Abstract
Male calls of the katydid Neoconocephalus triops exhibit substantial developmental plasticity in two parameters: (i) calls of winter males are continuous and lack the verse structure of summer calls and (ii) at equal temperatures, summer males produce calls with a substantially higher pulse rate than winter males. We raised female N. triops under conditions that reliably induced either summer or winter phenotype and tested their preferences for the call parameters that differ between summer and winter males.
Neither generation was selective for the presence of verses, but females had strong preferences for pulse rates: only a narrow range of pulse rates was attractive. The attractive ranges did not differ between summer and winter females. Both male pulse rate and female preference for pulse rate changed with ambient temperature, but female preference changed more than the male calls.
As a result, the summer call was attractive only at 25°C, whereas the slower winter call was attractive only at 20°C. Thus, developmental plasticity of male calls compensates for differences in temperature dependency between calls and preferences and enables the communication system to function in heterogeneous environments. The potential role of call plasticity during the invasion of new habitats is discussed.
Keywords: phenotypic plasticity, Neoconocephalus triops, phonotaxis, temperature dependency, communication
1. Introduction
Phenotypes are often strongly influenced by the environment during development. Identical genotypes may result in significantly different phenotypes depending on the environmental conditions (West-Eberhard 2003). The potential of such developmental (or phenotypic) plasticity has recently been recognized, as a mechanism for the evolution of novel traits (review in West-Eberhard 2003). Furthermore, developmental plasticity may be crucial for living in variable environments (seasonal and non-seasonal; Cook & Johnson 1968; Shapiro 1976; Trussel & Smith 2000; Agrawal 2001) and may facilitate the spread of a species into new environments (Losos et al. 2000; Agrawal 2001; Yeh & Price 2004).
One important function of acoustic communication in insects and anurans is to identify conspecific mates. Accordingly, call parameters used by the receiver to identify conspecific signals exhibit little within-male variation and between-male variation (Gerhardt 1991; Gerhardt & Huber 2002). This situation is complicated by the temperature dependency of many call parameters (e.g. pulse rate), which increase or decrease in value with changing ambient temperatures. In case of temperature-dependent parameters used for species identification, the mean value changes with temperature, while variation at any given temperature remains small (Gerhardt & Huber 2002). Correct species identification is then assured by matching the temperature dependency of the receiver (call recognizer) to that of the sender throughout the relevant temperature range (Gerhardt 1978; Helversen & Helversen 1981; Doherty 1985).
In the katydid Neoconocephalus triops (Orthoptera: Tettigoniidae), females identify and approach calling males for mating. In North America, this species occurs as two seasonally distinct generations, one in the summer and the other in the winter (Whitesell 1974; Whitesell & Walker 1978). In general, the offspring of one generation produce the next generation, so that both generations constitute one gene pool (Whitesell & Walker 1978). For a detailed description of natural history of N. triops, see §2.
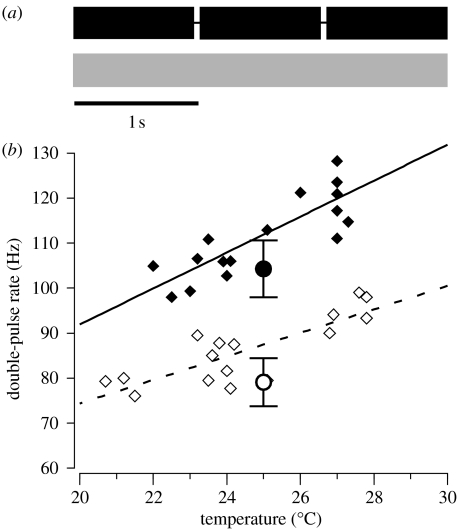
Male calls of N. triops are significantly influenced by the photoperiod experienced during juvenile development (Whitesell & Walker 1978). This developmental plasticity results in striking differences between the calls of the two generations. First, winter males produce continuous calls, whereas the calls of summer males are structured in verses (Whitesell & Walker 1978; Greenfield 1990). Second, at equal ambient temperature, the average double-pulse rate in calls of summer males was more than 20% higher than that of winter males with low between-male variation within each generation (CV: 6.02–6.72%, figure 1). In fact, the call differences are so striking that seasonal generations of N. triops were originally considered as separate species (Walker 1964; Whitesell 1974).
Figure 1.
Developmental plasticity of male calls of N. triops. (a) Schematic call structures of versed summer call (black trace) and continuous winter call (grey trace). (b) Temperature dependencies of summer and winter calls. Filled diamonds indicate amplitude modulation (AM) rates (double-pulse rates) of summer calls and open diamonds indicate rates of winter calls (data and regression lines from Whitesell & Walker 1978). Filled circle indicates mean AM rate (±s.d.) of calls of summer males (n=25) and the open circle indicates that of winter males (n=15) raised in this study.
Here we investigate the consequences of the plasticity of male calls for the communication system of N. triops. Females were raised under environmental conditions that induce different call phenotypes. We tested female preferences of both generations for the call parameters that differ between summer and winter generations. Female preferences did not differ between generations; that is, they did not exhibit developmental plasticity that parallels the plasticity of male calls. Rather, male call plasticity enables communication in seasonally variable environments.
2. Material and methods
(a) Natural history of N. triops
The distribution of N. triops ranges from the tropics in South America to the temperate regions of North America. In North America, two distinct reproductive generations occur per year. One generation reproduces during the summer and the other generation reproduces in late winter/early spring (Whitesell & Walker 1978). The two generations do not form distinct gene pools, as each generation largely comprises the offspring of the previous generation (Whitesell 1974). The offspring of the summer generation diapause as adults during the coldest months of the year before they reproduce in the late winter. Offspring of the winter generation mature without diapause and reproduce in the following summer. Males of these populations express the call plasticity described above. In the tropics, reproductively active individuals of N. triops can be found all year round. Males of these populations express only the summer call (Whitesell 1974; Greenfield 1990).
(b) Rearing of animals
Adult N. triops were collected in Gainesville, FL, in winter and summer of 2004 and brought to the laboratory at the University of Missouri for egg collection. Males and females were housed on grass that was used by females for oviposition. We collected eggs from the grass and stored them in Petri dishes on moist filter paper. We sprayed the eggs with 0.5% methylparaben solution to inhibit the growth of fungi. Eggs were kept in the dark at 20–30°C until eclosion.
All insects were raised on potted wheat seedlings and fed with puppy chow (Purina, MO), rolled oats and apples. Cages were kept in incubators at 50–70% RH, with daily temperature and light cycles. The high temperature was maintained from 1 hour after lights came on until 1 hour before dark, during which the temperature was reduced. The specific temperature and light cycles are given below.
The phenotypic variability of male calls in N. triops is induced by the photoperiod experienced during juvenile development (Whitesell 1974; Whitesell & Walker 1978). A 15 L : 9 D cycle results in summer calls (discontinuous calls, fast double-pulse rate), whereas 11 L : 13 D cycle results in continuous winter calls with slow double-pulse rate (Whitesell & Walker 1978).
After eclosion, we assigned first-instar nymphs randomly to summer or winter rearing conditions (Whitesell 1974). Under summer conditions, animals were reared to adulthood with 15 L : 9 D cycle and high/low temperatures of 30/20°C. We refer to these animals as ‘summer generation’ or ‘summer animals’. Male calls were recorded two to three weeks after the final moult. Females were tested in phonotaxis experiments starting three to four weeks after the final moult. Some summer females used in phonotaxis experiments were not raised from eggs, but collected as nymphs in the field and raised to adulthood in the conditions described above. We could not detect any difference in preferences between the females raised from eggs and those collected as nymphs.
Under winter conditions, animals were reared to adulthood at 11 L : 13 D cycle and high/low temperatures of 23/17°C. Two to three weeks after adult moult, we transferred the animals to a cycle of 9.5 L : 14.5 D and temperatures of 18/12°C to secure diapause. After 3.5–5 months in diapause, insects were transferred to 13 L : 11 D cycle and high/low temperatures of 26/18°C in order to terminate diapause. We refer to these animals as ‘winter generation’ or ‘winter animals’. Male calls were recorded one to three weeks after the termination of diapause. Females were tested in phonotaxis experiments starting three to four weeks after the end of diapause. The photoperiods and temperatures used in this study correspond approximately to the natural conditions that each generation experiences in Gainesville, FL (www.wunderground.com).
We recorded calls of males raised under both summer and winter conditions (for details of call recording and analysis see Schul & Patterson 2003). Calls of the summer generation were structured in verses, whereas winter males produced continuous calls; Whitesell & Walker (1978) reported the same pattern for summer and winter calls. Calls of reared summer males had a significantly higher double-pulse rate (mean±s.d. at 25°C: 104.3±6.3 Hz, n=25) than that of reared winter males (79.1±5.3, n=15; t-test, p<0.001; see figure 1). Whitesell & Walker (1978) reported double-pulse rates at 25°C in the range of 103–112 and 78–88 Hz for summer and winter calls, respectively. Thus, our rearing conditions reliably resulted in the call phenotypes found in summer and winter animals.
(c) Female phonotaxis tests
We tested female phonotaxis using a walking compensator (Kramer–Kugel; Weber et al. 1981) in a temperature-regulated anechoic chamber at ambient temperatures of 20±1 and 25±1°C. In short, females were placed on top of a sphere, free to walk but kept in place by compensatory sphere rotations, while acoustic signals were presented from loudspeakers located in the animal's horizontal plane. The intended direction and speed of the animal were read from the control circuitry. All experiments were performed in the dark except for the infrared light used to monitor the insects' position (for details see Weber et al. 1981; Schul 1998). Females were tested for as long as six weeks; we did not detect any change in preference during this period.
(d) Stimulation
We generated synthetic signals using a custom-developed DA-converter/amplifier system with 250 kHz sampling rate and 16-bit resolution. We delivered the stimuli using one of two loudspeakers (Motorola KSN1218C) mounted at a distance of 150 cm in the horizontal plane of the animal and separated by an angle of 105°. Signal amplitude was set to 80±1 dB peak SPL (reference pressure 20×10−5 Pa) using a Bruel and Kjaer sound level meter (B&K 2231) and a 1/4″ condenser microphone (G.R.A.S. 40BF) that was positioned 1 cm above the sphere.
Most of the energy in the calls of N. triops is concentrated in a low-frequency band centred around 11 kHz, and frequency components in the ultrasound are at least 20 dB less intense than the low-frequency band. We used a pure tone of 11 kHz as a carrier signal to which we subsequently applied amplitude modulations (AMs).
We based temporal parameters of our stimuli on our analysis of calls from natural populations. The control stimulus of the 25°C experiments consisted of a train of paired pulses of 2 and 2.75 ms duration with an interval of 1.75 ms in between. These double pulses were repeated after an interval of 2.5 ms, resulting in a double-pulse rate of 111 Hz. For the control stimulus in 20°C experiments, we used pulse durations of 3 and 3.2 ms and interval durations of 2.8 and 4.4 ms, resulting in a double-pulse rate of 75 Hz. The amplitude of the first pulse of the pair was 50% of that of the second pulse in both control stimuli. In experiments with summer animals, the control stimulus was structured in verses with durations of 950 ms separated by silent intervals of 50 ms. In experiments with winter animals, the control stimulus was continuous. For both winter and summer females, responses to the control stimulus did not differ significantly in response strength from responses to the natural calls.
We determined in preliminary tests the relevant call parameters for this study (data shown in the electronic supplementary material). We found that female N. triops did not require the elaborate double-pulse structure of the male call: stimuli with the double pulse replaced by one long pulse of the duration of the double pulse (merged pulses) were as attractive as stimuli with the double-pulse pattern (see Schul (1998) and Deily & Schul (2004) for similar results in other katydid species). This result indicates that the relevant AM of N. triops calls is the rate at which double pulses are repeated. In the tests presented in this study, we therefore use such merged pulses (equivalent to double pulses in natural calls). We refer to the rate of these merged pulses and to the equivalent double-pulse rate in natural calls as AM rate.
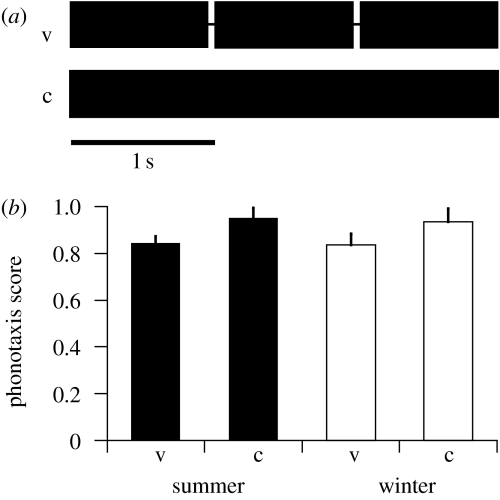
In the first set of tests, we determined female preference for call structure (figure 2). We used stimuli (AM rate of 110 Hz) that were either continuous (winter call structure) or structured in verses with durations of 950 ms and intervals of 50 ms (summer call structure). We compared the responses of summer and winter generations at an ambient temperature of 25°C.
Figure 2.
Female preference to the verse structure of male calls in N. triops. (a) Schematic of tested call model with verse structure (v, verse duration: 950 ms; interval duration: 50 ms), and continuous call model (c). (b) Mean phonotaxis scores (±s.e.m.) of summer females (n=11; filled bars) and winter females (n=10; open bars) to the versed call models (v) and the continuous call models (c).
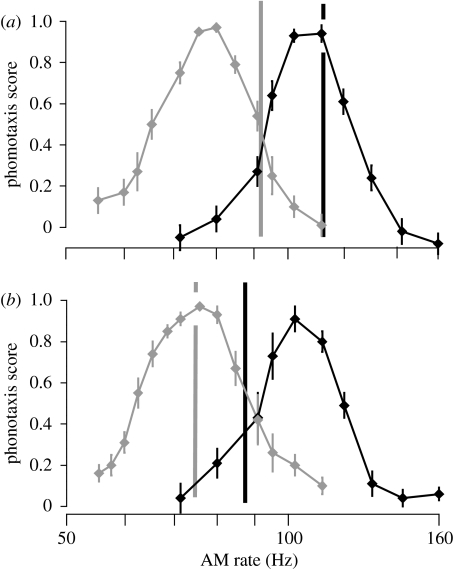
The second set of tests examined female preference for AM rate (figure 3) at two ambient temperatures (20 and 25°C). All stimuli had a duty cycle of 72% (duty cycle=pulse duration/pulse period). AM rates were varied between 55 and 160 Hz. In preliminary experiments we confirmed that AM rate rather than pulse duration or interval duration was the critical parameter evaluated by female N. triops (see electronic supplementary material).
Figure 3.
Preference for AM rate in (a) summer and (b) winter females at 20°C (grey) and 25°C ambient temperature (black). Each curve gives the mean (±s.e.m.) of normalized phonotaxis scores (summer females: 20°C, n=11; 25°C, n=10; winter females: 20°C, n=7; 25°C, n=9). Vertical lines indicate AM rates (adapted from Whitesell & Walker 1978) of male calls at 20°C (grey) and 25°C (black) for each generation.
(e) Experimental protocol
Each stimulus tested in this study was presented for 60–90 s from each of two different loudspeaker positions. The first stimulus of each test series was the control stimulus, followed by two to three test stimuli, another control, etc. A period of 60 s of silence was given between stimuli. The experimental series lasted between 30 and 60 min and consisted of up to six test stimuli (plus up to four controls). We randomly varied the sequence of stimuli within a series among individual females (for a detailed description of the experimental protocol, see Schul 1998; Bush et al. 2002).
(f) Data analysis
We quantified female response to a given stimulus by calculating a phonotaxis score (Schul 1998). The phonotaxis score included three measures describing the relative strength of phonotaxis: (i) the walking speed relative to that during the control stimulus, describing the elicited locomotion activity, (ii) the vector length, describing the accuracy of orientation, and (iii) the angular orientation relative to that during the control stimulus. The score can range from approximately +1 to −1 representing perfect positive or negative phonotaxis, respectively. Phonotaxis scores close to 0 indicate random orientation or no response (for details see Schul 1998).
In the first experiment, we compared female responses to continuous and versed calls (figure 2) using Wilcoxon signed-rank tests (Zar 1984). To compare female preference for AM rate between summer and winter generations in the second experiment (figure 3), we plotted phonotaxis scores as a function of AM rate. These response functions were smoothed by calculating a gliding average through three values and then normalizing. We determined upper and lower cut-off rates by fitting a parabolic function (y=c−ax×(x−b)2) to each individual's response function by minimizing the sum of squared errors. Upper and lower cut-off rates were defined as the AM rate where the fitted function fell below 70%. We compared the upper and lower cut-offs between generations at the same temperature using Mann–Whitney U-tests (Zar 1984). The application of cut-off frequencies in our study is only meant to emphasize the relative attractiveness of stimuli and do not classify stimuli as ‘recognized’ or ‘not recognized’ (for a detailed discussion, see Bush et al. 2002). Unless otherwise stated, data are given as mean±s.e. of the mean (s.e.m.).
3. Results
First, we tested the preferences of summer and winter females for the call structure (figure 2). For this parameter, we could not detect differences in female preference between summer and winter generations. In both generations, responses were high to the continuous call model (winter call) and to the call model with verse structure (summer call). Continuous calls elicited slightly higher scores than did the versed calls (summer females: 0.95±0.04 versus 0.84±0.03, mean±s.e.m., n=11; winter females: 0.94±0.05 and 0.84±0.05, n=10). These differences were marginally significant for both generations (Wilcoxon signed-rank test, 0.1≥p>0.05).
In the second set of experiments, we compared the preference function for AM rate between summer and winter females at two ambient temperatures (20 and 25°C). At 20°C, phonotaxis scores of both generations were the highest for AM-rates of approximately 75 Hz, and decreased towards higher and lower AM rates (figure 3a,b). The lower and upper cut-off frequencies did not differ significantly between the summer and winter generations (lower cut-off: summer 67.4±1.09 Hz, winter 64.7±0.47 Hz; upper cut-off: summer 88.9±1.23 Hz, winter 86.4±1.65 Hz; n=11 (summer) or 7 (winter); Mann–Whitney U-test, all tests p>0.05).
At 25°C, females of both generations responded strongest to AM rates close to 105 Hz; higher and lower AM rates elicited weaker responses (figure 3a,b). Lower and upper cut-off frequencies did not differ significantly between generations (lower cut-off: summer 95.4±1.45 Hz, winter 89.6±3.01 Hz; upper cut-off: summer 121.3±1.96 Hz, winter 116.7±1.96 Hz; n=10 (summer) or 9 (winter); Mann–Whitney U-test, all tests p>0.05). Thus, we could not detect any differences between the two generations in their preference for AM rates.
Comparing female preferences for AM rate with that of male calls demonstrates distinct mismatches at some temperatures. At 20°C, preference of both summer and winter females was tuned to the AM rate of the winter calls at this temperature. However, the model of the summer call at 20°C evoked only weak responses (phonotaxis score<0.5; figure 3) in females of either generation. Conversely, at 25°C preference of both summer and winter females was tuned to the AM rate of the summer call, while the AM rate of the winter call at the same temperature was much less attractive (phonotaxis score<0.4; figure 3). Thus, male calls and female preference were well matched only at temperatures corresponding to the season when each generation calls: at 25°C only the summer call was attractive, while at 20°C only the winter call elicited strong responses.
4. Discussion
The calls of the summer and winter generations of N. triops differ in two temporal aspects. First, summer calls are structured in verses while winter calls are continuous (figure 1). Second, summer calls have a substantially higher AM rate than winter calls at equal temperatures. By contrast, there were no differences in female preference between summer and winter generations: females of both generations responded readily to calls with and without verse structure (figure 2) and we could not detect differences in their tuning for AM rate (figure 3).
The presence or absence of a verse structure in the calls had little influence on their attractiveness: both the models were highly attractive to females of both generations (figure 2). Thus, this call characteristic is most likely not relevant for the attraction of females. A potential function of the verse pattern of the summer calls could be to provide males with silent windows during which they can detect the calls of other signallers (Greenfield 1990). The selective disadvantage of the absence of such a detection window in winter calls is likely to be small, because population densities are lower during the winter, and no calling congeners are present (Whitesell 1974).
In several cricket species, pulse rate of male calls was influenced by the temperature during development. The pulse rates of males raised at lower temperatures were 5–10% lower when recorded at the same temperature (e.g. Walker 2000; Grace & Shaw 2004). In one species (Laupala cerasina), female preferences changed according to the temperature during development. Females raised at low temperatures preferred lower rates than those raised at higher temperatures (Grace & Shaw 2004). By contrast, alternative call phenotypes in N. triops were induced by the photoperiod during development and not the temperature (Whitesell 1974). Also, in N. triops, developmental plasticity was limited to male calls, while female preferences were not affected by the photoperiod or temperature during development.
In N. triops, the developmental plasticity of AM rates results in a match between the calls of the summer males and female preference at high temperatures, and between the calls of winter males and female preference at low temperatures. Therefore, we argue that developmental plasticity of AM rates is most likely a mechanism to match the temperature dependencies of calls and female preferences at substantially different temperature ranges.
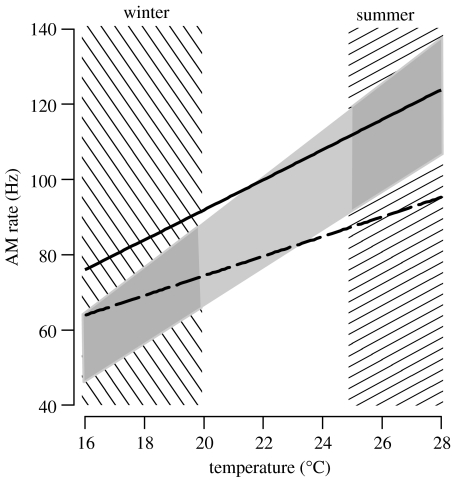
Female preference for AM rates changed with temperature, as is expected for poikilothermic animals (Randall et al. 1997); an increase in ambient temperature of 10°C results in approximately a doubling of the most attractive AM rate (figure 4). The temperature dependency of the AM rate of male calls, however, is considerably less steep than that of female preferences (figure 4). Owing to the exceptionally fast AM rates in Neoconocephalus (Greenfield 1990), muscular activity during calling produces considerable amounts of heat, elevating thorax temperature by up to 15°C above ambient temperature (Heath & Josephson 1970; Josephson 1984). The increase in thoracic temperature is larger for lower ambient temperatures, so that thorax temperature during calling is almost constant (Heath & Josephson 1970), resulting in only a weak dependence of male AM rates on ambient temperature (Walker 1975).
Figure 4.
Importance of call plasticity for the communication system of N. triops. Temperature dependency of summer call (solid line), winter call (dashed line), and female preference for AM rates (light grey area). Temperature dependency of preference for AM rate based on upper and lower cut-off frequencies (at 20 and 25°C) from pooled data of summer and winter females. The preference for AM rate below 20°C and above 25°C (dark grey areas) was extrapolated from our data assuming a linear relationship between temperature and preference. Hatched vertical bars indicate approximate temperature ranges at sunset between February and mid-March (left bar, winter), and between July and mid-August (right bar, summer) in Gainesville, FL (from www.wunderground.com). Call data from Whitesell & Walker (1978).
Because male calls and female preferences differ in their temperature dependency, male AM rates and female preferences are matched only in a narrow temperature range in N. triops. For populations in the tropics, which express only the summer call (Greenfield 1990), this narrow temperature range appears sufficient, as ambient temperatures fluctuate little throughout the year. Moreover, because N. triops calls after sunset, the influence of radiant heat is negligible.
Temperate populations of N. triops that encounter distinct seasons overwinter as adults. Their eggs develop directly without diapause (Whitesell 1974), allowing for two distinct generations per year, one reproducing in the summer and the other in late winter/early spring. Since summer and winter generations experience different ambient temperatures, the narrow range of temperature matching between calls and call preference seems to be insufficient: summer calls were attractive only at temperatures typical for their season (approx. 25–28°C; figure 4); at temperatures encountered during late winter and early spring (approx. 16–20°C; figure 4), females responded only weakly to summer calls. Conversely, winter calls were only attractive at low, but not high temperatures. The developmental plasticity of the mating call solves this problem by allowing two narrow ranges of temperature matching, one optimized for summer temperatures and the other for winter temperatures (figure 4).
Whether call plasticity in N. triops originated in a temperate or tropical population remains unclear. Call plasticity could have evolved as an adaptation to the seasonal temperatures encountered by populations in North America. Alternatively, call plasticity could have been a latent trait shared among all populations but only induced in those that live in a suitable environment. In either case, call plasticity most likely explains today's ecological success of N. triops. This species has the most extensive distribution range of any Neoconocephalus species (Walker & Greenfield 1983) and is the only member of the genus to inhabit both tropical and temperate regions (Greenfield 1990). All other species are confined to one region or the other. Developmental plasticity of the male calls enables N. triops to communicate over a wide range of temperatures. Male call plasticity seems to represent an adaptation of the communication system to the environmental variability encountered by N. triops in temperate regions and probably facilitated the spread into new habitats.
Acknowledgments
This research adhered to the Association for the Study of Animal Behaviour, Animal Behavior Society Guidelines for the Use of Animals in Research, the legal requirements of the USA and all institutional guidelines.We thank Sarah Bush and Carl Gerhardt for their critical comments and editing of the manuscript. We thank Thomas Walker for his support during our fieldwork in Gainesville, FL. This research was supported by a grant from the National Science Foundation (IOB-0445286) to J.S. and a grant of Sigma Xi to O.M.B. who received a Life Sciences Fellowship of the University of Missouri.
Supplementary Material
Importance of pulse structure structure/interval and pulse duration for female preference
References
- Agrawal A.A. Phenotypic plasticity in the interactions and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. doi:10.1126/science.1060701 [DOI] [PubMed] [Google Scholar]
- Bush S.L, Gerhardt H.C, Schul J. Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim. Behav. 2002;63:7–14. doi:10.1006/anbe.2001.1880 [Google Scholar]
- Cook S.A, Johnson M.P. Adaptation to heterogeneous environments. I. Variation in heterophylly in Ranunculus flammula L. Evolution. 1968;22:496–516. doi: 10.1111/j.1558-5646.1968.tb03988.x. doi:10.2307/2406876 [DOI] [PubMed] [Google Scholar]
- Deily J.A, Schul J. Recognition of calls with exceptionally fast pulse rates: female phonotaxis in the genus Neoconocephalus (Orthoptera: Tettigoniidae) J. Exp. Biol. 2004;201:3523–3529. doi: 10.1242/jeb.01179. doi:10.1242/jeb.01179 [DOI] [PubMed] [Google Scholar]
- Doherty J.A. Temperature coupling and “trade-off” phenomena in the acoustic communication system of the cricket, Gryllus bimaculatus De Geer (Gryllidae) J. Exp. Biol. 1985;114:17–35. [Google Scholar]
- Gerhardt H.C. Temperature coupling in the vocal communication system of the gray treefrog Hyla versicolor. Science. 1978;199:992–994. doi: 10.1126/science.199.4332.992. doi:10.1126/science.199.4332.992 [DOI] [PubMed] [Google Scholar]
- Gerhardt H.C. Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim. Behav. 1991;42:615–635. doi:10.1016/S0003-3472(05)80245-3 [Google Scholar]
- Gerhardt H.C, Huber F. University of Chicago Press; Chicago, IL: 2002. Acoustic communication in insects and anurans. [Google Scholar]
- Grace J.L, Shaw K.L. Effects of developmental environment on signal-preference in a Hawaiian cricket. Evolution. 2004;58:1627–1633. doi: 10.1111/j.0014-3820.2004.tb01744.x. doi:10.1554/04-042 [DOI] [PubMed] [Google Scholar]
- Greenfield M.D. Evolution of acoustic communication in the genus Neoconocephalus: discontinuous songs, synchrony, and hetereospecific interactions. In: Bailey W.J, Rentz D.C.F, editors. The Tettigoniidae: biology, systematics and evolution. Springer; Heidelberg, Germany: 1990. pp. 71–97. [Google Scholar]
- Heath J.E, Josephson R.K. Body temperature and singing in the katydid Neoconocephalus robustus (Orthoptera, Tettigoniidae) Biol. Bull. 1970;138:272–285. doi:10.2307/1540212 [Google Scholar]
- Helversen D, Helversen O. Korrespondenz zwischen Gesang und auslösendem Schema bei Feldheuschrecken. Nova Acta Leopold. 1981;54:449–462. [Google Scholar]
- Josephson R.K. Contraction dynamics of flight and stridulatory muscles of tettigoniid insects. J. Exp. Biol. 1984;108:77–96. [Google Scholar]
- Losos J.B, Creer D.A, Glossip D, Goellner R, Hampton A, Roberts G, Haskell N, Taylor P, Ettling J. Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution. 2000;54:301–305. doi: 10.1111/j.0014-3820.2000.tb00032.x. doi:10.1111/j.0014-3820.2000.tb00032.x [DOI] [PubMed] [Google Scholar]
- Randall D, Burggren W, French K. 4th edn. W. H. Freeman and Company; New York, NY: 1997. Eckert animal physiology: mechanisms and adaptations. [Google Scholar]
- Schul J. Song recognition by temporal cues in a group of closely related bushcricket species (genus Tettigonia) J. Comp. Physiol. A. 1998;183:401–410. doi:10.1007/s003590050266 [Google Scholar]
- Schul J, Patterson A.C. What determines the tuning of hearing organs and the frequency of calls? A comparative study in the katydid genus Neoconocephalus (Orthoptera; Tettigoniidae) J. Exp. Biol. 2003;206:141–152. doi: 10.1242/jeb.00070. doi:10.1242/jeb.00070 [DOI] [PubMed] [Google Scholar]
- Shapiro A.M. Seasonal polyphenism. Evol. Biol. 1976;9:259–333. [Google Scholar]
- Trussel G.C, Smith L.D. Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc. Natl Acad. Sci. USA. 2000;97:2123–2127. doi: 10.1073/pnas.040423397. doi:10.1073/pnas.040423397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T.J. Cryptic species among sound-producing ensiferan Orthoptera (Gryllidae and Tettigoniidae) Q. Rev. Biol. 1964;39:345–355. doi:10.1086/404325 [Google Scholar]
- Walker T.J. Effects of temperature on rates in poikilotherm nervous systems: evidence from the calling songs of meadow katydids (Orthoptera: Tettigoniidae: Orchelimum) and reanalysis of published data. J. Comp. Physiol. 1975;101:57–69. doi:10.1007/BF00660119 [Google Scholar]
- Walker T.J. Pulse rates in the songs of trilling field crickets (Orthoptera: Gryllidae: Gryllus) Entomol. Soc. Am. 2000;93:565–572. doi:10.1603/0013-8746(2000)093[0565:PRITSO]2.0.CO;2 [Google Scholar]
- Walker T.J, Greenfield M.D. Songs and systematics of Caribbean Neoconocephalus (Orthoptera: Tettigoniidae) Trans. Am. Entomol. Soc. 1983;109:357–389. [Google Scholar]
- Weber T, Thorson J, Huber F. Auditory behaviour of the cricket. I. Dynamics of compensated walking and discrimination paradigms on the Kramer treadmill. J. Comp. Physiol. 1981;141:215–232. doi:10.1007/BF01342668 [Google Scholar]
- West-Eberhard M.J. Oxford University Press, Inc.; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]
- Whitesell, J. J. 1974 Geographic variation and dimorphisms in song, development, and color in a katydid: field and laboratory studies (Tettigoniidae, Orthoptera). PhD dissertation, University of Florida, Gainesville.
- Whitesell J.J, Walker T.J. Photoperiodically determined dimorphic calling songs in a katydid. Nature. 1978;274:887–888. doi:10.1038/274887a0 [Google Scholar]
- Yeh P.J, Price T.D. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Nat. 2004;164:531–542. doi: 10.1086/423825. doi:10.1086/423825 [DOI] [PubMed] [Google Scholar]
- Zar J.H. Prentice Hall; Upper Saddle River, NJ: 1984. Biostatistical analysis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Importance of pulse structure structure/interval and pulse duration for female preference