Abstract
The distribution of marine bivalve species among genera and higher taxa takes the form of the classic hollow curve, wherein few lineages are species rich and many are species poor. The distribution of species among genera (S/G ratio) varies with latitude, with temperate S/G's falling within the null expectation, and tropical and polar S/G's exceeding it. Here, we test several hypotheses for this polar overdominance in the species richness of small numbers of genera. We find a significant positive correlation between the latitudinal range of a genus and its species richness, both globally and within regions. Genus age and species richness are also positively related, but this relationship breaks down when the analysis is limited to genera endemic to climate zones or with narrow latitudinal ranges. The data suggest a link between speciation and range-expansion, with genera expanding out of the tropical latitudinal bins tending to speciate more prolifically, both globally and regionally. These genera contain more species within climate zones than taxa endemic to that zone. Range expansion thus appears to be fundamentally coupled with speciation, producing the skewed distribution of species among genera, both globally and regionally, whereas clade longevity is achieved through extinction—resistance conferred by broad geographical ranges.
Keywords: geographic range, species–genus ratio, bivalves, latitudinal diversity gradient
Abbreviations: S/G, species–genus ratio, LDG, latitudinal diversity gradient, Myr, million years
1. Introduction
The taxonomic structure of clades and biotas, often measured as the ratio of species to genera, genera to families and so on, does not vary randomly on a global scale. As Simberloff (1970) showed in one of the earliest uses of null models in ecology, species–genus (S/G) ratios are sensitive to diversity, such that the null expectation is a monotonic increase in S/G with the number of species (Järvinen 1982; Gotelli & Colwell 2001). However, Roy et al. (1996) found a significant latitudinal increase in the S/G ratio for marine molluscs along the northeastern Pacific coast, even as the total number of species drops sharply with increasing latitude, and Fenner et al. (1997) found a comparable inverse relationship in regional and island angiosperms. Such trends, clearly divergent from the null expectation, require explanation.
Early workers, usually focusing on low-diversity island faunas, generally interpreted S/G ratios in terms of competitive exclusion—fewer congeneric taxa would coexist in the face of intense competition. However, most results did not differ significantly from null expectations (Simberloff 1970; Harvey et al. 1983; Chase & Leibold 2003), and some analyses found more congeners than expected by chance (Tofts & Silvertown 2000; Daehler 2001; Enquist et al. 2002). A very different hypothesis would involve spatial variation in evolutionary dynamics, with S/G ratios being positively related to regional speciation or diversification rates (e.g. Floeter et al. 2004). The counter-intuitive latitudinal increase in S/G ratios could be taken to indicate that the few genera that manage to occupy the highest latitude regions actually diversify at higher rates there than they do in low latitudes, here termed the high-latitude diversification hypothesis (see Weir & Schluter (2007) on high avian speciation rates at high latitudes). More generally, a fuller understanding of spatial variation in S/G is needed in the light of the ongoing use of genus-level patterns as proxies for species diversity in living and fossil biotas (e.g. Sepkoski 1992; Anderson 1995; Balmford et al. 2000; Gaston 2000; Brummitt 2005; Gladstone & Alexander 2005; Villasenor et al. 2005; Heino & Soininen 2007).
To evaluate the patterns described in previous work, and the high-latitude diversification hypothesis in particular, we analyse S/G ratios for marine bivalves of the global continental shelves, and quantify within-clade trends among climate zones on a global scale. We find that genera whose ranges extend from equator to poles are not richer in species at high rather than low latitudes, but their S/G ratios do exceed the null expectation. We argue that the latitudinal trend in taxonomic structure results from a link between speciation within genera and the expansion of generic ranges, a process ultimately derived from the evolutionary and spatial dynamics that also underlie the latitudinal diversity gradient (LDG).
2. Methods and data
A global, taxonomically standardized database of spatial occurrences of extant shallow-water (less than 200 m) marine bivalves was used for this analysis (Jablonski & Flessa 1986; Flessa & Jablonski 1995, 1996; Jablonski et al. 2006; Valentine et al. 2006). This group is one of the most diverse in the oceans, occurs at all latitudes and has a rich fossil record. Data were compiled primarily from an exhaustive literature search but were also drawn from museum collections. The database currently includes 769 genera and subgenera (herein simply termed genera, out of a total of 1293 living genera, deep-sea is also included), 5428 species, and 20 816 occurrences from 228 localities around the world (figure 1 in the electronic supplementary material). These data were taxonomically standardized using both recent taxonomic revisions in the literature and independent analysis of individual specimens. Geological ages of genera were taken from an extensively revised and updated version of Sepkoski's database (see Sepkoski 2002; Jablonski et al. 2003, 2006). Of the 769 genera, 82% are known from the fossil record. Despite considerable expansion and taxonomic revision, this percentage is similar to that reported in the previous analyses of these data (76–88%; Flessa & Jablonski 1996; Valentine et al. 2006), as well as in a regional study of bivalves from the Pleistocene of the Californian Province (84%; Valentine 1989), suggesting that we are exploring a robust representation of the bivalve fossil record.
S/G ratios were determined for 5° latitudinal bins and climate zones. For both binning schemes, S/G is calculated simply as the average of the number of species per genus in a given bin. Climate zone diversity was determined by designating each locality within the database as either tropical, temperate or polar, generally following the hydrographic compartments of Longhurst (1998). The sole exception is that our Okhotsk Sea localities, which Longhurst (1998) included in his polar Bering Sea region, are classified as temperate following Spalding et al. (2007), who considered this region to be more similar to the Sea of Japan and Kuril Islands. This zonal approach is important for global analyses, as the boundaries between climate zones, especially the tropical and temperate boundary, vary among coasts with respect to latitude.
Using climate zones, several subsets of genera were differentiated. Genera that are located in only one climate zone are termed ‘climatic endemics’. Because all polar genera occur in at least one temperate locality, only tropical and temperate climatic endemics are recognized. Genera that range from tropical to polar climate zones are termed ‘climatic cosmopolitans’. The terms ‘endemic’ and ‘cosmopolitan’ generally refer to taxa at smaller spatial scales (continents, provinces, habitats, etc.), but we use them here to designate genera in single versus multiple zones, which is much preferable to the definition of new terms. Genera shared only between tropical and temperate climate zones are referred to as ‘warm-water’ genera and those shared only between temperate and polar climate zones are termed ‘cold-water’ genera.
Because S/G ratios correlate with species richness, spatial variation was assessed against a null model derived from a randomization of the taxonomic structure of the dataset. The null model was generated as follows. First, the geographical range of a species in this study was calculated as the degrees of latitude over which that species ranged, and is therefore essentially a one-dimensional line segment. These line segments remain unchanged in the randomization. Second, the assignments of species to genera were shuffled, while maintaining the global taxonomic structure of the database (i.e. the number of species within each genus). This was done by drawing species randomly (without replacement) from the original dataset and assigning them to genera until each genus had its original number of species. Every genus, therefore, maintains its species richness, but its species composition, and therefore its composition of latitudinal ranges is random. Using the new taxonomic assignments, S/G ratios were determined for each 5° latitudinal bin. This process was repeated 1000 times, the results were averaged and standard error was calculated. The null model therefore maintains both the original spatial distribution of species and the exact observed numerical distribution of species per genus. This null model represents the expected number of genera within a latitudinal bin given the number of species within that bin and the spatial autocorrelation of the dataset. The purpose of this model is to test the significance of spatial variations in S/G given that S/G is expected to vary as a function of species richness. Deviations from this null will indicate that the number of genera within a bin is determined by factors other than simply species richness. As will be discussed below, such factors include genus age, genus geographical range, speciation potential and dispersal ability of species.
Because geological age, geographical range and species richness are all highly skewed distribution, correlations among them were evaluated using non-parametric Spearman's rank tests. The shapes of distributions of species among genera within climate zones were compared using Kolmogorov–Smirnov tests.
3. Global patterns
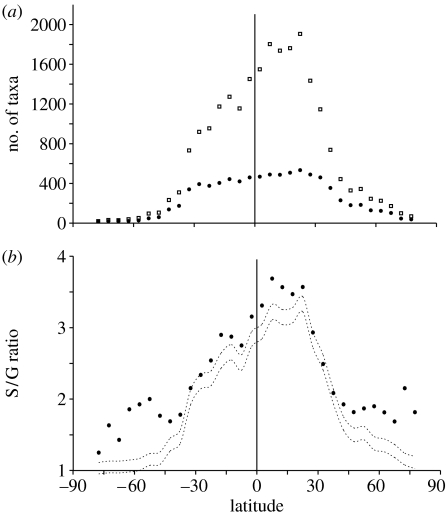
The distribution of species within genera for marine bivalves forms a hollow curve, with many species-poor genera and few species-rich genera, as is true for most taxa (Willis & Yule 1922; Anderson 1974; Williams & Gaston 1994; Roy et al. 1996; Cardillo et al. 2003; Hilu 2007). This distribution holds for all data partitions, including genera within latitudinal and climatic bands, and genera with large and small geographical ranges. The number of species and genera shows a standard LDG, with peaks in the tropics and low diversity in the poles (figure 1a). S/G ratios (figure 1b) also show a strong latitudinal gradient at the global scale, with high tropical ratios decreasing significantly into the temperate zone. Above roughly 40° in both hemispheres, S/G ratios level off at approximately 1.7 species per genus, despite further declines in diversity (figure 1b).
Figure 1.
(a) LDG for species (squares) and genera (circles). Numbers of taxa were determined at the global scale for 5° latitudinal bins. (b) Species–genus (S/G) ratios within 5° latitudinal bins for all living shelf-depth marine bivalve genera contained in the database. Dashed lines represent 95% CIs on the expected S/G ratios derived from a randomization of the taxonomic structure of the dataset (see §2). Vertical line marks the equator. Negative latitudes indicate the southern hemisphere.
Given that S/G ratios are sensitive to diversity, the decreasing species richness towards high latitudes will bring a decline in the S/G ratio as described previously. However, S/G ratios in both low- and high-latitude bins significantly exceed the null expectation for their species numbers (figure 1b). At the global scale, the null expectation is met only in mid-latitudes, from approximately 25 to 40° in both hemispheres, and nowhere are S/G ratios lower than expected. The complete absence of lower-than-expected S/G ratios in any latitudinal bin may seem counterintuitive given that higher-than-expected ratios occur often. However, this pattern is due to the hollow curve distribution of species among genera. Because the taxonomic structure of the dataset is highly skewed towards species-poor genera, and the null S/G ratio is predicted using this skewed distribution, lower-than-expected S/G ratios in one latitudinal bin are not expected to balance higher-than-expected S/G ratios in others.
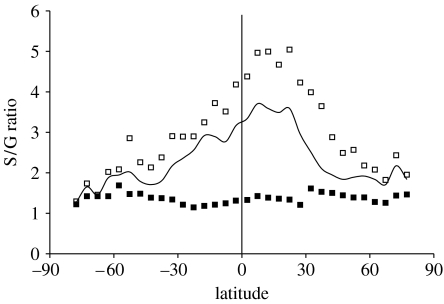
Dissecting the faunal trends into their constituent, climatically defined subsets of genera show that genera extending from tropical to polar climate zones (climatic cosmopolitans) do not increase in species richness with latitude, contrary to the high-latitude diversification hypothesis (table 1). Of the 128 genera recorded in polar climates in this database (N and S poles combined), 95 (74%) range into the tropics. S/G ratios for these genera decline monotonically from low to high latitudes (figure 2). When binned by climate zone (table 1), cosmopolitans have significantly more species in tropical zones than they do in polar zones (KS test, p=4.133 e−8; figure 2 in the electronic supplementary material). Genera endemic to a single climate zone (tropical versus extratropical) have consistently fewer species in each latitudinal bin than do cosmopolitans (figure 2), and their S/G ratios show little variation among climatic zones despite declining numbers of species (table 1). Distributions of species among genera endemic to tropical and extratropical climate zones are statistically indistinguishable (KS test, p=0.84; figure 2 in the electronic supplementary material). Warm- and cold-water genera have S/G ratios intermediate between cosmopolitan and endemic genera in each climatic zone, and these ratios decline towards higher latitudes as well (table 1).
Table 1.
Mean number of species per genus within biodistributional categories. (Values for cosmopolitan genera in the temperate zones are the average of the Northern and Southern Hemispheres.)
| N | tropical | temperate | polar | |
|---|---|---|---|---|
| all genera | 769 | 5.1 | 4.0 | 2.0 |
| cosmopolitan genera | 95 | 7.5 | 5.5 | 2.2 |
| warm-water genera | 444 | 5.3 | 3.6 | — |
| cold-water genera | 33 | — | 2.7 | 1.4 |
| tropical endemics | 87 | 1.54 | — | — |
| temperate endemics | 110 | — | 1.72 | — |
Figure 2.
S/G ratios for genera within 5° latitudinal bins. Open squares are the S/G ratio for climatic cosmopolitans (genera extending from tropical to polar climate zones). Solid line is the S/G ratio for all marine bivalves in the database. Filled squares are the S/G ratios for climate-zone endemics. Vertical line marks the equator.
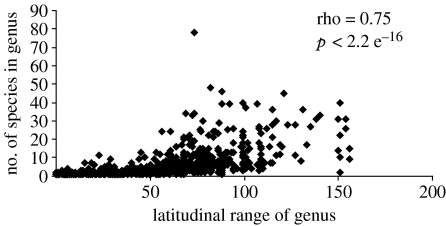
These results are consistent with a highly significant relationship between latitudinal range and S/G for all marine bivalve genera (Spearman's rank order correlation, p=2.2 e−16, figure 3). However, climatic cosmopolitans are not only more species rich overall but also tend to have more species in each latitudinal and climatic bin than genera endemic to that climate zone (figure 2, table 1).
Figure 3.
The latitudinal range of a marine bivalve genus (in degrees) is significantly related to the number of species within that genus (N=769 genera). Significance of the relationship was tested by Spearman's rank correlation.
4. Geological ages
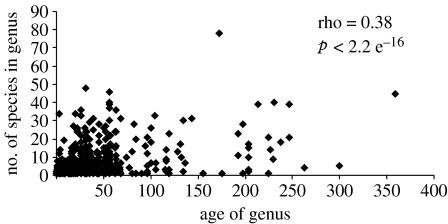
A significant positive relationship exists between the age of a genus and the number of species within it (p=2.2 e−16, figure 4). Subdividing genera into climatic subsets also finds a significant relation between age and species richness for climatic cosmopolitans and both cold- and warm-water taxa. However, this relationship does not hold for genera endemic to a climate zone, despite the presence of several endemic genera dating back to the Mesozoic (e.g. Pectinella, Plicatula and Pholadomya), again indicating that high S/G ratios are not created by the regional accumulation of species within endemic genera.
Figure 4.
The number of species within a marine bivalve genus is significantly related to its age. The significance of the relationship was tested by Spearman's rank correlation.
5. S/G ratios in geographical range classes
Hollow-curve distributions can be generated by stochastic processes, leading some to treat the taxonomic distribution of species in purely random terms (Raup et al. 1973; Anderson 1974; Anderson & Anderson 1975). However, a few taxa often overdominate biotic assemblages and clades, containing more species than expected under a variety of null models (Simberloff 1970; Dial & Marzluff 1989; Gotelli & Colwell 2001; Cardillo et al. 2003). Such overdominance, seen also in our results, is perhaps unsurprising in the tropics, which have high species and genus origination rates. However, the polar zone also tends to contain more species per genus than expected at the global scale, despite the low number of taxa at high latitudes (figure 1b). In fact, nowhere in the global S/G curve are ratios lower than the random expectation, and such non-random diversification suggests a natural cause. We briefly discuss three hypotheses.
Higher-than-expected S/G ratios are the opposite of those predicted under competitive exclusion models, wherein congeneric species are less likely to coexist owing to their similar ecological requirements (Darwin 1859; Elton 1946; see review by Daehler 2001). The observed S/G ratios imply phylogenetic clumping at high latitudes rather than overdispersion. Given the coarse spatial scale of these analyses, completely rejecting models based on competitive interactions may be inappropriate, as these interactions may only be detected at finer spatial resolution. However, given the large degree of homogeneity of species composition within polar localities and that the Eastern Pacific coast independently confirms the global pattern (see §7), these results suggest that competitive exclusion, even if it regularly occurs on a fine scale, is unlikely to be the primary mechanism behind the large-scale taxonomic pattern.
Higher-than-expected S/G ratios near the poles might result from relatively high net rates of speciation (the high-latitude diversification hypothesis), wherein the few lineages that manage to reach the poles are free to diversify there, producing more species per lineage than expected. However, this hypothesis is not supported when we dissect faunal trends taxonomically. Climatic cosmopolitans tend to decline in species richness with increasing latitude, as do their S/G ratios. Genera endemic to the temperate zone have S/G ratios lower than cosmopolitan genera and similar to tropical endemic genera (table 1, although the ratios may be influenced by the tendency of tropical genera to expand into higher latitudes after some amount of speciation). Finally, the suite of genera in polar zones tends to be older than elsewhere in the world (Jablonski et al. 2006), a finding inconsistent with rapid high-latitude diversification. Therefore, although S/G ratios exceed the null expectation at high latitudes, there is little evidence that this pattern is driven by high diversification rates within the polar zone. Every genus at the poles has more species elsewhere, but the genera that reach the poles are not a random subset of the global biota—they are the genera that tend to accumulate species in every region.
Higher-than-expected S/G ratios might arise from spatial patterns of diversification and range expansion through geological time. The strong positive correlation for cosmopolitans between the latitudinal range of a genus and its species richness suggests that genus range expansion occurs primarily by speciation, rather than through range expansion of existing species. If species were simply expanding their latitudinal ranges (and ‘carrying’ genera with them), then S/G ratios would track the number of species among regions, and should therefore conform to the null expectation for low-diversity regions. Additionally, a strong correlation between the latitudinal ranges of species and genera would be produced, but none apparently exists, at least for marine molluscs (Jablonski 2005). Although species from a few lineages might randomly invade certain zones and drive S/G ratios above the expectation, this is unlikely to be common at a global scale, and cannot explain the tendency for genera with large ranges to be more species rich globally throughout their range.
Genera of marine bivalves originate preferentially in the tropics and subsequently expand their geographical range into higher latitudes while maintaining their tropical positions (Jablonski et al. 2006). Genera that extend from the tropics to the poles not only have more species overall but also have more species per latitudinal bin (figure 2) and climate zone (table 1) than do bivalves with narrower ranges. Warm- and cold-water genera, which are limited to two climate zones, conform to this trend, having consistently intermediate numbers of species in each climatic zone occupied by them (table 1). Thus, the tendency for genera with large latitudinal ranges to be more species rich within each latitudinal bin suggests a link between traits that promote speciation within climate zones and those that promote range expansion.
Both dispersal and adaptation hypotheses might be framed in ways consistent with our data. For example, taxa having intermediate dispersal abilities might be capable of breaching biogeographic barriers but still might be subject to allopatric speciation within regions or climatic zones. The fact that the most powerful dispersers (species ranging from equator to poles) tend not to belong to the most diverse genera (figure 3 in the electronic supplementary material) supports this hypothesis, but more direct observations are needed. Alternatively, the ability of a species to adapt to new environments might enhance the likelihood of both within-region speciation and successful invasion of new regions, a hypothesis that might be tested by evaluating the frequencies of ecological speciation (Schluter 2000) in zonal endemics versus climatic cosmopolitans.
These results rather suggest that genera expanding their latitudinal ranges preferentially contain species with high speciation potential. Previous studies have suggested that both organismic (e.g. small body size, reduced mobility) and species-level traits (e.g. narrow geographical range) can promote speciation (Mayr 1963; Maurer & Nott 1998; Hubbell 2001; Jablonski & Roy 2003; Coyne & Orr 2004) and are both heritable (Vrba & Gould 1986; Jablonski 1987; Hunt et al. 2005; Jablonski & Hunt 2006; Waldron 2007). Therefore, a species-level trait that promotes speciation (such as geographical range) will probably be conserved through a lineage. Owing to the tendency for range-expanding lineages to branch in both new and previously occupied areas, these results suggest that heritability plays a vital role in the preferential diversification of lineages, at least at a global scale.
In polar waters, the higher-than-expected S/G ratios are due to the predominance of genera with large latitudinal ranges (74% of polar genera extend to the tropics). The latitudinal ranges of genera in polar zones are significantly greater than the ranges of genera located in tropical zones (KS test, p<2.2 e−16), despite the shared ranges of genera existing simultaneously in both the zones, which would act to make the distributions more similar. These findings are consistent with those of Goldberg et al. (2005) and Roy & Goldberg (2007), who combined an earlier version of this database with integrative models incorporating origination, extinction and dispersal to show that polar diversity reflects the accumulation of taxa that evolved elsewhere in the world. Additionally, their models support high tropical origination rates, low polar extinction rates, and the preferential spread of genera from low to high latitudes (Goldberg et al. 2005; Roy & Goldberg 2007). Although these studies have focused on the origination and spread of genera rather than species within genera, the parallels between their results and those reported here are nevertheless striking.
In a sense, the global trends in bivalve S/G ratios are a consequence of the different slopes of the species- and genus-level diversity trends. As expected, the genus-level LDG correlates highly with the species-level LDG (p=2.2 e−16, linear correlation) but is considerably less steep (figure 1a). The difference between these LDGs arises from the link between speciation and range expansion at the global scale. That is, range expansion is overwhelmingly unidirectional, from the tropics towards polar zones (Goldberg et al. 2005; Jablonski et al. 2006), so a genus that expands its range into high latitudes will tend to produce many species in the tropics and few in the polar zones. This leads to a latitudinal decline in S/G ratios, and produces much of the well-known LDG. This process indicates a fundamental link between the formation of LDGs at the genus and species levels, with range expansion at the genus-level integral to the formation of LDG's at both taxonomic scales.
6. Species richness and age
The weak but highly significant correlation in our data between the age of a genus and its species richness (figure 4) has been recorded in many other taxa (Willis & Yule 1922; McPeek & Brown 2007). Although time might be a primary control on the accumulation of species within genera (McPeek & Brown 2007), old age might also be conferred upon a widespread genus by its resistance to extinction. In simple null models, species poor and geographically restricted genera tend to be extinction prone (Raup 1978, 1985; Jablonski 1986; Patzkowsky 1995). Among animals, species richness tends to vary with other attributes (including geographical range) so that species-poor genera also tend to be extinction-prone (Purvis et al. 2000; Jablonski 2007), whereas among plants species-poor genera often contain widespread, abundant, presumably extinction-resistant species (Schwartz & Simberloff 2001; Lozano & Schwartz 2005).
If age were the primary factor in determining the species richness of a genus, then the positive relationship between the two would remain when geographical range is held constant. However, neither tropical nor extratropical endemics show a significant relationship between age and species richness of genera (data not shown); indeed tropical endemics actually show a weak inverse relation, with species-rich genera generally being younger than species-poor genera, although the relationship is not statistically significant. The lack of correlation between species richness and genus age for endemics suggests that factors other than time account for the accumulation of species. Rather, the accumulation of species and subsequent range expansion make a genus less extinction prone.
Because age and range of genera are not independent variables, we further tested their relative contributions to species richness using linear models. Because age and range have skewed distributions, the data were log-transformed prior to analysis. Independently, the results of the linear regressions were consistent with the use of Spearman's rank correlations, with both variables significantly correlated with species richness but with range explaining more of the variance in the data (table 1 in the electronic supplementary material). Using a multiple regression of age and range simultaneously against species richness explains only 5% more of the variability in the data than does a regression of range alone (table 1 in the electronic supplementary material). Thus, geographical range again emerges as the variable fundamentally linked to the accumulation of species within bivalve genera.
7. Global versus regional patterns
Our global results differ from the northeastern Pacific patterns in Roy et al. (1996), who reported higher S/G ratios in polar regions than in the tropics, with minimal values at mid-latitudes, and the northeastern Pacific segment of our data confirms the regional pattern, with S/G ratios increasing consistently from 30° N towards the poles. The increase in S/G ratios from 30° in the northeastern Pacific derives from exceptionally low S/G ratios (1.2 S/G) in the temperate zone; north of 60° the S/G ratio rebounds to 1.7 matching the global S/G ratio at this latitude. This rules out exceptional diversification in the northernmost Pacific, but does suggest variations in diversification patterns along different continental coastlines. Nevertheless, the northeastern Pacific dataset also shows the positive, significant relationship between latitudinal range and species richness of genera (ρ=0.71, p<2.2 e−16), and a randomization test confirms that northeastern Pacific S/G ratios at high latitudes exceed the null expectation, as in Roy et al. (1996). The northeastern Pacific thus conforms to the first-order processes governing global within-genus diversification, with the mid-latitude low in S/G ratios—contrasting with the continuous decline seen at the global scale—probably deriving from regional effects.
8. Conclusions
The distribution of species among genera varies non-randomly with latitude, with both tropical and polar genera containing more species than expected given a randomization of the taxonomic structure of the fauna. The unexpected polar deviation is apparently not related to higher diversification rates in high latitudes because (i) genera that extend from the tropical to polar zones have monotonically decreasing S/G ratios when binned latitudinally and (ii) genera limited to extratropical climate zones have similar or lower S/G ratios than do genera endemic to the tropical zone. Rather, high polar S/G ratios for marine bivalves appear to be related to a dynamic of tropical origination, extratropical range expansion and within-region speciation. Genera that extend from tropical to polar climate zones consistently have higher S/G ratios in all climate zones, and constitute the bulk of genera that exist in polar seas today. Species within these cosmopolitan genera may have life-history traits or other attributes that allow for rapid adaptation to new environments, promoting both speciation and range expansion of a lineage; but data do not yet exist to test this hypothesis directly. Nevertheless, the global, latitudinal and climatic distributions of bivalve species among genera seem to be derived from the same underlying evolutionary principles of diversification and range expansion which produce the LDG.
Acknowledgments
We thank the following for advice, assistance and/or access to collections in their care: L. C. Anderson, K. Amano, A. G. Beu, R. Bieler, J. G. Carter, R. v. Cosel, J. S. Crampton, E. V. Coan, T. A. Darragh, H. H. Dijkstra, E. M. Harper, C. S. Hickman, S. Kiel, K. Lam, K. Lamprell, K. A. Lutaenko, N. Malchus, P. A. Maxwell, P. M. Mikkelsen, P. Middelfart, N. J. Morris, G. Paulay, F. Scarabino, J. A. Schneider, P. V. Scott, J. T. Smith, J. D. Taylor, J. D. Todd, T. R. Waller, A. Warén and F. P. Wesselingh. We also thank P.G. Harnik for computational advice and assistance, and S. M. Kidwell, K. Roy, R. K. Colwell and an anonymous reader for reviews. This research was funded by NASA.
Supplementary Material
Additional figures and data in support of the manuscript
References
- Anderson S. Patterns of faunal evolution. Q. Rev. Biol. 1974;49:311–322. doi: 10.1086/408171. doi:10.1086/408171 [DOI] [PubMed] [Google Scholar]
- Anderson A.L. Measuring more of biodiversity: genus richness as a surrogate for species richness in Australian ant faunas. Biol. Conserv. 1995;73:39–43. doi:10.1016/0006-3207(95)90059-4 [Google Scholar]
- Anderson S, Anderson C.S. Three Monte Carlo models of faunal evolution. Am. Mus. Novit. 1975;2563:1–6. [Google Scholar]
- Balmford A, Lyon A.J.E, Lang R.M. Testing the higher-taxon approach to conservation planning in a megadiverse group: the macrofungi. Biol. Conserv. 2000;93:209–217. doi:10.1016/S0006-3207(99)00140-8 [Google Scholar]
- Brummitt N.A. Patterns in the global distribution of flowering plant genera. Biol. Skr. 2005;55:539–564. [Google Scholar]
- Cardillo M, Huxtable J.S, Bromham L. Geographic range size, life history and rates of diversification in Australian mammals. J. Evol. Biol. 2003;16:282–288. doi: 10.1046/j.1420-9101.2003.00513.x. doi:10.1046/j.1420-9101.2003.00513.x [DOI] [PubMed] [Google Scholar]
- Chase J.M, Leibold M.A. University of Chicago Press; Chicago, IL: 2003. Ecological niches. [Google Scholar]
- Coyne J.A, Orr H.A. Sinauer Press; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Daehler C.C. Darwin's naturalization hypothesis revisited. Am. Nat. 2001;158:324–330. doi: 10.1086/321316. doi:10.1086/321316 [DOI] [PubMed] [Google Scholar]
- Darwin C. John Murray; London, UK: 1859. The origin of species. [Google Scholar]
- Dial K.P, Marzluff J.M. Nonrandom diversification within taxonomic assemblages. Syst. Zool. 1989;38:26–37. doi:10.2307/2992433 [Google Scholar]
- Elton C. Competition and the structure of ecological communities. J. Anim. Ecol. 1946;15:54–68. doi:10.2307/1625 [Google Scholar]
- Enquist B.J, Haskell J.P, Tiffney B.H. General pattern of taxonomic and biomass partitioning in extant and fossil plant communities. Nature. 2002;419:610–613. doi: 10.1038/nature01069. doi:10.1038/nature01069 [DOI] [PubMed] [Google Scholar]
- Fenner M.W, Lee W.G, Wilson J.B. A comparative study of the distribution of genus size in twenty angiosperm families. Biol. J. Linn. Soc. 1997;62:225–237. [Google Scholar]
- Flessa K.W, Jablonski D. Biogeography of recent marine bivalve mollusks and its implications for paleobiogeography and the geography of extinction: a progress report. Hist. Biol. 1995;10:24–47. [Google Scholar]
- Flessa K.W, Jablonski D. The geography of evolutionary turnover: a global analysis of extant bivalves. In: Jablonski D, Erwin D.H, Lipps J.H, editors. Evolutionary paleobiology. University of Chicago Press; Chicago, IL: 1996. pp. 376–397. [Google Scholar]
- Floeter S.R, Ferreira C.E.L, Dominici-Arosemena A, Zalmon I.R. Latitudinal gradients in Atlantic reef fish communities: trophic structure and spatial use patterns. J. Fish Biol. 2004;64:1680–1699. doi:10.1111/j.0022-1112.2004.00428.x [Google Scholar]
- Gaston K.J. Biodiversity: higher taxon richness. Prog. Phys. Geogr. 2000;24:117–127. [Google Scholar]
- Gladstone W, Alexander T. A test of the higher-taxon approach in the identification of candidate sites for marine reserves. Biodiv. Conserv. 2005;14:3151–3168. doi:10.1007/s10531-004-0383-y [Google Scholar]
- Goldberg E.E, Roy K, Lande R, Jablonski D. Diversity, endemism, and age distributions in macroevolutionary sources and sinks. Am. Nat. 2005;165:623–633. doi: 10.1086/430012. doi:10.1086/430012 [DOI] [PubMed] [Google Scholar]
- Gotelli N.J, Colwell R.K. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001;3:379–391. doi:10.1046/j.1461-0248.2001.00230.x [Google Scholar]
- Harvey P.H, Colwell R.K, Silvertown J.W, May R.M. Null models in ecology. Annu. Rev. Ecol. Evol. Syst. 1983;14:189–211. [Google Scholar]
- Heino J, Soininen J. Are higher taxa adequate surrogates for species-level assemblage patterns and species richness in stream organisms? Biol. Conserv. 2007;137:78–89. doi:10.1016/j.biocon.2007.01.017 [Google Scholar]
- Hilu K. Skewed distribution of species number in grass genera: is it a taxonomic artifact? In: Hodkinson T.R, Parnell J.A.N, editors. Reconstructing the tree of life: taxonomy and systematics of large and species rich taxa. CRC Press; Boca Raton, FL: 2007. pp. 165–176. [Google Scholar]
- Hubbell S.P. Princeton University Press; Princeton, NJ: 2001. The unified theory of biodiversity and biogeography. [Google Scholar]
- Hunt G, Roy K, Jablonski D. Species-level heritability reaffirmed: a comment on “on the heritability of geographic range sizes”. Am. Nat. 2005;166:129–135. doi: 10.1086/430722. doi:10.1086/430722 [DOI] [PubMed] [Google Scholar]
- Jablonski D. Background and mass extinctions: the alternation of macroevolutionary regimes. Science. 1986;231:129–133. doi: 10.1126/science.231.4734.129. doi:10.1126/science.231.4734.129 [DOI] [PubMed] [Google Scholar]
- Jablonski D. Heritability at the species level: analysis of geographic ranges of Cretaceous mollusks. Science. 1987;238:360–363. doi: 10.1126/science.238.4825.360. doi:10.1126/science.238.4825.360 [DOI] [PubMed] [Google Scholar]
- Jablonski D. Mass extinctions and macroevolution. In: Vrba E.S, Eldredge N, editors. Macroevolution: diversity, disparity, contingency. The Paleontological Society; Lawrence, KS: 2005. pp. 192–210. [Google Scholar]
- Jablonski D. Scale and hierarchy in macroevolution. Palaeontology. 2007;50:87–109. doi:10.1111/j.1475-4983.2006.00615.x [Google Scholar]
- Jablonski D, Flessa K.W. The taxonomic structure of shallow-water marine faunas: implications for Phanerozoic extinctions. Malacologia. 1986;27:43–66. [Google Scholar]
- Jablonski D, Hunt G. Larval ecology, geographic range, and species survivorship in Cretaceous mollusks: organismic versus species-level explanations. Am. Nat. 2006;168:556–564. doi: 10.1086/507994. doi:10.1086/507994 [DOI] [PubMed] [Google Scholar]
- Jablonski D, Roy K. Geographical range and speciation in fossil and living molluscs. Proc. R. Soc. B. 2003;270:401–406. doi: 10.1098/rspb.2002.2243. doi:10.1098/rspb.2002.2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski D, Roy K, Valentine J.W, Price R.M, Anderson P.S. The impact of the pull of the recent on the history of marine diversity. Science. 2003;300:1133–1135. doi: 10.1126/science.1083246. doi:10.1126/science.1083246 [DOI] [PubMed] [Google Scholar]
- Jablonski D, Roy K, Valentine J.W. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314:102–106. doi: 10.1126/science.1130880. doi:10.1126/science.1130880 [DOI] [PubMed] [Google Scholar]
- Järvinen O. Species-to-genus ratios in biogeography: a historical note. J. Biogeogr. 1982;9:363–370. doi:10.2307/2844723 [Google Scholar]
- Longhurst A. Academic Press; San Diego, CA: 1998. Ecological geography of the sea. [Google Scholar]
- Lozano F.D, Schwartz M.W. Patterns of rarity and taxonomic group size in plants. Biol. Conserv. 2005;126:146–154. doi:10.1016/j.biocon.2005.04.024 [Google Scholar]
- Maurer B.A, Nott M.P. Geographic range fragmentation and the evolution of biological diversity. In: McKinney M.L, Drake J.A, editors. Biodiversity dynamics. Columbia University Press; New York, NY: 1998. pp. 31–50. [Google Scholar]
- Mayr E. Harvard University Press; Cambridge, MA: 1963. Animal species and evolution. [Google Scholar]
- McPeek M.A, Brown J.M. Clade age and not diversification rate explains species richness among animal taxa. Am. Nat. 2007;169:E97–E106. doi: 10.1086/512135. doi:10.1086/512135 [DOI] [PubMed] [Google Scholar]
- Patzkowsky M.E. A hierarchical branching model of evolutionary radiations. Paleobiology. 1995;21:440–460. [Google Scholar]
- Purvis A, Agapow P.M, Gittleman J.L. Nonrandom extinction and the loss of evolutionary history. Science. 2000;288:328–330. doi: 10.1126/science.288.5464.328. doi:10.1126/science.288.5464.328 [DOI] [PubMed] [Google Scholar]
- Raup D.M. Cohort analysis of generic survivorship. Paleobiology. 1978;4:1–15. [Google Scholar]
- Raup D.M. Mathematical models of cladogenesis. Paleobiology. 1985;11:42–52. [Google Scholar]
- Raup D.M, Gould S.J, Schopf T.J.M, Simberloff D.S. Stochastic models of phylogeny and the evolution of diversity. J. Geol. 1973;81:525–542. [Google Scholar]
- Roy K, Goldberg E.E. Origination, extinction and dispersal: integrative models for understanding present-day diversity gradients. Am. Nat. 2007;170:S71–S85. doi: 10.1086/519403. doi:10.1086/519403 [DOI] [PubMed] [Google Scholar]
- Roy K, Jablonski D, Valentine J.W. Higher taxa in biodiversity studies: patterns from eastern Pacific marine molluscs. Phil. Trans. R. Soc. B. 1996;351:1605–1613. doi:10.1098/rstb.1996.0144 [Google Scholar]
- Schluter D. Oxford University Press; New York, NY: 2000. The ecology of adaptive radiation. [Google Scholar]
- Schwartz M.W, Simberloff D. Taxon size predicts rates of rarity in vascular plants. Ecol. Lett. 2001;4:464–469. doi:10.1046/j.1461-0248.2001.00241.x [Google Scholar]
- Sepkoski J.J., Jr . Phylogenetic and ecologic patterns in the Phanerozoic history of marine biodiversity. In: Eldredge N, editor. Systematics, ecology, and the biodiversity crisis. Columbia University Press; New York, NY: 1992. pp. 77–100. [Google Scholar]
- Sepkoski J.J., Jr A compendium of fossil marine animal genera. Bull. Am. Paleontol. 2002;363:1–563. [Google Scholar]
- Simberloff D. Taxonomic diversity of island biotas. Evolution. 1970;24:23–47. doi: 10.1111/j.1558-5646.1970.tb01738.x. doi:10.2307/2406712 [DOI] [PubMed] [Google Scholar]
- Spalding M.D, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience. 2007;57:573–583. doi:10.1641/B570707 [Google Scholar]
- Tofts R, Silvertown J. A phylogenetic approach to community assembly from a local species pool. Proc. R. Soc. B. 2000;267:363–369. doi: 10.1098/rspb.2000.1010. doi:10.1098/rspb.2000.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J.W. How good was the fossil record? Clues from the California Pleistocene. Paleobiology. 1989;15:83–94. [Google Scholar]
- Valentine J.W, Jablonski D, Kidwell S, Roy K. Assessing the fidelity of the fossil record by using marine bivalves. Proc. Natl Acad. Sci. USA. 2006;103:6599–6604. doi: 10.1073/pnas.0601264103. doi:10.1073/pnas.0601264103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasenor J.L, Ibarra-Manriquez G, Meave J.A, Ortiz E. Higher taxa as surrogates of plant biodiversity in a megadiverse country. Conserv. Biol. 2005;19:232–238. doi:10.1111/j.1523-1739.2005.00264.x [Google Scholar]
- Vrba E.S, Gould S.J. The hierarchical expansion of sorting and selection: sorting and selection cannot be equated. Paleobiology. 1986;12:217–228. [Google Scholar]
- Waldron A. Null models of geographic range size evolution reaffirm its heritability. Am. Nat. 2007;170:221–231. doi: 10.1086/518963. doi:10.1086/518963 [DOI] [PubMed] [Google Scholar]
- Weir J.T, Schluter D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science. 2007;315:1574–1576. doi: 10.1126/science.1135590. doi:10.1126/science.1135590 [DOI] [PubMed] [Google Scholar]
- Williams P.H, Gaston K.J. Measuring more of biodiversity: can higher-taxon richness predict wholesale species richness? Biol. Conserv. 1994;67:211–217. doi:10.1016/0006-3207(94)90612-2 [Google Scholar]
- Willis J.C, Yule G.U. Some statistics of evolution and geographical distribution in plants and animals, and their significance. Nature. 1922;109:177–179. doi:10.1038/109177a0 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional figures and data in support of the manuscript