Abstract
Variation in the intensity of conspicuous displays raises three basic questions: (i) the relationship between internal state and display intensity, (ii) the relationship between display intensity and receiver response, and (iii) the effect of variation in receiver responsiveness on signaller behaviour. Here, I investigate the interaction between pups and helpers in the communally breeding banded mongoose (Mungos mungo), where each pup forms an exclusive relationship with a single adult helper (termed its ‘escort’). By experimentally manipulating pup need, I demonstrate that changes in begging rate correspond to changes in short-term need. The data then suggest that escorts in good condition may be more responsive to increased begging and that pups associating with them increase their begging more than do pups paired with escorts in poor condition. Escorts also appear more responsive to increased begging by female pups, and female pups increase their begging more than do male pups. These results suggest that banded mongoose pups may strategically adjust their investment in begging in relation to variation in the expected pay-off. I argue that such adjustment is likely to be a general phenomenon: wherever there is variation in responsiveness to signals, signallers will be selected to identify different categories of receiver and adjust their signals in order to maximize the pay-offs. Therefore, differences in signal intensity may be as much a product of context as an indication of variation in individual phenotypic or genotypic state.
Keywords: banded mongoose, Mungos mungo, parent–offspring-conflict, honest signalling, cooperative care, begging
1. Introduction
Conspicuous displays are seen as expressions of underlying conflicts of interest between signallers and receivers, with signallers attempting to maximize the response to a signal while minimizing investment in signalling and with receivers attempting to extract accurate information from signals and maximize the benefits of responding (Williams 1966; Zahavi 1975, 1987; Dawkins & Krebs 1978; Grafen 1990). However, communication is never one way, and in responding to a signal, receivers provide information about themselves which might be exploited by signallers in subsequent encounters (Acebo & Thoman 1995; Kedar et al. 2000; Kolliker & Richner 2004; Lotem & Winkler 2004).
Begging by dependent offspring has become a powerful paradigm for investigating conflict between signallers and receivers because offspring are expected to demand more food than carers are selected to provide (Trivers 1974; Parker & Macnair 1978; Macnair & Parker 1979; Godfray 1995). The majority of research has focused on the relationship between begging intensity and the internal state of offspring (the ‘honesty’ of begging), with a current consensus that begging intensity is a broadly accurate indicator of offspring need (reviewed in Kilner & Johnstone 1997; Royle et al. 2002; Wright & Leonard 2002). It is becoming increasingly clear, however, that numerous external factors influence the way that changes in state are expressed by changes in begging intensity (Kilner 1995; Kedar et al. 2000). In particular, differences between carers in the amount of care they are willing to provide may select offspring to beg in different ways to different carers (Stamps et al. 1989; Krebs & Magrath 2000; Kolliker & Richner 2004; Roulin & Bersier 2007). This will be especially important where begging is costly, since selection should act on offspring to maximize the return on their investment in begging.
An excellent way to address these issues is to investigate begging in cooperative societies where the majority of offspring care is carried out by non-breeding helpers. Helper contributions are expected to vary as a result of individual differences in the costs and benefits of investing in someone else's offspring (Heinsohn & Legge 1999; Clutton-Brock 2002; Griffin & West 2003), which means that there is likely to be considerable variation in responsiveness to changing signals of need. At the same time, offspring repeatedly interact with the same individuals over an extended period of time. This means that offspring have both the opportunity and the incentive to learn about differences in responsiveness and to adjust their begging in order to maximize the return.
To investigate whether there is variation among helpers in responsiveness to begging, and to determine how this affects the way offspring adjust their begging after a change in need, I carried out field observations and experiments on communally breeding banded mongooses (Mungos mungo) in western Uganda. Unique among cooperative mammals, individual pups form stable relationships with single helpers (termed ‘escorts’), with which they spend more than 70% of their time and from which they receive nearly all of their food (Cant 1998; Bell 2007a). This imposes an important restriction on the way variation in helper responsiveness might affect pups: rather than directing begging to more generous helpers, pups are probably forced to maximize the pay-offs of begging to one individual by adjusting their begging effort.
I first determine how changes in pup hunger affect begging by performing feeding and deprivation experiments on dependent pups, with the expectation that feeding should reduce and deprivation should increase begging rates. I then examine how escorts respond to changes in begging after pup deprivation. If escort investment in pups is influenced by variation in the costs and benefits of investing, then escorts should differ in their responsiveness to an increase in begging. Specifically, escorts for whom an increase in provisioning rate will be more costly should increase their provisioning rate by less than those for whom an increase in provisioning rate is less costly. Finally, I investigate whether changes in begging as a result of deprivation are adjusted with regard to the responsiveness of the escort. If escorts vary in their responsiveness, then I expect that pups associating with more responsive escorts will increase their begging by more than pups associating with less responsive escorts.
2. Material and methods
Between May 2003 and August 2005, I observed 565 banded mongooses and monitored 68 successful breeding attempts from 13 packs in Queen Elizabeth National Park, Uganda (0°12′ S; 27°54′ E). All individuals were habituated to close (less than 5 m) observation on foot, and accurate ages (±2 days) were known for most of the population (92%). Individuals aged 0–3 months were classified as pups and more than three months as helpers (animals as young as three months have been observed provisioning pups, Bell 2007b).
All animals were tattooed for permanent identification; while for field identification, fully grown animals were fitted with colour-coded plastic collars (weight 1.5 g). Growing animals and some well-habituated animals were given unique haircuts. Animals were trained to step on an electronic laboratory scale (accuracy±1.5 g) before foraging in the morning (approx. 07.30). To obtain a measure of individual condition, I calculated average weight from three successive mornings and obtained the residual from a linear regression of weight (g) over age (days). This provides a measure of weight relative to others for a given age, which has an effect on contributions to cooperative care by helpers (after Clutton-Brock et al. 2002).
This research was carried out under licence from Uganda National Council for Science and Technology, and all procedures approved by Uganda Wildlife Authority.
(a) Study animal
Banded mongooses live in large family groups (average number of adults=29, range 5–75) and are one of the few cooperative species where subordinates regularly breed (median breeding females=4, range 1–12). Females give birth in synchrony, producing large communal litters (median litter size=5, range 1–23), which remain in dens for three to four weeks. When pups emerge from the den, they spend 3–5 days approaching different helpers, after which individual pups form stable associations with a single adult helper (their escort) and remain associated with that animal until independence (approx. 9–13 weeks; Cant 1998; Gilchrist 2004). Adults who do not become escorts thereafter provide very little pup care (Gilchrist 2004; Bell 2007a). Escorts are generally young, non-breeding males (1–3 years old) or breeding females who contributed to the current litter (Gilchrist 2004; Hodge 2005). Associations are initiated and maintained by the pups, with escorts following a relatively passive ‘feed the nearest begging pup’ rule (Gilchrist 2004; Hodge 2005), and escorts do not preferentially associate with close relatives (S. J. Hodge 2007, unpublished data). During a foraging session, pups follow escorts closely (usually within 10 cm), begging constantly with a high pitched, bird-like chirp (average call rate=34.4 calls min−1±0.73 s.e., maximum=80).
(b) Pup feeding experiment
To determine whether begging rate reflects pup short-term need, I performed supplementary feeding experiments. On a control morning, I carried out a 10-min focal watch on a pup, then handled the pup for 5 min, before carrying out another 10-min focal watch. On an experimental morning, I repeated this protocol, but instead of handling the pup, I fed it 10 g of scrambled egg. I conducted the experiment on 20 pups and randomized the order of the trials.
(c) Pup deprivation experiment
To investigate variation in escort responsiveness to an increase in pup begging, and to determine whether such variation influenced the way pups changed their begging after a change in state, I carried out a series of pup deprivation experiments. On a control morning, I carried out focal watches on 21 pups when the pack started foraging. All focal watches were carried out within 1 hour of emergence from the den in the morning, minimizing the effects of provisioning through the morning, and standardizing as far as possible the state of all pups observed. On an experimental morning, I removed these pups (either singly or in pairs) when packs emerged from the den (approx. 07.00). The removed pups were provided with ad libitum water, but no food. I released them at the start of afternoon foraging (approx. 17.00) and carried out a second set of focal watches. Observations on 81 unmanipulated pups revealed that both the begging and provisioning rates are significantly lower in the afternoon than in the morning (paired t-tests, begging rate: t81=3.34, p=0.001; provisioning rate: t81=3.38, p=0.001), so any increase in begging or provisioning is unlikely to be a time of day effect.
(d) Statistical analysis
Where possible I carried out matched comparisons of individual behaviour (all tests two tailed). To investigate the variables influencing changes in pup begging rates and changes in escort provisioning rates, I used linear mixed models (LMM; see electronic supplementary material). Means±s.e. are presented throughout.
3. Results
(a) Pup feeding experiment
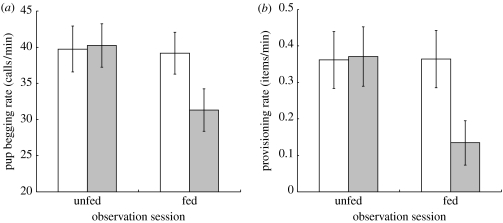
Pups begged at a significantly lower rate after being fed 10 g of scrambled egg, while the same pups showed no change in begging rate on an unfed control day (two-way ANOVA: significant interaction between observation session and treatment, F2,19=4.02, p=0.02; figure 1a). Pups were fed at a significantly lower rate after being fed 10 g of scrambled egg, while the same pups received food at the same rate on an unfed control day (two-way ANOVA: significant interaction between observation session and treatment, F2,19=10.30, p<0.001; figure 1b).
Figure 1.
Changes in (a) focal pup begging rate (n=20) and (b) focal escort provisioning rate (n=20) on an unfed control day and on a day when pups were fed 10 g of scrambled egg (mean±s.e.). White bar, before treatment; grey bar, after treatment.
(b) Pup deprivation experiment
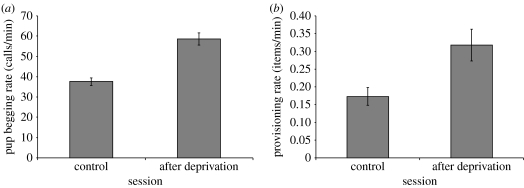
After a period of experimental deprivation, pups begged at a significantly higher rate (paired t-test, t20=9.01, p<0.0001; figure 2a), and received food at a significantly higher rate (t20=3.70, p=0.002; figure 2b) than they did on unmanipulated control mornings.
Figure 2.
(a) Focal pup begging rate (n=21) and (b) focal escort provisioning rate (n=21) on an unmanipulated control day and after pups were experimentally deprived (mean±s.e.).
(c) Pup deprivation experiment, changes in escort feeding
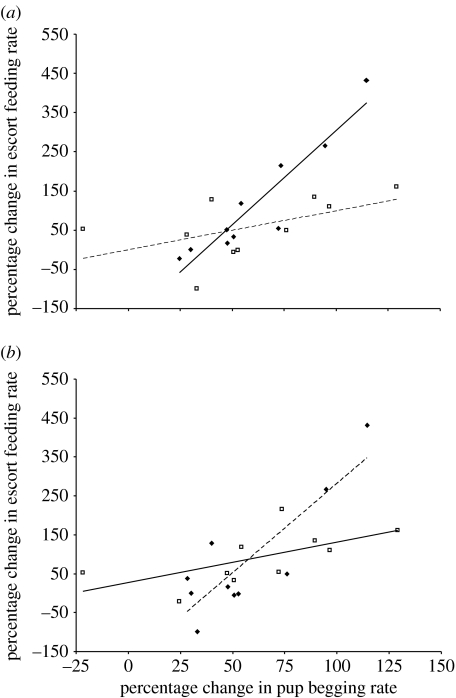
In response to changes in pup begging after deprivation, escorts increased their provisioning rate by more to pups who increased their begging by more (LMM: Χ2=23.17, p<0.001; table 1). However, there were significant interactions with both escort condition (LMM: Χ2=22.33, p<0.001) and pup sex (LMM: Χ2=6.14, p=0.013; see table 1 for all variables tested). Escorts who were in better condition appeared to be more responsive to a given increase in begging and they increased their provisioning more than escorts in poor condition (figure 3a). This is unlikely to be due to assortative pairing between escorts in good condition with pups in good condition: there is no evidence for a correlation between pup and escort condition at the start of the association period (LMM: Χ2=0.29, p=0.6; from 50 pup–escort pairs 16 litters in five packs, analysis restricted to individuals who were weighed within the first 2 days after the associations had formed). There is also no correlation between pup and escort condition within the experimental sample (linear regression: F1,19=1.41, p=0.25).
Table 1.
Linear mixed model of variables affecting the percentage change in feeding rate by focal escorts after pups have been experimentally deprived. (Effects only given for significant terms in the minimal model. Analysis was conducted on 20 pups with 20 escorts from 11 litters in five packs.)
| explanatory terms | Wald statistic (Χ1,192) | p |
|---|---|---|
| percentage increase in begging rate | 23.17 | <0.001 |
| escort condition | 8.67 | 0.003 |
| percentage increase in begging rate×escort condition | 22.33 | <0.001 see figure 3a |
| percentage increase in begging rate×pup sex | 6.14 | 0.013 see figure 3b |
| pup condition | 2.66 | 0.103 |
| escort age (days) | 2.32 | 0.128 |
| litter size | 2.09 | 0.148 |
| rainfall in last 30 days (mm) | 2.08 | 0.149 |
| escort sex | 1.79 | 0.181 |
| pup age (days) | 1.59 | 0.207 |
| previous feed rate | 0.01 | 0.921 |
| minimal model | effect size | s.e. |
|---|---|---|
| constant | 1.613 | 0.234 |
| percentage increase in begging rate | 2.32 | 0.48 |
| escort condition | 0.0045 | 0.0015 |
| percentage increase in begging rate×escort condition | 0.0211 | 0.000444 |
| percentage increase in begging rate×pup sex (F) | 1.743 | 0.174 |
Figure 3.
Changes in focal escort provisioning rate in response to experimental pup deprivation. (a) The effect of percentage change in pup begging rate and escort condition. To illustrate this interaction, I converted escort condition into a two-level factor by splitting about the median. Escort condition was calculated as the residual from a polynomial regression of weight (in g) over age (in days). Solid line, escort in good condition; dashed line, escort in poor condition. (b) The effect of percentage change in pup begging rate and pup sex. Points are observed values, lines are regression lines. Solid line, male pups; dashed line, female pups.
Escorts also appeared to be more responsive to increases in begging by female pups (figure 3b). Because this effect could potentially be driven by the fact that female pups increase their begging more than males, I repeated the analysis including only those data points that fell within the range of changes in begging shown by pups of both sex (n=9 females, 8 males). The interaction between change in begging and pup sex remained significant (LMM: Χ2=5.90, p=0.015). The results are not affected by sex differences in condition among the escorts (no significant difference between male and female escorts: two-sample t-test, t10,10=0.13, p=0.90), or pre-existing sex differences in provisioning rate (two-sample t-test, t10,10=0.02, p=0.98).
(d) Pup deprivation experiment, changes in pup begging
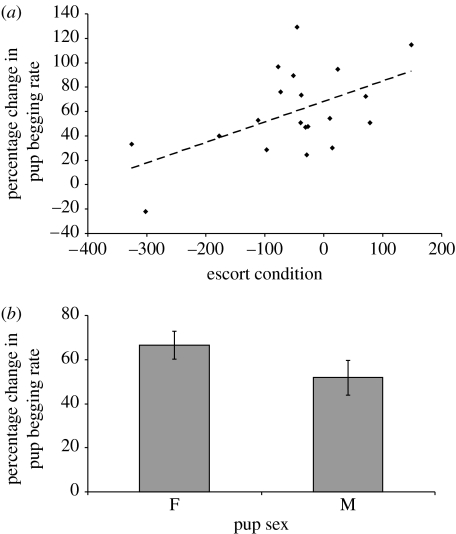
Pups associated with escorts in better condition increased their begging rate more than those in poorer condition (general linear model: F1,19=17.8, p<0.001; figure 4a; table 2), and female pups increased their begging rate more than did male pups (general linear regression: F1,19=4.64; p=0.048; figure 4b), after controlling for the significant negative effect of litter size (F1,19=8.18, p=0.01; See table 2 for all variables tested).
Figure 4.
Variables affecting the percentage change in pup begging after experimental deprivation. (a) Escort condition, calculated as the residual of a polynomial regression of weight (in g) over age (in days). Points are observed values, line is regression line (b) pup sex (means±s.e).
Table 2.
Linear model of variables affecting the percentage change in focal pup begging rate after a period of experimental deprivation. (Effects only given for significant terms in the minimal model. Analysis was conducted on 20 pups with 20 escorts from 11 litters in five packs.)
| explanatory terms | F1,19 | p |
|---|---|---|
| escort condition | 17.8 | <0.001 |
| litter size | 8.18 | 0.01 |
| pup sex | 4.64 | 0.048 |
| rainfall in last 30 days | 4.03 | 0.064 |
| pup condition | 1.71 | 0.21 |
| escort age (days) | 1.65 | 0.22 |
| pup age (days) | 0.07 | 0.79 |
| escort sex | 0.00 | >0.98 |
| minimal model | effect size | s.e. |
|---|---|---|
| constant | 174.0 | 35.0 |
| escort condition | 0.20 | 0.047 |
| litter size | −5.17 | 1.81 |
| pup sex (male) | −20 | 9.28 |
In general, these results must be treated cautiously because it was impossible to manipulate pup and escort characteristics independently.
4. Discussion
Although it is becoming clear that begging intensity is a broadly accurate indicator of offspring need (Kilner & Johnstone 1997; Royle et al. 2002; Wright & Leonard 2002), we lack an understanding of how factors other than the magnitude of a change in begging intensity influence changes in carer investment, and it is not clear how much variation in begging intensity is actually strategic adjustment by offspring in order to exploit variation in the way carers respond to begging. This study revealed that changes in begging rate by banded mongoose pups accurately indicated changes in short-term need; experimentally fed pups reduced and experimentally deprived pups increased their begging rates. However, it also suggested that responses to changes in begging may be influenced by not only the magnitude of the change, but also individual variation related to the costs and benefits of changing investment in pup care: escorts in good condition and those paired with female pups appeared to be more responsive to increases in begging. This variation in responsiveness in turn may have influenced the way pups changed their begging after deprivation: female pups and pups paired with escorts in good condition appeared to increase their begging more than male pups and pups paired with escorts in poor condition.
The apparent reduction in responsiveness to increases in pup begging by escorts in poor condition is probably because increases in investment are costlier to individuals who are themselves in greater need. The previous studies have shown lower absolute contributions to cooperative care by carers for whom the costs are greater (Clutton-Brock et al. 2002; Field et al. 2006). Moreover, experimentally increasing the cost of care is known to reduce carer contributions in biparental species (Wright & Cuthill 1989), while previous experiments in banded mongooses showed that supplementary feeding increases and deprivation reduces escort provisioning (Bell 2007b; Hodge 2007). Changes in current investment in offspring are known to have consequences for both future contributions to care (Russell et al. 2003) and future reproductive success (Heaney & Monaghan 1996; Monaghan et al. 1998). Wherever carers differ in the extent to which changes in current investment affect their future fitness, they are likely to differ in their responsiveness to changes in signals of offspring need (Mock et al. 2005).
It is less clear why escorts might be more responsive to increases in begging by female pups, though it may be because changes in juvenile condition have greater long-term fitness consequences for female banded mongooses. In this species, the intensity of intra-sexual competition over mating opportunities appears to be more intense among females than males, and measures of female reproductive success are strongly influenced by juvenile condition (Bell 2007b; Hodge 2007). In particular, small female pups face a significantly increased probability of being evicted during later life, and start breeding at a later age, while there are no such effects for males (Bell 2007b). These sex-specific effects of juvenile condition on later fitness may provide a greater incentive for escorts to increase investment in hungry female pups, as predicted by recent theory (Lessells 1998, 2002).
Such variation in responsiveness to changes in begging is likely to exert selection on offspring to use information gained during previous interactions to adjust their begging in a way that best exploits this variation. There is growing evidence that this occurs. For example, nestlings learn to modify their begging intensity in relation to variation in experimental hand-rearing regimes (Kedar et al. 2000; Rodriguez-Girones et al. 2002), while in several species nestlings discriminate between parents and beg more intensely to the more generous sex (Stamps et al. 1985, 1989; Kolliker et al. 1998; Roulin & Bersier 2007). It is important to note that the way in which offspring adjust to variation in carer responsiveness is liable to be affected by the cost of begging. Where begging is costly, offspring may be selected to maximize the energetic return for a given investment in begging, and are therefore likely to increase their begging more when begging to more responsive carers. However, where there is a negligible cost to begging, offspring may be selected to increase their begging more when begging to less responsive carers. In banded mongooses, the former seems to be the case, since previous work suggests that begging carries an energetic cost (Bell 2007a), and pups are subject to frequent predation by marabou storks, who appear to use begging calls to track victims (Bell 2007b).
In banded mongooses, the escort system imposes restrictions on the way variation in carer responsiveness affects offspring. In most species, other carers seem able to compensate to some extent for reduced investment by an individual (e.g. Wright & Cuthill 1989; reviewed in Hinde 2006), whereas banded mongoose pups receive negligible amounts of care from helpers other than their escort (Bell 2007a), and helpers who are not escorting a specific pup do not respond to increases in begging after experimental deprivation (Bell 2007b). In non-escorting species, therefore, offspring can direct their begging to the most responsive helpers, whereas banded mongoose pups do not have this option and are forced to adjust their begging effort to best exploit the responsiveness of their individual escorts. However, it is not yet clear how banded mongoose pups learn about the responsiveness of their escorts, nor is it known whether they monitor changes in escort responsiveness and adjust their begging accordingly. Further experiments to manipulate escort state by feeding (known to increase provisioning; Hodge 2007) would reveal whether deprived pups increase their begging to a greater extent when their escorts become more responsive.
Finally, it is important to consider how differences in carer responsiveness affect changes in offspring state, and the effect this has on changes in begging intensity. The rate at which offspring approach satiation should differ depending on the responsiveness of their carers, so the rate at which their begging intensity changes should also differ. Assuming that offspring are attempting to maximize the energetic return from begging, this means that, after a similar period of deprivation, offspring begging to more responsive carers should start begging at higher intensity than those begging to less responsive carers. However, their state will change faster, so they will quickly reach a point where they beg at lower intensity than those begging to less responsive carers. This means that when interpreting differences in begging intensity, it is imperative to consider the interaction between offspring state and carer responsiveness, and the time elapsed since the last period of deprivation ended.
More generally, strategic adjustment of signalling intensity in relation to variation in receiver responsiveness is likely to be very common, particularly wherever there is a cost to signalling. In fact, any social or ecological variation that alters the pay-off of signalling should alter the optimal signalling intensity for a given state. This means that individual differences in signal intensity may be as much a product of context as an indication of variation in individual phenotypic or genotypic state. Moreover, changes in state may sometimes not be indicated by changes in signal intensity, if the context has also changed. Such variation is evident in sexual signals, where female responses to male courtship displays can be influenced by variation in the pay-offs of responding (Moore & Moore 2001; Hunt et al. 2005; Cotton et al. 2006); and where males may adjust displays in relation to variation in female responsiveness (West & King 1988; Patricelli et al. 2002; Dukas 2005).
Acknowledgments
Francis Mwanguhya helped collect data and carry out experiments. Corsin Muller provided advice in the field. The Uganda National Council for Science and Technology and the Uganda Wildlife Authority provided permission to carry out research in QENP, Uganda, as part of a long-term study supported by Mike Cant, Tim Clutton-Brock and Marta Manser. I am grateful to Andy Radford, Rebecca Kilner, Rufus Johnstone, Tim Clutton-Brock, Joah Madden, and Nikki Raihani and two anonymous reviewers for valuable comments. This work was funded by a NERC studentship.
Supplementary Material
Additional details of field methodology and statistical procedure
References
- Acebo C, Thoman E.B. Role of infant crying in the early mother–infant dialogue. Physiol. Behav. 1995;57:541–547. doi: 10.1016/0031-9384(94)00345-6. doi:10.1016/0031-9384(94)00345-6 [DOI] [PubMed] [Google Scholar]
- Bell M.B.V. Cooperative begging in banded mongoose pups. Curr. Biol. 2007a;17:717–721. doi: 10.1016/j.cub.2007.03.015. doi:10.1016/j.cub.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Bell, M. B. V. 2007b Communication, cooperation and conflict in Banded Mongooses. PhD thesis. University of Cambridge.
- Cant, M. A. 1998 Communal breeding in banded mongooses and the theory of reproductive skew. PhD thesis, University of Cambridge.
- Clutton-Brock T.H. Behavioural ecology—breeding together: kin selection and mutualism in cooperative vertebrates. Science. 2002;296:69–72. doi: 10.1126/science.296.5565.69. doi:10.1126/science.296.5565.69 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Russell A.F, Sharpe L.L, Young A.J, Balmforth Z, McIlrath G.M. Evolution and development of sex differences in cooperative behaviour in meerkats. Science. 2002;297:253–256. doi: 10.1126/science.1071412. doi:10.1126/science.1071412 [DOI] [PubMed] [Google Scholar]
- Cotton S, Small J, Pomiankowski A. Sexual selection and condition-dependent mate preferences. Curr. Biol. 2006;16:R755–R765. doi: 10.1016/j.cub.2006.08.022. doi:10.1016/j.cub.2006.08.022 [DOI] [PubMed] [Google Scholar]
- Dawkins R, Krebs J.R. Animal signals: information of manipulation. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. Blackwell; Oxford, UK: 1978. pp. 282–309. [Google Scholar]
- Dukas R. Experience improves courtship in male fruit flies. Anim. Behav. 2005;69:1203–1209. doi:10.1016/j.anbehav.2004.08.012 [Google Scholar]
- Field J, Cronin A, Bridge C. Future fitness and helping in social queues. Nature. 2006;441:214–217. doi: 10.1038/nature04560. doi:10.1038/nature04560 [DOI] [PubMed] [Google Scholar]
- Gilchrist J.S. Pup escorting in the communally breeding banded mongoose. Behav. Ecol. 2004;15:952–960. doi:10.1093/beheco/arh071 [Google Scholar]
- Godfray H.C.J. Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 1995;146:1–24. doi:10.1086/285784 [Google Scholar]
- Grafen A. Biological signals as handicaps. J. Theor. Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Griffin A.S, West S.A. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science. 2003;302:634–636. doi: 10.1126/science.1089402. doi:10.1126/science.1089402 [DOI] [PubMed] [Google Scholar]
- Heaney V, Monaghan P. Optimal allocation of effort between reproductive phases: the trade-off between incubation costs and subsequent brood rearing capacity. Proc. R. Soc. B. 1996;263:1719–1724. doi:10.1098/rspb.1996.0251 [Google Scholar]
- Heinsohn R, Legge S. The cost of helping. Trends Ecol. Evol. 1999;14:53–57. doi: 10.1016/s0169-5347(98)01545-6. doi:10.1016/S0169-5347(98)01545-6 [DOI] [PubMed] [Google Scholar]
- Hinde C.A. Negotiation over offspring care?—A positive response to partner provisioning rate in great tits. Behav. Ecol. 2006;17:6–12. doi:10.1093/beheco/ari092 [Google Scholar]
- Hodge S.J. Helpers benefit offspring in both the short and long-term in the cooperatively breeding banded mongoose. Proc. R. Soc. B. 2005;272:2479–2484. doi: 10.1098/rspb.2005.3255. doi:10.1098/rspb.2005.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S.J. Counting the costs: the evolution of male biased care in the cooperatively breeding banded mongoose. Anim. Behav. 2007;74:911–919. doi:10.1016/j.anbehav.2006.09.024 [Google Scholar]
- Hunt J, Brooks R, Jennions M.D. Female choice as a condition-dependent life history trait. Am. Nat. 2005;166:79–92. doi: 10.1086/430672. doi:10.1086/430672 [DOI] [PubMed] [Google Scholar]
- Kedar H, Rodriguez-Girones M.A, Yedvab S, Winkler D.W, Lotem A. Experimental evidence for offspring learning in parent–offspring communication. Proc. R. Soc. B. 2000;267:1723–1727. doi: 10.1098/rspb.2000.1201. doi:10.1098/rspb.2000.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R.M. When do canary parents respond to nestling signals of need. Proc. R. Soc. B. 1995;260:343–348. doi:10.1098/rspb.1995.0102 [Google Scholar]
- Kilner R.M, Johnstone R.A. Begging the question: are offspring solicitation behaviours signals of need? Trends. Ecol. Evol. 1997;12:11–15. doi: 10.1016/s0169-5347(96)10061-6. doi:10.1016/S0169-5347(96)10061-6 [DOI] [PubMed] [Google Scholar]
- Kolliker M, Richner H. Chick positioning in great tit, Parus major, nests. Anim. Behav. 2004;68:941–948. doi:10.1016/j.anbehav.2003.11.019 [Google Scholar]
- Kolliker M, Richner H, Werner I, Heeb P. Begging signals and biparental care: nestling choice between parental feeding locations. Anim. Behav. 1998;55:215–222. doi: 10.1006/anbe.1997.0571. doi:10.1006/anbe.1997.0571 [DOI] [PubMed] [Google Scholar]
- Krebs E.A, Magrath R.D. Food allocation in crimson rosella broods: parents differ in their responses to chick hunger. Anim. Behav. 2000;59:739–751. doi: 10.1006/anbe.1999.1375. doi:10.1006/anbe.1999.1375 [DOI] [PubMed] [Google Scholar]
- Lessells C.M. A theoretical framework for sex biased parental care. Anim. Behav. 1998;56:395–407. doi: 10.1006/anbe.1998.0764. doi:10.1006/anbe.1998.0764 [DOI] [PubMed] [Google Scholar]
- Lessells C.M. Parentally biased favouritism: why should parents specialise in caring for different offspring? Phil. Trans. R. Soc. B. 2002;357:381–403. doi: 10.1098/rstb.2001.0928. doi:10.1098/rstb.2001.0928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem A, Winkler D.W. Can reinforcement learning explain variation in early infant crying? Behav. Brain Sci. 2004;27:468. [Google Scholar]
- Macnair M.R, Parker G.A. Models of parent offspring conflict. III. Intrabrood conflict. Anim. Behav. 1979;27:1202–1209. doi:10.1016/0003-3472(79)90067-8 [Google Scholar]
- Mock D.W, Schwagmeyer P.L, Parker G.A. Male house sparrows deliver more food to experimentally subsidised offspring. Anim. Behav. 2005;70:225–236. doi:10.1016/j.anbehav.2004.10.020 [Google Scholar]
- Monaghan P, Nager R.G, Houston D.C. The price of eggs: increased investment in egg production reduces the offspring rearing capacity of parents. Proc. R. Soc. B. 1998;265:1731–1735. doi:10.1098/rspb.1998.0495 [Google Scholar]
- Moore P.J, Moore A.J. Reproductive aging and mating: the ticking of the biological clock in female cockroaches. Proc. Natl Acad. Sci. USA. 2001;98:9171–9176. doi: 10.1073/pnas.161154598. doi:10.1073/pnas.161154598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A, Macnair M.R. Models of parent–offspring conflict 1: monogamy. Anim. Behav. 1978;26:97–110. doi: 10.1016/0003-3472(78)90009-x. doi:10.1016/0003-3472(78)90009-X [DOI] [PubMed] [Google Scholar]
- Patricelli G.L, Uy J.A.C, Walsh G, Borgia G. Male displays adjusted to female's response. Macho courstship by the satin bowerbird is tempered to avoid frightening the female. Nature. 2002;415:279–280. doi: 10.1038/415279a. doi:10.1038/415279a [DOI] [PubMed] [Google Scholar]
- Rodriguez-Girones M.A, Zuniga J.M, Redondo T. Feeding experience and relative size modify the begging strategies of nestlings. Behav. Ecol. 2002;13:782–785. doi:10.1093/beheco/13.6.782 [Google Scholar]
- Roulin A, Bersier L. Nestling barn owls beg more intensely in the presence of their mother than in the presence of their father. Anim. Behav. 2007;74:1099–1106. doi:10.1016/j.anbehav.2007.01.027 [Google Scholar]
- Royle N.J, Hartley I.R, Parker G.A. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. 2002;17:434–440. doi:10.1016/S0169-5347(02)02565-X [Google Scholar]
- Russell A.F, Sharpe L.L, Brotherton P.N.M, Clutton-Brock T.H. Cost minimisation by helpers in cooperative vertebrates. Proc. Natl Acad. Sci. USA. 2003;100:3333–3338. doi: 10.1073/pnas.0636503100. doi:10.1073/pnas.0636503100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps J.A, Clark A, Arrowood P, Kus B. Parent–offspring conflict in budgerigars. Behaviour. 1985;94:1–40. [Google Scholar]
- Stamps J.A, Clark A, Arrowood P, Kus B. Begging behaviour in budgerigars. Ethology. 1989;81:177–192. [Google Scholar]
- Trivers R.L. Parent offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- West M.J, King A.P. Female visual displays affect the development of male song in the cowbird. Nature. 1988;334:244–246. doi: 10.1038/334244a0. doi:10.1038/334244a0 [DOI] [PubMed] [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection: a critique of some current evolutionary thought. [Google Scholar]
- Wright J, Cuthill I. Manipulation of sex differences in parental care. Behav. Ecol. Sociobiol. 1989;25:171–181. doi:10.1007/BF00302916 [Google Scholar]
- Wright J, Leonard M.L, editors. The evolution of begging: competition, cooperation and communication. Kluwer; Dordrecht, The Netherlands: 2002. [Google Scholar]
- Zahavi A. Mate selection—a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. doi:10.1016/0022-5193(75)90111-3 [DOI] [PubMed] [Google Scholar]
- Zahavi, A. 1987 The theory of signal detection and some of its implications. In Int. Symp. of Biological Evolution (ed. V. P. Delfino). Bari, Italy: Adriatica Editirice.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional details of field methodology and statistical procedure