Abstract
Adhesive organs on the legs of arthropods and vertebrates are strongly direction dependent, making contact only when pulled towards the body but detaching when pushed away from it. Here we show that the two types of attachment pads found in cockroaches (Nauphoeta cinerea), tarsal euplantulae and pretarsal arolium, serve fundamentally different functions. Video recordings of vertical climbing revealed that euplantulae are almost exclusively engaged with the substrate when legs are pushing, whereas arolia make contact when pulling. Thus, upward-climbing cockroaches used front leg arolia and hind leg euplantulae, whereas hind leg arolia and front leg euplantulae were engaged during downward climbing. Single-leg friction force measurements showed that the arolium and euplantulae have an opposite direction dependence. Euplantulae achieved maximum friction when pushed distally, whereas arolium forces were maximal during proximal pulls. This direction dependence was not explained by the variation of shear stress but by different contact areas during pushing or pulling. The changes in contact area result from the arrangement of the flexible tarsal chain, tending to detach the arolium when pushing and to peel off euplantulae when in tension. Our results suggest that the euplantulae in cockroaches are not adhesive organs but ‘friction pads’, mainly providing the necessary traction during locomotion.
Keywords: adhesion, locomotion, tribology, biomechanics, direction dependence, robotics
1. Introduction
Many insects, spiders and some vertebrates are capable of climbing and walking upside down on diverse substrates, using adhesive structures on their legs (Scherge & Gorb 2001). The performance of adhesive organs is striking because of the animals' capability of controlling attachment rapidly during locomotion. The detailed mechanisms of how they combine the conflicting tasks of running and making stable adhesive contacts are still largely unclear (Federle & Endlein 2004).
One fundamental property of most tarsal adhesive structures used for locomotion is their direction dependence. Attachment systems of many different animals maximize forces when legs are pulled towards the body but detach when moved in the opposite direction; examples include flies (Niederegger & Gorb 2003), bush crickets (Gorb & Scherge 2000), ants (Federle et al. 2001; Federle & Endlein 2004), tree frogs (Hanna & Barnes 1991), spiders (Hill 1977) and geckos (Autumn et al. 2000, 2006a). Despite its omnipresence among adhesive systems, direction dependence appears to be achieved by different structures and mechanisms. For example, in fibrillar adhesive systems, distally oriented seta shafts and asymmetrically structured tips make setae non-adhesive by default, only making full contact when pulled proximally (Autumn & Hansen 2006; Autumn et al. 2006a; Federle 2006; Gravish et al. 2008). The mechanisms of direction dependence are less clear in smooth pads. In hymenopteran insects, adhesive pads unfold passively, increasing contact area, when pulled towards the body (Federle et al. 2001). In Tettigonia viridissima, the anisotropy of pad friction forces was proposed to result from the orientation of cuticular fibres (Gorb & Scherge 2000). However, it is still unclear whether the directionality of adhesive pads is based on changes in contact area or pad efficiency (shear stress).
Direction dependence allows animals to control attachment and detachment via shear forces, by pulling their legs towards or pushing their legs away from the body. The proximal pull required to maximize adhesion is often achieved passively, e.g. during inverted walking, where the sprawled posture of insects generates a strong in-plane force component. However, there are many situations where legs need to attach while the force vector is pointing away from the body. This is the case for all legs during level walking and for legs below the centre of gravity in vertically climbing insects (Goldman et al. 2006). Here the force away from the body should cause the pads to detach. So far, no study has investigated how animals solve this intrinsic problem of direction-dependent pads. The observation that tree frogs are unable to adhere to smooth surfaces in the head-down orientation indicates that direction dependence can indeed give rise to locomotory constraints (Hanna & Barnes 1991).
Species with multiple toes such as geckos could avoid detachment by aligning some of their toes opposite to the force vector (Autumn et al. 2006b; Goldman et al. 2006), but how do insects without ‘toes’ overcome this problem? In this study we demonstrate that in cockroaches, pushing and pulling are achieved by different parts of the tarsus, which are specialized for these tasks. Many insects possess one or two adhesive organs located on a single segment, in most cases the pretarsus (Beutel & Gorb 2001). However, there are several insect orders that have evolved additional pads on the more proximal tarsus segments. These structures on the ventral side of one or more tarsomeres are called (eu-)plantulae and occur not only in cockroaches (order Dictyoptera) but also in the Phasmatodea, Mantophasmatodea, Orthoptera, Grylloblattodea, as well as some Plecoptera, Dermaptera and Hymenoptera (Beutel & Gorb 2001, 2006; Schulmeister 2003).
Here we investigate the biological function of the pretarsal arolium and the tarsal euplantulae in cockroaches by addressing the following questions: (i) how are arolium and euplantulae used during locomotion? (ii) how do friction forces, contact area and shear stress of both pad types depend on sliding direction? and (iii) how is the biological function of arolium and euplantulae related to their morphology?
2. Material and methods
(a) Study animals
Adult cockroaches (Nauphoeta cinerea, Blaberidae; body mass: 541±25 mg; mean±s.e., n=31) were taken from a laboratory colony kept at 24°C with food and water ad libitum.
(b) Morphology
Pads were studied using scanning electron microscopy (SEM) and freeze-fracture techniques. Legs from adult cockroaches were amputated and immediately transferred into fixative (4% glutaraldehyde in 0.1 M PIPES buffer at pH 7.4) for 48 hours at 4°C. Legs were then washed with de-ionized water and gradually dehydrated with 100% ethanol. Legs were frozen in liquid nitrogen, fractured using a cooled razor blade, transferred back to 100% ethanol and critical point dried. All preparations were mounted on SEM stubs, sputter-coated with gold 20 nm thick, and examined in a FEI XL30-FEG SEM at 10 kV.
(c) Video observation of climbing
To examine how the arolium and euplantulae are used during locomotion, cockroaches were filmed while running up or down a vertical, clear Perspex square tube at 250 fps, using two Redlake PCI 1000 B/W or Hot Shot 1280PCI cameras (see figure 1S in the electronic supplementary material). Only stereotyped runs that had passed completely through the field of view were used (running speeds ranged from 0.04 to 0.30 m s−1). One camera viewed the insect from a lateral perspective, showing the legs and adhesive pads as they came into contact with the surface. A cut-out in the tube was covered with a glass cover-slip to image adhesive contact area from below using reflected light. We used a stereo microscope with coaxial illuminator (Wild M3C, Leica) to obtain high-contrast images of the pad contact zone (Federle et al. 2002). Video analysis was performed with custom-made programs written in Matlab (The Mathworks).
We determined whether the arolium or the euplantulae or both made contact with the substrate. When euplantulae and arolium were in contact simultaneously, we determined for each frame the proportion of the total contact area contributed by each pad. This ‘relative’ contact area was then averaged over all frames in a single stride. Results are shown as the relative contact area of the euplantulae; results for the arolium are simply the difference to 100%.
(d) Single-leg force measurements
To measure friction forces of tarsal and pretarsal pads, cockroaches were briefly anaesthetized using CO2 and fastened to a mount using parafilm tape. Nauphoeta cinerea hind legs were used for all measurements and were tested in two different conditions, ‘fixed’ and ‘footloose’. In the fixed condition, all tarsal segments as well as the dorsal side of the pretarsus were immobilized by attaching them to the mount using paraffin wax or dental cement (ESPE Protemp II, 3 M). The footloose condition was devised to test the tarsus in a more natural state. To examine euplantulae in the footloose condition, hind legs were fixed at the tibia so that the tarsus was free to move. For the arolium, however, it was impossible to leave the whole tarsus unfixed, because even moderate normal forces (greater than 0.4 mN, n=3) brought the euplantulae into surface contact (see video 3 in the electronic supplementary material). To test the arolium footloose, we therefore fixed the tarsus at the fourth tarsal segment so that pretarsus and fifth tarsal segment remained free.
Forces were measured using a two-dimensional bending beam equipped with 350 Ω foil strain gauges (see figure 2S in the electronic supplementary material). The hind leg was brought into contact with a glass cover-slip (12×12×0.1 mm) attached to the distal end of the bending beam. The contact area was recorded under reflected light using a Redlake PCI 1000 B/W camera at 10 Hz. Force input signals were amplified (GSV1T8, ME-Systeme) and recorded to a data acquisition board (PCI-6035E, National Instruments) with a sampling frequency of 100 Hz. The bending beam was mounted on a computer-controlled three-dimensional positioning stage (M-126PD, C-843, Physik Instrumente). Motor movements, video trigger and force recording were synchronized by a custom-made LabVIEW program (National Instruments).
Before a friction measurement, the pad was brought into contact with the glass plate for 10 s with a normal force of 2 mN, using force feedback (frequency=10 Hz). This force was chosen since it approximately represents the load on a single leg during tripod locomotion of a 600 mg cockroach. Sliding movements covering 3 mm were performed with a velocity of 0.2 mm s−1 either in the proximal direction (imitating the leg pulling towards the body) or in the distal direction (imitating the leg pushing away from the body). In the fixed condition, the normal force was kept constant during each slide via force feedback. In the footloose condition, force feedback would cause the tarsal chain to buckle during distal slides. We therefore performed footloose trials with a constant z-position of the motor after an initial preload of 2 mN. Results for all analyses are presented as mean±s.e.
3. Results
(a) Morphology
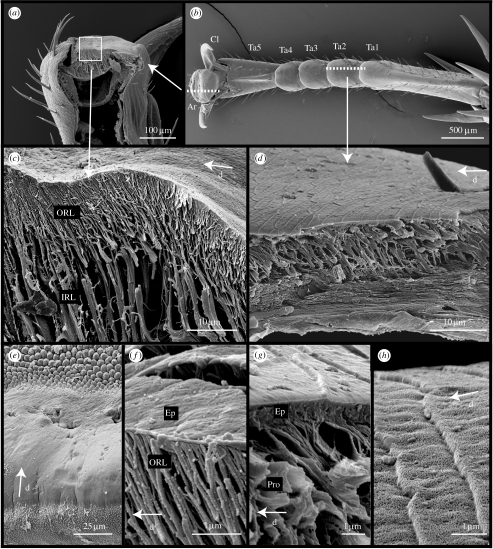
The tarsus of N. cinerea cockroaches is similar in structure to that of previously studied cockroaches (Roth & Willis 1952; Arnold 1974; Frazier et al. 1999). Each of the five tarsomeres, except the fifth, distal one (Ta5), bear on the ventral surface soft pad-like structures, the euplantulae (length=263.2±19 μm, width=309.0±10.3 μm, n=6; figure 1b). The fifth tarsal segment articulates with the pretarsus, which bears the claws and the arolium (length=133.9±14 μm, width=413.2±17.5 μm, n=3; figure 1a). The arolium itself appears smooth under light microscopy, but SEM revealed areas on its surface with different microstructures (figure 1e). The surface of the adhesive contact zone appeared smooth, but distal to the contact zone the cuticular surface is specialized into a region of knobs, as described in the desert locust (Kendall 1970). Proximal from the contact area, the pad is covered in regularly spaced protuberances of less than 0.5 μm height termed cuticular spines by Kendall (1970).
Figure 1.
Morphology of arolium and euplantulae in N. cinerea. (a,b) Arrangement of attachment structures on the tarsus. (c) Freeze fracture of the arolium contact zone showing branched cuticle fibres. (d) Cuticle structure of the euplantulae. (e) Surface morphology of the arolium. (f) Epicuticle of the arolium and fine rods of the procuticle. (g) Epicuticle of the euplantulae and procuticular rods. (h) Surface profile of the euplantulae. Ar, arolium; Cl, claws; PrT, pretarsus; Ta1-5, tarsal segments 1–5; ORL, outer rod layer; IRL, inner rod layer; Ep, epicuticle; Pro, procuticle; d, distal direction.
Freeze-fracture techniques showed that the inner cuticle of the arolium contact zone (and of the region of knobs distal to this) is made up of numerous branched rods oriented distally at an angle to the surface (angle α=57.15±1.53, n=5; figure 1c) originating from the deeper endocuticle layer. Deeper below the surface these rods are thicker (diameter 1.44±0.10 μm, n=31) and branch into finer rods near the surface (diameter 0.36±0.03 μm, n=41). The inner layer of thick rods varies in height from 61 μm distally to 14.5 μm proximally. The outer layer of thin rods is only present under the adhesive contact zone and has a more constant height (13.68±0.97 μm, n=10). The fibrous procuticle is covered by an epicuticle of less than 100 nm thickness (figure 1f).
The euplantulae contain regularly spaced pits on the ventral surface (four per pad) holding in their centre two sensilla, similar to those described for desert locusts (Kendall 1970) and other roach species (Roth & Willis 1952). The surface of the pad is covered by a regular pattern of oblong ‘platelets’ (lateral length 11.58±0.59 μm, n=13, proximal–distal length 4.32±0.30 μm, n=13), separated by small ridges. The distal facing edge of these ridges is steeper than the proximal facing edge, giving the impression that each platelet overlaps the one preceding it (figure 1h). Similar to the adhesive cuticle of the arolium, the cuticle of the euplantulae contains a layer of rod-like structures which are oriented distally (figure 1d diameter 0.57±0.01 μm, n=24). However, the procuticle layer is thinner (height 9.56±0.32 μm, n=7) and the rods are not branched and are more intimately connected with each other, so that the structure appears ‘spongy’. The amorphous epicuticle of the euplantulae is much thicker than that of the arolium (figure 1g, thickness 1012±48 nm, n=6).
(b) Use of arolium and euplantulae during vertical climbing
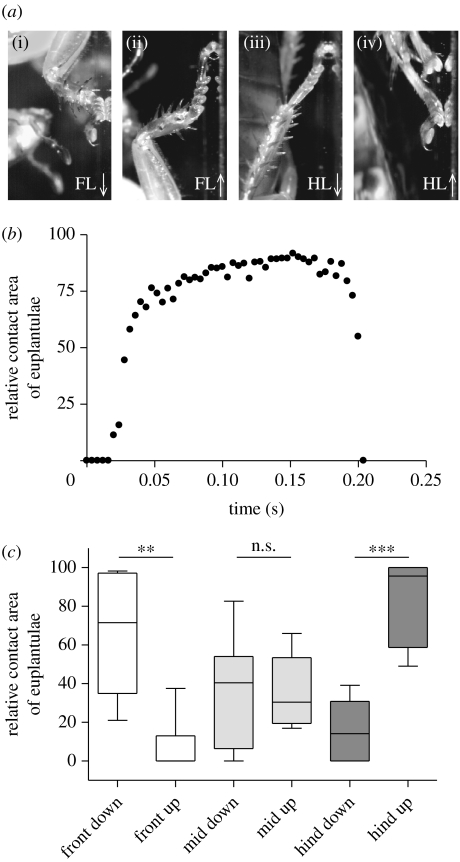
We investigated the movements of the tarsus and the adhesive contact area of arolium and euplantulae when cockroaches climbed upward or downward. We discovered that the position of the legs relative to the body determined which pad was mainly employed (table 1). As a rule, legs that were above the body centre of mass used the arolium while legs below the centre of mass used the euplantulae. Thus, the front legs used mainly the arolium when climbing upward but mainly the euplantulae when going head first downward (see videos 1 and 2 in the electronic supplementary material). The exact opposite was found in the hind legs, where the euplantulae were engaged when climbing upward but the arolium when going downward (figure 2a). The frequency of pad use was significantly different for the front legs (Craddock–Flood's Χ2,222=10.72, p=0.005) and the hind legs (Craddock–Flood's Χ2,212=10.79, p=0.004) but not for the middle legs (Craddock–Flood's Χ2,242=3.49, p=0.174).
Table 1.
Contact of adhesive pads in strides of vertically climbing cockroaches.
| front legs | middle legs | hind legs | ||||
|---|---|---|---|---|---|---|
| up | down | up | down | up | down | |
| arolium only | 8 | 0 | 3 | 2 | 0 | 4 |
| euplantulae only | 0 | 3 | 1 | 0 | 6 | 0 |
| both | 5 | 6 | 10 | 8 | 4 | 7 |
| total strides | 13 | 9 | 14 | 10 | 10 | 11 |
Figure 2.
(a) Video captures of tarsal contacts for front legs (FL) and hind legs (HL) of cockroaches climbing downward (↓) and upward (↑). (b) Relative contact area recorded for the euplantulae during one stride (in this example, the mean relative contact area for the euplantulae is 68.4%). (c) Relative contribution of the euplantulae for each leg during downward and upward climbing. **p<0.010, ***p<0.001, n.s. not significant.
In some cases, both euplantulae and arolium made contact. In these strides, we measured the relative contact area contribution of each pad (figure 2b,c). Again, the contribution of each pad differed strongly between upward and downward climbing. In the front legs, the relative contact area of the euplantulae was significantly larger during downward climbing (Mann–Whitney U6,9=52, p=0.002) whereas in the hind legs, it was significantly higher during upward climbing (Mann–Whitney U7,6=42, p=0.0012); however, no significant difference was found in the middle legs (Mann–Whitney U7,6=22, p=0.945).
The striking difference in pad use between upward and downward climbing in front and hind legs can be explained by a difference between pushing and pulling. Legs above the body centre of mass mainly pull, whereas legs below it mainly push to balance the force of gravity.
(c) Direction dependence of arolium and euplantulae
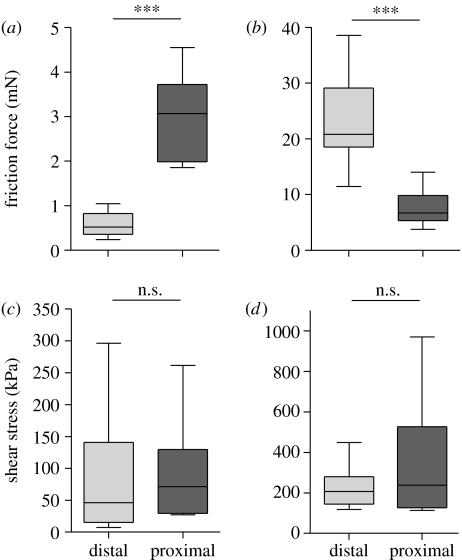
We investigated whether arolium and euplantulae are direction dependent by quantifying single-pad shear forces in the proximal and distal directions. When pads were tested in the footloose condition (table 2), arolium and euplantulae showed an opposite directionality, consistent with the observed use of both pads during climbing (see above). In the euplantulae, the friction force was significantly higher during distal slides than during proximal slides (figure 3). By contrast, arolium friction was significantly higher for proximal slides than distal slides. However, the direction dependence was no longer present when we considered shear stress (force per contact area). Neither arolium nor euplantulae showed a significant difference between slide directions. The direction-dependent forces of both arolium and euplantulae resulted from significant changes in contact area.
Table 2.
Single-leg force measurements for arolia and euplantulae of cockroach hind legs in the footloose condition.
| distal slide mean±s.e. | proximal slide mean±s.e. | n | test statistic | ||
|---|---|---|---|---|---|
| arolium | force (mN) | 0.57±0.08 | 2.94±0.29 | 10 | paired t-test T9=8.16, p<0.001 |
| contact area (μm2) | 12 233±5114 | 52 022±6859 | 9 | Wilcoxon paired T9=0, p=0.004 | |
| shear stress (kPa) | 124±38.7 | 81.26±25.5 | 9 | Wilcoxon paired T9=16, p=0.496 | |
| euplantulae | force (mN) | 22.83±2.22 | 7.80±0.99 | 12 | paired t-test T11=7.48, p<0.001 |
| contact area (μm2) | 109 635±8557 | 32 920±5448 | 11 | paired t-test T10=6.63, p<0.001 | |
| shear stress (kPa) | 215±29.1 | 336±80.7 | 11 | paired t-test T10=1.88, p=0.088 |
Figure 3.
Direction dependence of (a,c) arolium and (b,d) euplantulae. Maximal friction and shear stress during distal and proximal slides measured in the footloose condition. ***p<0.001, n.s. not significant.
When pads were tested in the fixed condition (table 3), the directionality of arolium and euplantulae partly disappeared or even reversed. In the fixed arolium, forces were significantly higher in the proximal direction. As in the footloose condition, this difference disappeared when we considered shear stress. In the fixed euplantulae, there was even a trend for greater forces and a significantly higher shear stress during the proximal pulls.
Table 3.
Single-leg force measurements for arolia and euplantulae of cockroach hind legs in the fixed condition.
| distal slide mean±s.e. | proximal slide mean±s.e. | n | test statistic | ||
|---|---|---|---|---|---|
| arolium | force (mN) | 1.69±0.19 | 2.94±0.39 | 12 | paired t-test T11=3.29, p=0.007 |
| contact area (μm2) | 21 086±3641 | 32 016±3929 | 11 | Wilcoxon paired T11=14, p=0.102 | |
| shear stress (kPa) | 213±115 | 121±14.4 | 11 | Wilcoxon paired T11=26, p=0.577 | |
| euplantulae | force (mN) | 14.40±0.97 | 20.23±2.45 | 11 | paired t-test T10=2.19, p=0.053 |
| contact area (μm2) | 60 337±5627 | 60 009±7730 | 11 | paired t-test T10=0.03, p=0.973 | |
| shear stress (kPa) | 262±28.8 | 392±57 | 11 | paired t-test T10=2.35, p=0.040 |
Tables 2 and 3 show that the shear stress for the euplantulae was generally higher than for the arolium in both the fixed and footloose conditions (e.g. footloose proximal, T11.6=2.98, p=0.012).
4. Discussion
All animals running with adhesive structures must have efficient ways of controlling surface contact to satisfy the conflicting demands of attachment and detachment. One widespread mechanism to control attachment and detachment is the directionality of attachment devices (Hanna & Barnes 1991; Autumn et al. 2000, 2006a; Federle et al. 2002; Niederegger & Gorb 2003). Most structures attach or interlock with a proximal pull and detach with a distal push (Autumn et al. 2000; Federle et al. 2002). However, a direction dependence of adhesive structures may be disadvantageous when the force vector of the leg tends to detach a pad, e.g. during level walking.
Our findings show that cockroaches (N. cinerea) have overcome this problem by employing two different pads with opposite directional dependence. In free walking cockroaches, the most distal pad, the arolium, is used primarily when the leg pulls towards the body, while the tarsal pads (euplantulae) are used when the leg is pushing away from it. This ‘division of labour’ between the two different pad types is consistent with the results of the single-leg force measurements. When the leg was secured in the more natural footloose position, friction forces of the arolium were significantly higher during a proximal slide than a distal slide, while the opposite was true for the euplantulae (figure 3).
The separate use of arolium and euplantulae for inverted and upright running was noted by other authors (Roth & Willis 1952; Arnold 1974; Larsen et al. 1997; Frazier et al. 1999), but its functional significance as an adaptation for the different tasks of pulling and pushing has not been recognized. Roth & Willis (1952) and Arnold (1974) noted that in several cockroach species, the arolium was kept away from the surface at rest and during level walking while the euplantulae are in contact. In mantophasmids (Mantophasma zephyra) the arolium is conspicuously folded away from the surface during normal walking, but is quickly brought into contact for emergency situations, such as additional loading or sudden wind pulses (Beutel & Gorb 2006). For most insects, a contraction of the claw flexor muscle brings the pretarsus into surface contact (Snodgrass 1935; Roth & Willis 1952) and it folds away by the recoil of elastic resilin cuticle at the penultimate tarsal joint (Frazier et al. 1999). Thus, our findings show that the claw flexor muscle is relaxed during push strides and probably contracted during pull strides. This suggests that insects can easily change the tarsus configuration for a push or a pull stride via the claw flexor muscle.
Our findings show that the cockroach arolium and euplantulae possess an opposed direction dependence, which corresponds to the usage of different pads for pushing and pulling. Two mechanisms may explain the existence of frictional anisotropy in adhesive structures: (i) a change of contact area or (ii) a change in the pad efficiency (shear stress).
The shear stress measurement in N. cinerea does not support the latter mechanism. When shear stress was considered, there was no longer a significant difference between the proximal and distal slides for the arolium and the euplantulae (tables 2 and 3). One exception was the fixed euplantulae, which had higher shear stress during proximal slides. This, however, is the opposite of what would be expected if changes in shear stress were the cause of the directional dependence. Thus, the anisotropic friction of both arolium and euplantulae in cockroaches is caused by changes in adhesive contact area, which increases significantly for the arolium during a proximal pull and for the euplantulae during a distal push.
As the arolium of cockroaches is not unfoldable (Roth & Willis 1952), an increase in contact area by unfolding, as shown for hymenopteran, mecopteran and trichopteran insects, is not possible (Snodgrass 1956; Heming 1971; Beutel & Gorb 2001). Similarly, lateral views of the tarsus indicate that neither the surface of the arolium nor the euplantulae are sloped like the tips of many adhesive setae, which would maximize contact area in the preferred direction by bending. However, the characteristic changes of contact area in both arolium and euplantulae can be explained by the mechanics of the tarsal chain.
In all insects, the tarsal chain consists of up to five segments linked via flexible joints (Snodgrass 1935). In most running animals, including sprawled-posture insects, joint torques are minimized by keeping ground reaction force vectors approximately aligned along the legs (Full et al. 1991). During a pulling stride, if both euplantulae and arolium are in contact with the surface, the force vector will tend to lift and pull the tarsal pads off the surface. In this situation, the peel force will be concentrated on the most proximal pad in contact, causing the euplantulae to detach from the surface. The arolium may be the most resistant to this peel force, because it is wider than the euplantulae (and peel force is directly proportional to width). If no adhesive structures are present on the pretarsus, as in beetles and bush crickets (Stork 1980; Gorb et al. 2000), it is usually the most distal tarsal pad which is conspicuously wider than the more proximal pads (Beutel & Gorb 2001), indicating a similar function.
In contrast to proximal pulls, a distal push is always coupled with a force directed towards the surface (for climbing cockroaches see Goldman et al. 2006). Thus, there will be a greater normal force for each euplantula in contact, increasing the contact area. However, a distal push on the arolium may cause the tarsus to buckle upwards, due to the flexibility of the tarsal and tarso-pretarsal joints. This instability caused by the arolium contact may provide a functional explanation as to why the last tarsal segment is often kept conspicuously off the surface during a pushing stride (Roth & Willis 1952; Arnold 1974). In general, the possibility of using a distal pre-(tarsal) attachment structure for pushing is limited owing to the chain-like construction of the tarsus. More proximally located attachment structures such as the euplantulae are better adapted for pushing because the tarsus is less susceptible to buckling. The stability of the proximal tarsus is enhanced by the extensive overlap between the proximal tarsal segments Ta1–Ta4 on their lateral walls, limiting the mobility of the intersegmental joints (Frazier et al. 1999).
While the distal arolium acts as an adhesive pad, resisting peeling, the function of the more proximal euplantulae is clearly different. Cockroaches use their euplantulae only when the foot is pressed onto the surface, when no adhesive forces are needed. For insects with a sprawled posture, however, sufficient friction forces are needed to prevent slipping. Thus, our results show that the euplantulae of cockroaches are not adhesive organs but mainly serve as friction pads.
The division of labour for pushing and pulling between proximal and distal tarsus appears to be widespread among arthropods. Many groups without euplantulae possess distally directed hairs or bristles on the ventral surface of the tarsus (e.g. Hymenoptera: Schulmeister 2003, Frantsevich & Gorb 2004). Tarsal segments covered by these hairs probably have the same direction dependence as the euplantulae, though they may only interlock on sufficiently rough surfaces. In jumping spiders, pretarsal scopulae were reported to make contact when pulled towards the body (Hill 1977), whereas a higher friction in the distal direction was found for the tarsal and metatarsal scopulae of bird and hunting spiders (Niederegger & Gorb 2006).
Our morphological analysis of the arolium and the euplantulae in N. cinerea confirms earlier findings obtained by light microscopy (Roth & Willis 1952) and supports the division of labour between the euplantulae and the arolium. The procuticle of the arolium is composed of many branched cuticular rods which have been noted in adhesive structures of many other insects (Slifer 1950; Kendall 1970; Gorb et al. 2000; Beutel & Gorb 2001). The branched fibre morphology may allow close moulding to surface structures of different length scales, thereby increasing adhesive contact area (Gorb et al. 2000).
By contrast, the euplantulae show a much thicker epicuticle and a thinner procuticle in which the fibres are not branched. This supports the conclusion by Roth & Willis (1952) that the euplantulae are not as soft as the arolium and less well adapted for adhering to rough substrates. For sufficient friction, however, it is less critical to make full contact to a rough surface, as high friction forces can be achieved even with very little contact area, especially if some interlocking is involved (Yoshizawa et al. 1993). Moreover, the cuticular surface of the euplantulae is characterized by a series of microscopic steps, with steeper sides facing distally. The advantages of such a surface profile are obvious. A distal push would reduce slipping on a rough surface, by catching small irregularities in the surface. The microstructured topography of the euplantulae may also account for the higher shear stress in the euplantulae when compared with the arolium. As the pad profile will facilitate drainage of the fluid secretion (Federle et al. 2006; Persson 2007), the highest peaks of the pad surface may come into closer contact with the substrate, which may be essential for generating high friction forces.
Direction dependence of attachment structures is an important biological principle that promises to be very useful for technical applications, where a dynamic control of surface attachment forces is required. Only recently, several bio-inspired robots have been developed, which are capable of climbing up vertical walls (Provancher et al. 2004; Asbeck et al. 2006; Autumn et al. 2006a). However, some climbing robots appear to have difficulty climbing head down, i.e. they have the same orientation during upward and downward climbing. This may be because their ‘legs’ are designed mainly for pulling. An insect-inspired robot foot that can generate both pushing and pulling forces might help to achieve better manoeuvrability.
Acknowledgments
We thank Thomas Endlein for his help with preliminary experiments for this project and Jeremy Skepper for advice on freeze-fracture techniques. This study was funded by research grants of the Deutsche Forschungsgemeinschaft (Emmy-Noether Fellowship FE 547/1 to W.F.), the UK Biotechnology and Biological Sciences Research Council as well as the Cambridge Isaac Newton Trust.
Supplementary Material
Cockroach (Nauphoeta cinerea) running upward on a smooth surface (velocity 0.17 m s−1, recorded at 500 fps, using a Hot Shot 1280PCI camera). Note that the front leg makes contact with the arolium, whereas the hind leg engages the euplantulae
Cockroach (Nauphoeta cinerea) running downward on a smooth surface (velocity 0.25 m s−1, 500 fps). Here, the front leg makes contact with the euplantulae, and the hind leg now uses the arolium
Loading of the arolium in the ‘footloose’ condition (fixed at the tibia). Even moderate normal forces (>0.4 mN) bring the euplantulae into surface contact
Setup for filming free walking roaches. (a) Lateral view of hind leg of an upward climbing roach. (b) Contact area captured using reflected light
Setup for measuring single leg forces (see text)
References
- Arnold J.W. Adaptive features on the tarsi of cockroaches (Insecta: Dictyoptera) Int. J. Insect Morphol. Embryol. 1974;3:317–334. doi:10.1016/0020-7322(74)90026-9 [Google Scholar]
- Asbeck A.T, Kim S, Cutkosky M.R, Provancher W.R, Lanzetta M. Scaling hard vertical surfaces with compliant microspine arrays. Int. J. Robot. Res. 2006;25:1165–1179. doi:10.1177/0278364906072511 [Google Scholar]
- Autumn K, Hansen W. Ultrahydrophobicity indicates a non-adhesive default state in gecko setae. J. Comp. Physiol. A. 2006;192:1205–1212. doi: 10.1007/s00359-006-0149-y. doi:10.1007/s00359-006-0149-y [DOI] [PubMed] [Google Scholar]
- Autumn K, Liang Y.A, Hsieh S.T, Zesch W, Chan W.P, Kenny T.W, Fearing R, Full R.J. Adhesive force of a single gecko foot-hair. Nature. 2000;405:681–685. doi: 10.1038/35015073. doi:10.1038/35015073 [DOI] [PubMed] [Google Scholar]
- Autumn K, Dittmore A, Santos D, Spenko M, Cutkosky M. Frictional adhesion: a new angle on gecko attachment. J. Exp. Biol. 2006a;209:3569–3579. doi: 10.1242/jeb.02486. doi:10.1242/jeb.02486 [DOI] [PubMed] [Google Scholar]
- Autumn K, Hsieh S.T, Dudek D.M, Chen J, Chitaphan C, Full R.J. Dynamics of geckos running vertically. J. Exp. Biol. 2006b;209:260–272. doi: 10.1242/jeb.01980. doi:10.1242/jeb.01980 [DOI] [PubMed] [Google Scholar]
- Beutel R.G, Gorb S.N. Ultrastructure of attachment specializations of hexapods (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J. Zool. Syst. Evol. Res. 2001;39:177–207. doi:10.1046/j.1439-0469.2001.00155.x [Google Scholar]
- Beutel R.G, Gorb S.N. A revised interpretation of attachment structures in Hexapoda with special emphasis on Mantophasmatodea. Arthropod Syst. Phylogeny. 2006;64:3–25. [Google Scholar]
- Federle W. Why are so many adhesive pads hairy? J. Exp. Biol. 2006;209:2611–2621. doi: 10.1242/jeb.02323. doi:10.1242/jeb.02323 [DOI] [PubMed] [Google Scholar]
- Federle W, Endlein T. Locomotion and adhesion: dynamic control of adhesive surface contact in ants. Arthropod Struct. Dev. 2004;33:67–75. doi: 10.1016/j.asd.2003.11.001. doi:10.1016/j.asd.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Federle W, Brainerd E.L, McMahon T.A, Hölldobler B. Biomechanics of the movable pretarsal adhesive organ in ants and bees. Proc. Natl Acad. Sci. USA. 2001;98:6215–6220. doi: 10.1073/pnas.111139298. doi:10.1073/pnas.111139298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle W, Riehle M, Curtis A.S.G, Full R.J. An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integr. Comp. Biol. 2002;42:1100–1106. doi: 10.1093/icb/42.6.1100. doi:10.1093/icb/42.6.1100 [DOI] [PubMed] [Google Scholar]
- Federle W, Barnes W.J.P, Baumgartner W, Drechsler P, Smith J.M. Wet but not slippery: boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface. 2006;3:689–697. doi: 10.1098/rsif.2006.0135. doi:10.1098/rsif.2006.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantsevich L, Gorb S. Structure and mechanics of the tarsal chain in the hornet, Vespa crabro (Hymenoptera: Vespidae): implications on the attachment mechanism. Arthropod Struct. Dev. 2004;33:77–89. doi: 10.1016/j.asd.2003.10.003. doi:10.1016/j.asd.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Frazier S.F, Larsen G.S, Neff D, Quimby L, Carney M, DiCaprio R.A, Zill S.N. Elasticity and movements of the cockroach tarsus in walking. J. Comp. Physiol. A. 1999;185:157–172. doi:10.1007/s003590050374 [Google Scholar]
- Full R.J, Blickhan R, Ting L.H. Leg design in hexapedal runners. J. Exp. Biol. 1991;158:369–390. doi: 10.1242/jeb.158.1.369. [DOI] [PubMed] [Google Scholar]
- Goldman D.I, Chen T.S, Dudek D.M, Full R.J. Dynamics of rapid vertical climbing in cockroaches reveals a template. J. Exp. Biol. 2006;209:2990–3000. doi: 10.1242/jeb.02322. doi:10.1242/jeb.02322 [DOI] [PubMed] [Google Scholar]
- Gorb S, Scherge M. Biological microtribology: anisotropy in frictional forces of orthopteran attachment pads reflects the ultrastructure of a highly deformable material. Proc. R. Soc. B. 2000;267:1239–1244. doi: 10.1098/rspb.2000.1133. doi:10.1098/rspb.2000.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorb S, Jiao Y, Scherge M. Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera Tettigoniidae) J. Comp. Physiol. A. 2000;186:821–831. doi: 10.1007/s003590000135. doi:10.1007/s003590000135 [DOI] [PubMed] [Google Scholar]
- Gravish N, Wilkinson M, Autumn K. Frictional and elastic energy in gecko adhesive detachment. J. R. Soc. Interface. 2008;5:339–348. doi: 10.1098/rsif.2007.1077. doi:10.1098/rsif.2007.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna G, Barnes W.J.P. Adhesion and detachment of the toe pads of tree frogs. J. Exp. Biol. 1991;155:103–125. [Google Scholar]
- Heming B.S. Functional morphology of the thysanopteran pretarsus. Can. J. Zool. 1971;49:91–108. doi: 10.1139/z71-014. [DOI] [PubMed] [Google Scholar]
- Hill D.E. The pretarsus of salticid spiders. Zool. J. Linn. Soc. 1977;60:319–338. [Google Scholar]
- Kendall M.D. The anatomy of the tarsi of Schistocerca gregaria Forskal. Zeitschrift für Zellforschung und mikroskopische Anatomie. 1970;109:112–137. doi: 10.1007/BF00364935. doi:10.1007/BF00364935 [DOI] [PubMed] [Google Scholar]
- Larsen G.S, Frazier S.F, Zill S.N. The tarso-pretarsal chordotonal organ as an element in cockroach walking. J. Comp. Physiol. A. 1997;180:683–700. doi:10.1007/s003590050083 [Google Scholar]
- Niederegger S, Gorb S.N. Tarsal movements in flies during leg attachment and detachment on a smooth substrate. J. Insect Physiol. 2003;49:611–620. doi: 10.1016/s0022-1910(03)00048-9. doi:10.1016/S0022-1910(03)00048-9 [DOI] [PubMed] [Google Scholar]
- Niederegger S, Gorb S.N. Friction and adhesion in the tarsal and metatarsal scopulae of spiders. J. Comp. Physiol. A. 2006;192:1223–1232. doi: 10.1007/s00359-006-0157-y. doi:10.1007/s00359-006-0157-y [DOI] [PubMed] [Google Scholar]
- Persson B.N.J. Biological adhesion for locomotion on rough surfaces: basic principles and a theorist's view. Mater. Res. Soc. Bull. 2007;32:488. [Google Scholar]
- Provancher, W. R., Clark, J. E., Geisler, B. & Cutkosky, M. R. 2004 Towards penetration-based clawed climbing. In CLAWAR 2004, Madrid, Spain, 22–24 September 2004
- Roth L.M, Willis E.R. Tarsal structure and climbing ability of cockroaches. J. Exp. Zool. 1952;119:483–517. doi:10.1002/jez.1401190307 [Google Scholar]
- Scherge M, Gorb S.N. Springer; Berlin, Germany; New York, NY: 2001. Biological micro- and nanotribology: nature's solutions. [Google Scholar]
- Schulmeister S. Morphology and evolution of the tarsal plantulae in Hymenoptera (Insecta), focussing on the basal lineages. Zool. Scr. 2003;32:153–172. doi:10.1046/j.1463-6409.2003.00118.x [Google Scholar]
- Slifer E.H. Vulnerable areas on the surface of the tarsus and pretarsus of the grasshopper (Acrididae, Orthoptera) with special reference to the arolium. Ann. Entomol. Soc. Am. 1950;43:173–188. [Google Scholar]
- Snodgrass R.E. McGraw-Hill Book Company; New York, NY; London, UK: 1935. Principles of insect morphology. [Google Scholar]
- Snodgrass R.E. Cornell University Press; Ithaca, NY: 1956. Anatomy of the honey bee. [Google Scholar]
- Stork N.E. A scanning electron microscope study of tarsal adhesive setae in the Coleoptera. Zool. J. Linn.Soc. 1980;68:173–306. [Google Scholar]
- Yoshizawa H, Chen Y.L, Israelachvili J. Fundamental mechanisms of interfacial friction. 1. Relation between adhesion and friction. J. Phys. Chem. 1993;97:4128–4140. doi:10.1021/j100118a033 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cockroach (Nauphoeta cinerea) running upward on a smooth surface (velocity 0.17 m s−1, recorded at 500 fps, using a Hot Shot 1280PCI camera). Note that the front leg makes contact with the arolium, whereas the hind leg engages the euplantulae
Cockroach (Nauphoeta cinerea) running downward on a smooth surface (velocity 0.25 m s−1, 500 fps). Here, the front leg makes contact with the euplantulae, and the hind leg now uses the arolium
Loading of the arolium in the ‘footloose’ condition (fixed at the tibia). Even moderate normal forces (>0.4 mN) bring the euplantulae into surface contact
Setup for filming free walking roaches. (a) Lateral view of hind leg of an upward climbing roach. (b) Contact area captured using reflected light
Setup for measuring single leg forces (see text)