Abstract
The productivity and biomass of pristine coral reef ecosystems is poorly understood, particularly in the Caribbean where communities have been impacted by overfishing and multiple other stressors over centuries. Using historical data on the spatial distribution and abundance of the extinct Caribbean monk seal (Monachus tropicalis), this study reconstructs the population size, structure and ecological role of this once common predator within coral reef communities, and provides evidence that historical reefs supported biomasses of fishes and invertebrates up to six times greater than those found on typical modern Caribbean reefs. An estimated 233 000–338 000 monk seals were distributed among 13 colonies across the Caribbean. The biomass of reef fishes and invertebrates required to support historical seal populations was 732–1018 g m−2 of reefs, which exceeds that found on any Caribbean reef today and is comparable with those measured in remote Pacific reefs. Quantitative estimates of historically dense monk seal colonies and their consumption rates on pristine reefs provide concrete data on the magnitude of decline in animal biomass on Caribbean coral reefs. Realistic reconstruction of these past ecosystems is critical to understanding the profound and long-lasting effect of human hunting on the functioning of coral reef ecosystems.
Keywords: historical ecology, coral reef, extinction, range restriction, monk seal, historical overfishing
1. Introduction
Historical analyses have revealed that coral reef communities are significantly altered due to human activity over the past 500 years and that historical data add a necessary dimension to the understanding of the structure and function of ecosystems without people (Jackson 1997; Jackson et al. 2001; Pandolfi et al. 2003; McClenachan et al. 2006). Quantitative data are often too incomplete to determine past ecosystem structure, so that historical reconstructions have been limited in either the temporal scale or the precision of the results. Historical and archaeological data for the Caribbean monk seal are unusually robust over the past 500 years, and they therefore provide an opportunity to reconstruct this component of Caribbean coral reef ecosystems.
Monk seals are large predators that feed on a variety of fishes and invertebrates, and their extinction undoubtedly contributed significantly to changes in Caribbean coral reef ecosystems. Such trophic-level omnivores are thought to have a disproportionate influence within tropical marine food webs, as their removal has consequences throughout the ecosystem (Bascompte et al. 2005). Thus, understanding how pristine coral reef ecosystems once functioned requires the inclusion of these formerly abundant predators. This study compiled historical and archaeological data on the extinct Caribbean monk seal and used these data to determine the location of breeding colonies, historical population size and the ecological consequences of removing this large animal from Caribbean coral reef communities.
The effects of the Caribbean monk seal's extinction in Caribbean coral reef ecosystems are clearly irreversible, but the precarious conservation status of the closely related Hawaiian and Mediterranean monk seals (Monachus schauinslandi and Monachus monachus) makes the understanding of the process and consequences of extinction important for current management regimes. Furthermore, quantifying the effects of this extinction contributes vital information necessary to understanding the various factors that have contributed to the historical degradation of Caribbean coral reef ecosystems.
(a) Historical background
The Caribbean monk seal is the only marine mammal to be driven extinct by humans in tropical seas. Hunting restricted the species' range and eliminated breeding colonies as early as the eighteenth century and the population was severely depleted at least 100 years prior to the extinction in 1952. Written accounts by Caribbean explorers and residents suggest that monk seal populations were historically widespread and abundant, so much so that particularly dense locations of seals were noted on nautical charts of the West Indies. Seals were a curiosity and source of food to early European explorers and castaways, including Christopher Columbus, who killed eight seals on the south coast of the Dominican Republic in 1494 (Colón 1959) and Juan Ponce de Leon, who killed 14 seals in the Dry Tortugas in 1512 (Herrera y Tordesillas 1725).
As settlers populated West Indian islands, they began to locate breeding colonies and hunt monk seals for oil, which was used to grease the machinery of sugar plantations. In the 1640s, Dutch settlers took regular sealing expeditions to Klein Curacao, a small island off the coast of Venezuela (van Grol 1934; Debrot 2000), and William Dampier, a well-known pirate and naturalist, noted in his travel diary in 1675 that both Spanish and British seal hunters frequented the Yucatan Peninsula (Dampier 1968). Jamaican plantation owners sent hunters north to the Bahamas, where they killed hundreds of seals nightly during the breeding season (Sloane 1707). Female seals were particularly vulnerable when they came onshore in winter to breed and nurse their pups (Proceedings of the Government and Council of the Bahamas 1722), but populations appeared to be robust. For example, after 50 years of intensive hunting, the seas of the Bahamas were said to abound with seals (Bruce 1970). By the mid-1800s, however, very few seals remained to sustain the industry (Gray 1850) and several breeding populations had been exterminated throughout the Caribbean (Allen 1880).
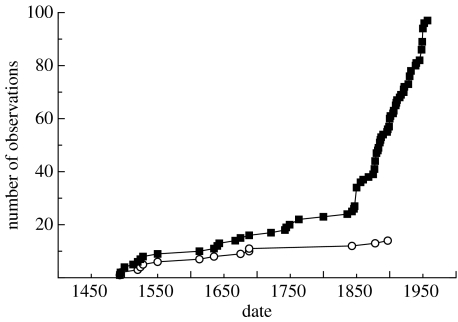
Naturalists and contemporary scientists began to describe the monk seal in the 1880s, so that the last six decades of the species' existence are remarkably well documented in scientific journals (e.g. Elliot 1884; Allen 1887; Townsend 1923). By this time, however, seals were found primarily on offshore atolls, so that the nineteenth-century scientists expended a large amount of effort looking for monk seals that were increasingly rare (figure 1). In fact, the species' range was severely restricted by 1900 (figure 2) and many subpopulations were probably already extinct. One naturalist observed that the few records of extant seal populations were accounts by fishermen and turtle hunters, and that the seals no longer existed in much of their former range (Allen 1880). Monk seal skeletons were valuable to natural history museums and other private collections, and, ironically, their collection by natural history enthusiasts—such as a 1911 expedition to Mexico that killed 200 seals and left few alive (Gaumer 1917)—drove the depleted population further towards extermination.
Figure 1.
Monk seal discovery curve. Interest in the monk seal in the mid-nineteenth century led to increasing numbers of observations, but few new discoveries (this does not include archaeological or data taken from maps). Squares, independent observations; circles, new populations observed.
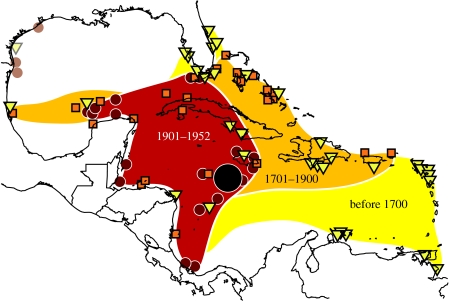
Figure 2.
Total extent of the Caribbean monk seal range over time. Early observations (triangles, before the eighteenth century) were recorded as far east as the Lesser Antilles and Guyana. Observations from the eighteenth and nineteenth centuries (squares) were recorded in most of the Caribbean basin, but, by 1900, observations (small circles) were restricted to the western Caribbean and Gulf of Mexico. The most persistent population (large circle, last colony) was found on the Serrana Bank. Observations in the western Gulf of Mexico are unconfirmed.
A small percentage of the total observations of seals were recorded by history, and reconstructing the historical population relies on these observations, as well as on limited archaeological data. These data are of varying usefulness. Many sources note the presence of monk seals, while a few describe breeding colonies and provide visual census data on the number of seals onshore. The geographical locations of seal observations and seal colonies through time give essential information on the rate of extinction in response to human hunting. Furthermore, existing quantitative data can be used to estimate historical population sizes within colonies and provide insights into the role played by these animals in coral reef ecosystems.
2. Material and methods
(a) Estimating the number of breeding colonies
Historical data on the locations of monk seals in the Caribbean were compiled from archival and published sources, which included both historical narratives and archaeological records. In total, 140 observations were found, ranging in time from pre-European archaeological sites to the last recorded sighting in 1952. To determine which observations most probably represented breeding populations, observations were ranked according to the data type. Eight data types were identified, in descending order of usefulness: (i) breeding colony observed, (ii) groups of seals observed on land during the breeding season, (iii) groups of seals observed on land, (iv) large abundance of seals observed, (v) presence observed, numbers unclear, (vi) seals observed in water or irregular presence noted, (vii) archaeological data, and (viii) place name or data from nautical chart.
It was assumed that only data of types 1–4 could be used to infer the existence of a breeding colony and that breeding colonies existed at a significant distance from each other. Therefore, information on maximum foraging distance and home range was used to estimate the number of independent breeding colonies from the highest quality data. While information on the Caribbean monk seal individuals' home range does not exist, data from closely related Hawaiian and Mediterranean monk seals provide insights into the probable behaviour of Caribbean seals. Hawaiian seals typically remain within 20 km of their home atolls (Stewart et al. 2006), but will travel to forage at distances ranging from 30 to 220 km (K. Abernathy 2006, personal communication). Estimates of the home range of Mediterranean monk seals are all less than 100 km (Berkes 1978; Gucu & Ok 2004). To estimate the number of observed breeding colonies, the locations for type 1–4 observations were mapped in a geographical information system database. Circles were used to represent the home range of a colony and first centred on each of the type 1 data locations. Additional circles were added until all type 1–4 observations were contained within a home range, with the goal being to minimize the number of circles. A radius of 150 km was used as the baseline case, but sensitivity analyses were performed using radii of 75–300 km. This implies a baseline minimum distance between groups of 300 km, with sensitivity analyses exploring distances ranging from 150 to 600 km.
The data that survived in the historical record are a small subset of the total number of actual observations throughout the five centuries of European occupation in the Caribbean. Likewise, archaeological evidence of monk seals has been uncovered in many locations, but data collection and species identification efforts have not been uniform across the Caribbean. Many colonies were probably unrecorded, and had disappeared entirely by the time organized scientific efforts to document the population began in the late nineteenth century. Because such cryptic colonies certainly existed, observation frequency data were used to estimate the number of unseen colonies. This method follows those used for estimating total species richness from a small sample size. Non-parametric estimators were developed to estimate total species richness when observational effort data are unreliable, as they avoid making assumptions about discovery rates and instead rely on the frequency of observations of rare species (Hellmann & Fowler 1999; Chao et al. 2005). The number of undiscovered colonies of Caribbean monk seals was thus estimated from the frequency of observations of rare colonies, using the following equation:
| (2.1) |
where C is the total number of colonies; D is the number of distinct colonies discovered in the sample; n is the sample size; and fk is the number of colonies that are represented exactly k times in the sample.
(b) Estimating the colony-level extinction rate
The rate of extinction of colonies and the probability of survival based on colony location were determined using temporal and spatial data. For each colony, the following data were compiled: (i) the date of first observation, (ii) the dates for some number of repeated observations including the date of last observation, and (iii) the date when an observation was attempted but the colony no longer existed. As such, the dates that bound the true date of extinction exist, but the precise date of extinction was unknown. This type of data is often used in studies of nest survival for birds where observational data exist over time (Dinsmore et al. 2002; Rotella et al. 2004). The primary goal of a nest survival model is to estimate the probability that a nest will survive from one day to the next, from the time eggs are laid until the time the chicks fledge, and to determine whether variables are statistically related to that daily survival rate. Data used in these models typically consist of the dates of first and repeated observations of the nest, with multiple days between observations. Nest survival models can be designed to specifically take account for the fact that researchers might not know the day on which the eggs were laid nor the exact day on which the nest failed (e.g. if it happens to fail between observations). For the monk seal analysis, we examine the probability that a monk seal colony will survive from one decade to the next, from the time it was first observed until the ultimate extinction of the species. This is analogous to a nest survival model where the time interval for survival is decades instead of days, colonies are only observed every few decades at best and all colonies happen to fail. The probability of survival was examined in relation to the colony's distance from the population's core area. Time was grouped into 10-year blocks and a set of a priori models were examined: (i) constant decadal survival rate across the entire time period, (ii) decadal survival rates constant within each century but different between centuries (sixteenth to twentieth centuries), (iii) decadal survival rates constant within each century except for the twentieth century when each decadal survival could differ, (iv) decadal survival being solely a function of distance, (v) decadal survival being a function of century and the colony's distance from the core, with the effect of distance constant across centuries and (vi) decadal survival being a function of century and distance, with the effect of distance varying by century. Models were fit using the nest survival function in the program Mark (v. 4.2, Colorado State University, Fort Collins, CO), and models were compared based on Akaike's information criterion (AICc) values.
(c) Estimating historical population size
Comprehensive, basin-wide survey data do not exist for the Caribbean monk seal, but limited quantitative data exist on population size and harvest rate for the northern Caribbean subpopulation. In 1688, hundreds of seals were killed per night (Sloane 1707); in the early eighteenth century, the seas still abounded with seals (Proceedings of the Government and Council of the Bahamas 1722; Bruce 1970); in 1836, a visual census of 500 individuals was made (Nesbitt 1836); in 1850, very few seals remained to make seal hunting a viable business (Gray 1850); and in 1922, there were no seals left in the entire northern Caribbean (Neill 1957). These data were used with natural population parameters from extant monk seal species to estimate the population size in a nearly unhunted breeding colony in the seventeenth century. While hunting certainly occurred before 1688, this is a reasonable baseline because intensive hunting for monk seal oil was related to the development of the sugar industry, which began in the Caribbean islands in the mid-seventeenth century. The majority of northern Caribbean seals were brought to Jamaica, whose sugar industry developed slowly between the 1660s and 1680s (Sheridan 1994). Therefore, by 1688, intensive hunting for oil had occurred for only a few years.
A simple age-structured pooled-sex density-independent model was constructed including five age classes: 0, 1, 2, 3, and 4+. Survival for each age class was assumed to be 0.80, 0.85, 0.90, 0.90 and 0.95, respectively (Gilmartin et al. 1993). Only those individuals aged 4 and above were assumed to be reproductive and fecundity was assumed to range from 0.1 to 0.2 per individual (Rice 1973). The annual timeline of the model was set up as follows: (i) individuals are counted, (ii) natural mortality occurs, (iii) harvesting occurs and (iv) surviving individuals reproduce and then age. Therefore, the number of monk seals born in a given year equals fecundity times the number of individuals aged 4 and above who survive natural causes and are not killed during the hunt. Mathematically, this is written as
| (2.2) |
The number of individuals in the colony aged 1–3 equals the number of individuals of previous age in the previous year, who survive natural mortality and are not killed during the hunt. For ages 1–3,
| (2.3) |
The number of individuals in the colony aged 4 and above equals the number of 3-year-olds in the previous year, who survive natural causes and are not killed during the hunt, plus the number of individuals aged 4 and above in the previous years, who survive natural causes and are not killed during the hunt. For ages 4 and above,
| (2.4) |
where N(i, t) is the number of individuals of age i at time t; S(i) is the survival rate from i to i+1; H(i, t) is the number of individuals harvested at age i time t; and R is the fecundity of the 4+ individuals. The age structure of the population in 1688 was assumed to equal the stable age distribution the population would achieve based on the assumed survivorship and fecundity estimates in the absence of harvest.
The total harvest across all ages in 1688 was assumed to equal 100 individuals per night for each night of the hunting season, which was assumed to range from 30 to 90 nights per year. We assumed this hunting duration because seals were probably hunted when the females were on land nursing their pups, as they would have been most vulnerable and visible to hunters during this time. In other species of monk seals, nursing is known to occur for approximately 30–50 days, with some degree of overlap among individuals (Johanos et al. 1994). Thus, a range of 30–90 days probably covers the length of time at which peak hunting occurred for Caribbean monk seals.
It was assumed that the hunters were non-selective, so that total harvest was parsed out by age, based on the relative abundance at age for that year. The number of years in which hunting occurred at the rate of 100 individuals per night is unknown, and applying that assumption to many years leads to the extinction of the species far earlier than the historical data suggest. Therefore, the total harvest was assumed to change in proportion to the change in total population size, such that
| (2.5) |
For example, if the population decreased by 10% from one year to the next, the total numbers killed during the harvest would also decrease by 10%. The total population size in 1688 was then estimated, such that the total population size in 1836 equalled 500 individuals.
A Caribbean-wide population size was estimated by calculating the expected number of monk seals per area of reef in the northern Caribbean and applying that density to the reef area within the foraging distance of each colony. It was assumed that the number of seals depended on the reef area available for foraging, so that regions with vast expanses of reefs supported larger populations of monk seals. The number of monk seals for the northern Caribbean population was estimated using the model previously mentioned. Total reef area within 300 km of the northern Caribbean population, as well as each of the 12 other breeding colonies, was obtained (Spalding et al. 2001). The number of seals in each colony was calculated by multiplying the estimated density for the northern Caribbean population by the reef area for each colony.
(d) Determining historical reef fish biomass
A food web model was used to determine the implications of historical monk seal populations on Caribbean coral reef communities. Specifically, the model was structured to address the question of how much fish and invertebrate biomass would have been required to support historical monk seal populations. Monk seal population size estimates derived previously were converted to biomass per habitat area using an average biomass of 245 kg for adult and 50 kg for juvenile seals (Adam & Garcia 2003) and a total reef area in the northern Caribbean of 4400 square kilometres (Spalding et al. 2001). A food web model was constructed using Ecopath software and published biomass, productivity and consumption values (Polovina 1984). Fifteen functional groups were included: tiger sharks; monk seals; birds; reef sharks; turtles; small pelagic fishes; jacks; reef fishes; lobsters and crabs; bottom fishes; near-shore fishes; zooplankton; phytoplankton; heterotrophic benthos; and benthic algae. Ecopath is a predator-driven mass balance model, so that adjusting the biomass values of monk seals affects their prey groups (reef fishes and invertebrates), as well as the groups that serve as prey for these animals (zooplankton, other reef fishes and heterotrophic benthos). The range of historical monk seal biomass values calculated previously was incorporated into the model and prey biomass was then adjusted so that the model maintained equilibrium.
3. Results
(a) Number of breeding colonies
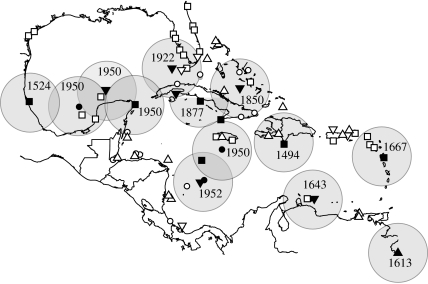
Of the 140 total historical and archaeological observations of monk seals, 37 were ranked to be of data types 1–4. Four observations were of a breeding colony, 11 were of groups of seals on land in the winter, 10 were of groups of seals on land and 12 were of large abundances of seals. A mean distance of 300 km between colonies provides an estimate of 13 breeding colonies (figure 3) with a range from 8 to 16 breeding colonies.
Figure 3.
Locations of breeding colonies. Observations were coded and ranked into eight data types. Data types 1–4 were used to infer the presence of a breeding colony, assuming a minimum distance of 300 km between groups. Large circles represent the area encompassed by a 300 km home range and the date of last observation is listed for each colony. Data type: 1, observed breeding colony (filled circles); 2, groups of seals on land in the winter (filled down triangles); 3, groups of seals on land (filled squares); 4, large abundance (filled up triangles); 5, presence observed (open circles); 6, seals in water/irregular presence noted (open down triangles); 7, archaeological data (open squares); 8, place name or data from map (open up triangles).
The distribution of observations among 13 estimated colonies was used to predict the number of unseen colonies. The number of observations per colony ranged from 1 to 23 with a mean of eight observations per colony (table 1). The species richness estimator provides an estimate of 14 total breeding colonies, suggesting that one colony was overlooked in the historical record.
Table 1.
Historical monk seal colonies. (Thirteen colonies were estimated using data types 1–4 and the assumption that colonies existed at a distance of at least 300 km from each other.)
| colony name | number of observations | highest ranked data type | latitude | longitude | date of last observation |
|---|---|---|---|---|---|
| Dry Tortugas | 23 | 2 | 24.67 | 82.85 | 1922 |
| A Triangulos | 19 | 1 | 20.95 | 92.27 | 1950 |
| Seal Cay, Ragged I | 13 | 2 | 22.62 | 75.88 | 1850 |
| Pedro | 12 | 1 | 17.00 | 77.83 | 1950 |
| A Alacran | 9 | 2 | 22.50 | 89.70 | 1950 |
| S Cuba | 5 | 3 | 19.90 | 77.20 | 1877 |
| Guadeloupe | 4 | 3 | 16.25 | 61.58 | 1667 |
| Curacao | 4 | 2 | 12.00 | 68.65 | 1643 |
| Anina | 4 | 3 | 21.20 | 86.72 | 1950 |
| Serrana | 3 | 1 | 15.83 | 79.83 | 1952 |
| Alta Vela | 3 | 3 | 17.47 | 71.63 | 1494 |
| Veracruz | 2 | 3 | 21.45 | 97.22 | 1524 |
| Guyana | 1 | 4 | 7.00 | 60.00 | 1613 |
| observations outside of colony | 38 |
(b) Colony-level extinction rate
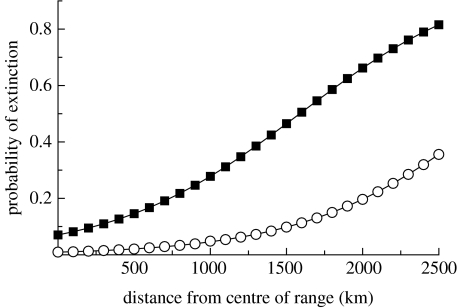
The most robust model of colony-level extinction rate held the decadal extinction rate constant within each century but differed it by century and as a function of distance. Holding the effect of distance constant had an AICc weight of 0.53, and allowing the effect to differ between centuries had an AICc weight of 0.35. The third best model kept allowed the decadal extinction rate to differ by century but did not include distance as a covariate, and had an AICc weight of only 0.08.
The probability of colony extinction was zero for the sixteenth, seventeenth and nineteenth centuries and varied as a function of distance in the eighteenth and twentieth centuries. Thus, the extinction of monk seal colonies followed a predictable pattern in space and occurred in two distinct phases. The first wave of extinction in the eighteenth century eliminated colonies at the periphery of the species' range; colonies within 1500 km of the centre or the range had less than a 10% chance of going extinct while those at the maximum distance from the centre had a 35% chance of extinction (figure 4). In the second wave of extinction, those at the periphery still had a greater probability of extinction, but the probability for all colonies was increased.
Figure 4.
Probability of extinction. The extinction of Caribbean monk seal colonies occurred in two distinct phases. The probability of extinction in each phase is a function of the distance from the centre of the range, with colonies on the periphery having a higher probability of extinction in both the phases. Phase 1, eighteenth century (circles); phase 2, twentieth century (squares).
(c) Historical population size
The total estimated number of seals in the northern Caribbean in the seventeenth-century population ranged from 48 156 to 227 648 individuals depending on the estimates of fecundity and number of nights of the harvest in 1688. Because the estimate of fecundity is considered low (Adam & Garcia 2003) and hunting at the highest rate of 100 seals per night probably occurred only during peak breeding season, the most conservative estimates of 48 156–69 810 seals in the northern Caribbean were selected. These estimates correspond to a hunting season of 30–45 days and a fecundity rate of 0.2. There are no variance estimates for the population sizes because the model was an algebraic one rather than a statistical one. It was flexible enough to always achieve the known population size of 500 in 1836.
The range of 48 156–69 810 seals on northern reefs is equivalent to 3.027–4.388 tons of monk seals per square kilometre of reef. The quantity of reef area available within 300 km of each colony ranged from 450 to 3150 km2 so that the number of seals per colony ranged from 6800 to 70 000. The total Caribbean monk seal population for the entire Caribbean was estimated to be between 233 000 and 338 000 individuals.
(d) Historical reef fish biomass
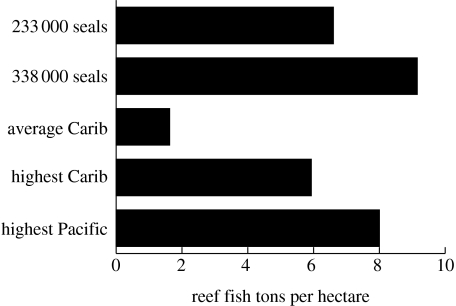
Monk seals were ubiquitous predators on Caribbean coral reefs and would have required a large biomass of fishes and invertebrates to sustain their populations at unexploited levels. Results from the Ecopath model suggest that the range of 3.027–4.388 g m−2 of monk seals would have consumed 660–915 g m−2 of reef fishes and from 71.5 to 103 g m−2 of lobsters and crabs (figure 5). These historical abundances are approximately three to five times more than those found on typical Caribbean coral reefs, more than twice that measured on the most pristine Caribbean reef and similar to those measured on the most remote coral reef atolls in the Pacific (figure 5).
Figure 5.
Historical reef fish biomass implied by monk seal population estimates. The biomass of reef fishes required to sustain the estimated population of historical monk seals (ranging from 233 000 to 338 000 seals in the entire Caribbean) is four to six times greater than the average Caribbean reef, which exceeds that found on the most pristine Caribbean coral reefs today (data from Newman et al. 2006) and is in the same range of the most pristine reefs worldwide (data from NOAA Fisheries, Pacific Islands Fisheries Science Center, Coral Reef Ecosystem).
4. Discussion
Before intensive human hunting, monk seals were found in dense colonies throughout the Caribbean, both on mainland coasts and offshore islands and atolls. Historical data describe 13 breeding colonies, but patterns in the data suggest that at least one colony escaped mention in the historical record. Three locations emerge as candidates for this unrecorded colony: the Little Bahama Bank; eastern Honduras; and eastern Venezuela. Large numbers of descriptions of seals in the water in the Little Bahama Bank and several distinct place names in eastern Honduras suggest the presence of colonies, and the distance between the colonies in Klein Curacao and Guyana make it probable that an intermediate colony existed in eastern Venezuela.
The most persistent monk seal populations were found on offshore atolls, far from human disturbance and in the centre of the range in the central–western Caribbean. The extinction of colonies followed a predictable pattern in time and space, with colonies far from the species' population centre having a significantly higher probability of extinction earlier in time (figure 4). This pattern can be explained by two factors. First, these colonies were on the edge of the species' range and therefore less likely to be repopulated if reduced by hunting. Second, the reef area in the eastern Caribbean is less than that in Central America and the western Caribbean islands, so colonies in the Lesser Antilles probably supported fewer individuals. Although colonies on the edge of the range were eliminated quickly, the persistence of those in the centre and on offshore atolls indicates that monk seals are resistant to moderate to intense levels of human disturbance, which suggests that proper protection has the potential to save the remaining Hawaiian and Mediterranean monk seal colonies from extinction.
The widespread presence of dense monk seal colonies and their prominent role in pristine reefs provide evidence that the entire reef community has suffered major declines in overall animal biomass. In the Caribbean, colonies were found on mainland coasts, islands and atolls close to productive coral reef communities, where high densities of fishes are known to have existed based on the historical data (e.g. Wallace 1955). Estimates of the historical fish biomass suggest that historical reefs were several times more productive than those in the Caribbean today. These results support hypotheses of total ecosystem effects of historical overfishing (Jackson et al. 2001; Pandolfi et al. 2003), and suggest that fishing and hunting has reduced animal biomass so that a once abundant predator such as the monk seals could not survive on the fish resources that remain in depleted Caribbean reefs. Values derived from Ecopath must be interpreted cautiously as the model does not respond dynamically and therefore cannot account for changes such as prey switching, which is a characteristic of omnivorous monk seals. Nevertheless, these results provide yet further evidence on the magnitude of decline that has occurred in fish populations. Observations of emaciation in the Hawaiian monk seal have caused speculation that intensive fishing has reduced the prey base for this species as well (Craig & Ragen 1999). Hawaiian monk seal recovery may be limited by food availability, even at severely depleted population levels, which suggests that intensive overfishing has lowered the carrying capacity for large predators across coral reef ecosystems. Thus, successful recovery plans must include efforts to reduce pressure on overexploited fish stocks used by these marine predators.
The reef fish and invertebrate biomass required to support Caribbean monk seal populations exceeds that observed in the most pristine and protected reef ecosystems in the Caribbean, and the average Caribbean reef has less than 25% of the fishes found on historical reefs (figure 5; Newman et al. 2006). Estimates of historical fish densities are more similar to those observed in the most remote Pacific coral reef atolls, a result which indicates that reefs in the Caribbean were as productive as pristine Pacific reefs today. The close agreement between historical analyses and modern empirical data from remote reefs (Friedlander & DeMartini 2002, Sandin et al. 2008, NOAA Fisheries, Pacific Islands Fisheries Science Center, Coral Reef Ecosystem 2002, 2004, unpublished data) suggests that pristine reef systems contain similar biomasses of reef fishes across ocean basins. The loss of productivity and dramatic change in overall biomass in Caribbean reef ecosystems underscores the continued need to rebuild fish populations, even in areas with the most protected and effective management regimes.
Acknowledgments
We are grateful to J. Jackson, G. Paredes, M. Hardt, A. Rosenberg, E. Sala and S. Sandin for their helpful discussions and two anonymous reviews for their insightful comments. This work was supported by the History of Marine Animal Populations component of the Census of Marine Life, Environmental Protection Agency STAR Graduate Fellowship and the Scripps Institution of Oceanography's William E. and Mary B. Ritter Chair.
References
- Adam P.J, Garcia G.G. New information on the natural history, distribution, and skull size of the extinct West Indian monk seal, Monachus tropicalis. Mar. Mam. Sci. 2003;19:297–317. doi:10.1111/j.1748-7692.2003.tb01110.x [Google Scholar]
- Allen, J. A. 1880 History of North American pinnipeds: a Monograph of the Walruses, SeaLions, SeaBears and Seals of North America, no. 12, pp. 1–785. US Geological and Geographical Survey of the Territories. Miscellaneous Publications.
- Allen J.A. The West Indian monk seal (Monachus tropicalis gray) Bull. Am. Mus. Nat. Hist. 1887;2:1–34. [Google Scholar]
- Bascompte J, Melian C.J, Sala E. Interaction strength combinations and the overfishing of a marine food web. Proc. Natl Acad. Sci. USA. 2005;102:5443–5447. doi: 10.1073/pnas.0501562102. doi:10.1073/pnas.0501562102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes F. Institute of Urban and Environmental Studies, Brock University; Ontario, Canada: 1978. The possibility of movements of Monachus monachus between the coastal waters of Greece and Turkey. [Google Scholar]
- Bruce P.H. J. and R. Byrn; Dublin, Ireland: 1970. Memoirs of Peter Henry Bruce. [Google Scholar]
- Chao A, Chazdon R.L, Colwell R.K, Shen T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005;8:148–159. doi:10.1111/j.1461-0248.2004.00707.x [Google Scholar]
- Colón F. Rutgers University Press; New Brunswick, Canada: 1959. The life of the Admiral Christopher Columbus by his son, Ferdinand. [Google Scholar]
- Craig M.P, Ragen T.J. Body size, survival, and decline of juvenile Hawaiian monk seals, Monachus schauinslandi. Mar. Mam. Sci. 1999;15:786–809. doi:10.1111/j.1748-7692.1999.tb00843.x [Google Scholar]
- Dampier W. Dover Publications; New York, NY: 1968. A new voyage round the world. [Google Scholar]
- Debrot A.O. A review of records of the extinct West Indian monk seal, Monachus tropicalis (Carnivora: Phocidae), for the Netherlands Antilles. Mar. Mam. Sci. 2000;16:834–837. doi:10.1111/j.1748-7692.2000.tb00977.x [Google Scholar]
- Dinsmore S, White G.C, Knopf F.L. Advanced techniques for modeling avian nest survival. Ecology. 2002;83:3476–3488. doi:10.1890/0012-9658(2002)083[3476:ATFMAN]2.0.CO;2 [Google Scholar]
- Elliot H.W. The monk seal of the West Indies, Monachus tropicalis gray. Science. 1884;3:752–753. doi: 10.1126/science.ns-3.72.752. doi:10.1126/science.ns-3.72.752 [DOI] [PubMed] [Google Scholar]
- Friedlander A.M, DeMartini E.E. Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Mar. Ecol. Prog. Ser. 2002;230:253–264. doi:10.1007/s00227-003-1288-0 [Google Scholar]
- Gaumer G.F. Departamento de Talleres Gráficos de la Secretaria de Fomento; Mexico City, Mexico: 1917. Monografia de los mamiferos de Yucatan. [Google Scholar]
- Gilmartin W.G, Johanos T.C, Eberbardt L.L. Survival rates for the Hawaiian monk seal (Monachus schauinslandi) Mar. Mam. Sci. 1993;9:407–420. doi:10.1111/j.1748-7692.1993.tb00473.x [Google Scholar]
- Gray J.E. British Museum; London, UK: 1850. Catalogue of the specimens of Mammalia in the collection of the British Museum. [Google Scholar]
- Gucu A.C, Ok M. Arab the pilgrim. Monach. Guard. 2004;7:1–4. [Google Scholar]
- Hellmann J.J, Fowler G.W. Bias, precision, and accuracy of four measures of species richness. Ecol. Appl. 1999;9:824–834. doi:10.1890/1051-0761(1999)009[0824:BPAAOF]2.0.CO;2 [Google Scholar]
- Herrera y Tordesillas A. N. Rodríguez FrancoN; Madrid, Spain: 1725. Descripción de las Indias Ocidentales. [Google Scholar]
- Jackson J.B.C. Reefs since Columbus. Coral Reefs. 1997;16:S23–S32. doi:10.1007/s003380050238 [Google Scholar]
- Jackson J.B.C, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. doi:10.1126/science.1059199 [DOI] [PubMed] [Google Scholar]
- Johanos T.C, Becker B.L, Ragen T.J. Annual reproductive-cycle of the female Hawaiian monk seal (Monachus Schauinslandi) Mar. Mam. Sci. 1994;10:13–30. doi:10.1111/j.1748-7692.1994.tb00386.x [Google Scholar]
- McClenachan L, Jackson J.B.C, Newman M.J.H. Conservation implications of historic sea turtle nesting beach loss. Front. Ecol. Environ. 2006;4:290–296. doi:10.1890/1540-9295(2006)4[290:CIOHST]2.0.CO;2 [Google Scholar]
- Neill W.T. The vanished sea wolves. Florida Wildl. 1957;10:16. (see also pages 17 and 38). [Google Scholar]
- Nesbitt C.R. On the Bahamas fisheries. J. Bah. Soc. Diff. of Knowl. 1836;11:126–136. [Google Scholar]
- Newman M.J.H, Paredes G.A, Sala E, Jackson J.B.C. Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol. Lett. 2006;9:1216–1227. doi: 10.1111/j.1461-0248.2006.00976.x. doi:10.1111/j.1461-0248.2006.00976.x [DOI] [PubMed] [Google Scholar]
- Pandolfi J.M, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. doi:10.1126/science.1085706 [DOI] [PubMed] [Google Scholar]
- Polovina J.J. Model of a coral reef ecosystem. I. The Ecopath model and its application to French Frigate Shoals. Coral Reefs. 1984;3:1–11. doi:10.1007/BF00306135 [Google Scholar]
- Proceedings of the Government and Council of the Bahamas 1722 Minutes of the Council. British Public Records Office CO 23/1, sec. 3, p. 17.
- Rice, D.W. 1973 Caribbean monk seal (Monachus tropicalis). In Proc. working meeting of seal specialists on threatened and depleted seals of the world. University of Guelph, Ontario, Canada, 18–19 August 1972 Gland, Switzerland: International Union for the Conservation of Nature and Natural Resources.
- Rotella J.J, Dinsmore S.J, Shaffer T.L. Modeling nest-survival data: a comparison of recently developed methods that can be implemented in Mark and SAS. Anim. Biodivers. Conserv. 2004;27:187–204. [Google Scholar]
- Sandin S.A, et al. Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE. 2008;3:e1548. doi: 10.1371/journal.pone.0001548. doi:10.1371/journal.pone.0001548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan R.B. Canoe Press; Kingston, Jamaica: 1994. Sugar and slavery: an economic history of the British West Indies, 1623–1775. [Google Scholar]
- Sloane H. British Museum; London, UK: 1707. A voyage to the islands Madera, Barbados, Nieves, S. Christophers, and Jamaica. [Google Scholar]
- Spalding M.D, Green E.P, Ravilious C. University of California Press; Berkeley, CA: 2001. World atlas of coral reefs. [Google Scholar]
- Stewart B.S, Antonelis G.A, Baker J.D, Yochem P.K. Foraging biogeography of Hawaiian monk seals in the Northwestern Hawaiian islands. Atoll Res. Bull. 2006;543:131–145. [Google Scholar]
- Townsend C.H. The West Indian seal. J. Mamm. 1923;4:55. [Google Scholar]
- van Grol G.J. De grondpolitiek in het West-Indisch domein der generaliteit. Algemeen historische inleiding. vol. 1. Landsdrukkerij; Den Haag, The Netherlands: 1934. pp. 1–52. [Google Scholar]
- Wallace F.W. Canadian Fisherman; Gardenvale, Quebec: 1955. Roving fisherman: recounting personal experiences in the commercial fishing fleets and fish industry of Canada and the United States 1911–1924. [Google Scholar]