functional morphology of the evolving hand and foot. [Google Scholar]
- Liu J, Tan Y, Zhuang Z, Shi Z, Chen B, Zhang J. Influence of iodine deficiency on human fetal thyroid gland and brain. In: DeLong R, Robbins J, Condliffe P.J, editors. Iodine and the brain. Plenum Press; New York, NY: 1989. pp. 249–257. [Google Scholar]
- Ma T, Lu T, Tan Y, Chen B, Zhu X. The present status of endemic goitre and endemic cretinism in China. Food Nutr. Bull. 1982;4:13–19. [Google Scholar]
- MacCallum W.G, Fabyan M. On the anatomy of a myxedematous idiot. Johns Hopkins Hosp. Bull. 1907;18:341–345. [Google Scholar]
- Madeline L.A, Elster A.D. Suture closure in the human chondrocranium: CT assessment. Radiology. 1995;196:747–756. doi: 10.1148/radiology.196.3.7644639. [DOI] [PubMed] [Google Scholar]
- Martin R.D, MacLarnon A.M, Phillips J.L, Dobyns W.B. Flores hominid: new species or microcephalic dwarf? Anat. Rec. A. 2006;288:1123–1145. doi: 10.1002/ar.a.20389. [DOI] [PubMed] [Google Scholar]
- Maslin B.R, Dunn J.E, Conn E.E. Cyanogenesis in Australian species of Acacia. Phytochemistry. 1988;27:421–428. doi:10.1016/0031-9422(88)83112-1 [Google Scholar]
- Melot C.J, Jeanmart-Michez L, Dumont J, Ermans A.M, Bastenie P. Les aspects radiologiques du crétinisme endémique. J. Belge Radiol. 1962;45:385–403. [PubMed] [Google Scholar]
- Miles A.E.W, Bulman J.S. Growth curves of immature bones from a Scottish population of sixteenth to mid-nineteenth century: shoulder girdle, ilium, pubis and ischium. Int. J. Osteoarch. 1995;5:15–27. doi:10.1002/oa.1390050103 [Google Scholar]
- Monk K.A, De Fretes Y, Reksodiharjo-Lilley G. Periplus; Hong Kong, China: 1997. The ecology of Nusa Tenggara and Maluku. [Google Scholar]
- Morwood M.J, et al. Further evidence for small-bodied hominins from the Late Pleistocene of Flores, Indonesia. Nature. 2005;437:1012–1017. doi: 10.1038/nature04022. doi:10.1038/nature04022 [DOI] [PubMed] [Google Scholar]
- O'Connor S. New evidence from East Timor contributes to our understanding of earliest modern human colonisation east of the Sunda Shelf. Antiquity. 2007;81:523–535. [Google Scholar]
- O'Rahilly R. A survey of carpal and tarsal anomalies. J. Bone Joint Surg. 1953;35:626–642. [PubMed] [Google Scholar]
- Ortner D.J, Hotz G. Skeletal manifestations of hypothyroidism from Switzerland. Am. J. Phys. Anthropol. 2005;127:1–6. doi: 10.1002/ajpa.20033. doi:10.1002/ajpa.20033 [DOI] [PubMed] [Google Scholar]
- Perloff W.H. A manifestation of juvenile hypothyroidism. J. Am. Med. Assoc. 1955;157:651–652. doi: 10.1001/jama.1955.02950250025006. [DOI] [PubMed] [Google Scholar]
- Quinn G.P, Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental design and data analysis for biologists. [Google Scholar]
- Rao A, Arathi R. Taurodontism of deciduous and permanent molars. J. Indian Soc. Pedod. Prev. Dent. 2006;24:42–44. doi: 10.4103/0970-4388.22836. [DOI] [PubMed] [Google Scholar]
- Schinz, H., Baensch, W., Friedl, E. & Uehlinger, E. 1952 Roentgen-diagnostics. Skeleton, vol. 2 (transl. Lehrbuch der Roentgendiagnostik, Band 2). New York, NY: Grune & Stratton.
- Schlaginhaufen O. Anthropologische Reminiszenzen von den Feni-Inseln in Bismarck-Archipel. Z. Morphol. Anthropol. 1954;46:282–287. [Google Scholar]
- Seibert, B. 1992 Ethnobotany and its relevance to IPAS. In Management and conservation of tropical forest ecosystems and biodiversity Reckershausen, Germany: Deutsche Forst-Consult and P.T. Hasfarm Dian Konsultan; Jakarta, Indonesia: PHPA and Ministry of Forestry, Republic of Indonesia.
- Silverman F.N, Currarino G. Roentgen manifestations of hereditary metabolic diseases in childhood. Metabolism. 1960;9:248–283. [PubMed] [Google Scholar]
- Soekirman, Atmarita, Olsen, J. L., Sanjaya, Elhusseiny, N. & Levinson, F. J. 2003 Indonesian micronutrient reference report. A report to the micronutrient initiative and the World Bank. Food policy and applied nutrition program discussion papers, Tufts University. See http://nutrition.tufts.edu/docs/pdf/fpan/wp23-indonesia_micro.pdf
- Stoccada F. Untersuchungen u¨ber die Synchondrosissphenooccipitalis und den Ossifikationsprozess bei Kretinismus und Athyreosis. Beitrage Pathologischen Anatomie und zur allgemeinen Pathologie. 1915;61:450–513. [Google Scholar]
- Taylor G.L, Appleton J.L.T. The dental aspects of a case of dwarfism (cretinism?) Dent. Cosmos. 1929;lxxi:124–131. [Google Scholar]
- Terry R.J. The clavicle of the American negro. Am. J. Phys. Anthropol. 1959;17:217–226. doi: 10.1002/ajpa.1330170307. doi:10.1002/ajpa.1330170402 [DOI] [PubMed] [Google Scholar]
- Thijn C.J.P. Springer; Berlin, Germany: 1986. Radiology of the hand. [Google Scholar]
- Tocheri M.W, et al. The primitive wrist of Homo floresiensis and its implications for hominin evolution. Science. 2007;317:1743–1745. doi: 10.1126/science.1147143. doi:10.1126/science.1147143 [DOI] [PubMed] [Google Scholar]
- Turner W. Some variations in the bones of the human carpus. J. Anat. Physiol. 1883;17:244–249. [PMC free article] [PubMed] [Google Scholar]
- Untoro J, Ruz M, Gross R. Low environmental selenium availability as an additional determinant for goiter in East Java, Indonesia? Biol. Trace Elem. Res. 1999;70:127–136. doi: 10.1007/BF02783854. doi:10.1007/BF02783854 [DOI] [PubMed] [Google Scholar]
- van Bommel L.B. Rijks-Universiteit/Eduard Ijdo; Leiden, The Netherlands: 1930. Struma Endemica en Cretinismus in Nederlandsch Oost-Indie: Meer in het Bijzonder in de Alaslanden. [Google Scholar]
- Vis H.L. Protein deficiency disorders. Postgrad. Med. J. 1969;45:107–115. doi: 10.1136/pgmj.45.520.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Werder K. Pituitary enlargement. Clin. Endocrinol. 1996;44:299–303. doi: 10.1046/j.1365-2265.1996.669490.x. doi:10.1046/j.1365-2265.1996.669490.x [DOI] [PubMed] [Google Scholar]
- Walker A, Leakey R. The skull. In: Walker A, Leakey R, editors. The Nariokotome Homo erectus skeleton. Harvard University Press; Cambridge, MA: 1993. pp. 63–94. [Google Scholar]
- Wang H, et al. A comparative study of endemic myxedematous and neurological cretinism in Hetian and Luopu, China. In: Ui N, Torizuka K, Nagataki S, Miyai K, editors. Second Asia and Oceania Thyroid Association Meeting. Excerpta Medica; Tokyo, Japan: 1982. pp. 349–355. [Google Scholar]
- Weber J, Czarnetzki A, Pusch C. Comment on ‘The Brain of LB1, Homo floresiensis’. Science. 2005;310:236b. doi: 10.1126/science.1117062. doi:10.1126/science.1114789 [DOI] [PubMed] [Google Scholar]
- Whitten T, Soeriaatmadja R.E, Afiff S.A. Periplus; Hong Kong: 1996. The ecology of Java and Bali. [Google Scholar]
- Wilken G.A. Struma en cretinisme in den Indischen Archipel. Bijdr. Taal-, Land-, Volkenkunde Ned.-Ind. 1890;5:349–425. [Google Scholar]
- Wilkins L. Epiphyseal dysgenesis associated with hypothyroidism. Am. J. Dis. Child. 1941;61:13–34. [Google Scholar]
- Wiriadinata H. Floristic distribution of Ruteng Nature Recreation Park. In: Simbolon H, editor. The natural resources of Flores Island. Research and Development Center for Biology, The Indonesian Institute of Sciences; Bogor, Indonesia: 1998. pp. 1–17. [Google Scholar]
- World Bank 2004 Implementation completion report (SCL-41250) See http://www-wds.worldbank.org/servlet/WDSContentServer/WDSP/IB/2004/07/01/000012009_20040701111003/Rendered/INDEX/29511.txt
- Yamada T, Tsukui T, Ikejiri K, Yukimura Y. Volume of sella turcica in normal subjects and in patients with primary hypothyroidism and hyperthyroidism. J. Clin. Endocrinol. Metab. 1976;42:817–822. doi: 10.1210/jcem-42-5-817. [DOI] [PubMed] [Google Scholar]
- Zeisz R.C, Nuckolls J. Mosby; St Louis, MO: 1949. Dental anatomy. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures S1–S8 and references
Supplementary tables S1–S12 and references
Bipartition of the trapezoid bone into a dorsal and palmar secondary trapezoid (translation)