Abstract
The discovery of a new dyrosaurid crocodylomorph from the well-dated Palaeocene deposits of northeastern Brazil sheds new light on the evolutionary history of this extinct group of marine crocodylomorphs that have survived the Cretaceous–Palaeogene (K–P) extinction crisis. Guarinisuchus munizi, the most complete member of this group collected in South America so far, is closely related to the African forms, and this fact suggests that dyrosaurids had crossed the Atlantic Ocean before the K–P boundary and dispersed from there to North America and other parts of South America. This discovery also suggests that on the coast of northeastern Brazil, dyrosaurids replaced the pre-existing Late Cretaceous fauna of diversified mosasaurs, a group of marine lizards, after the K–P extinction event, becoming the main predators, together with sharks, in shallow marine Palaeocene environments. More detailed stratigraphic records and detailed dating of the deposits with dyrosaurids are necessary to correlate this particular pattern found in the ancient northeastern Brazilian coast within the evolution of the group, especially in Africa.
Keywords: crocodylomorph, Dyrosauridae, Palaeocene, K–P boundary, extinction
1. Introduction
Among the few vertebrates that survived the mass extinction event documented at the Cretaceous–Palaeogene boundary are dyrosaurids (Buffetaut 1990). These long-snouted extinct marine crocodylomorphs are known mainly from the Maastrichtian deposits of New Jersey (Owen 1849; Denton et al. 1997) and the Late Cretaceous to Early Palaeogene units formed by the Tethys Sea in northern and western Africa (Swinton 1950; Halstead 1975; Buffetaut 1978a, 1981; Brochu et al. 2002; Jouve 2005; Jouve et al. 2005a). Incomplete material is also known from the Palaeocene and Eocene strata of Pakistan (Buffetaut 1978b; Storrs 1986). In South America, dyrosaurids were previously known only by fragmentary material (Cope 1886; Argollo et al. 1987; Buffetaut 1991; Gayet et al. 1991) and some cranial material briefly mentioned in the literature (Hastings & Bloch 2007). Despite being of great interest, in part due to their survivorship across the Cretaceous–Palaeogene boundary (Buffetaut 1990), the fragmentary nature of most specimens hampers a better knowledge of the diversity and evolutionary history of the group. Furthermore, the majority of the occurrences have no detailed stratigraphic record or accurate information regarding their age (Argollo et al. 1987; Buffetaut 1991; Jouve et al. 2005a; Jouve 2007), a common feature particularly for the African deposits (e.g. Kellner & Mader 1997).
Fieldwork carried out in the Poty Quarry, a limestone quarry located close to Recife in northeastern Brazil, has revealed the remains of a dyrosaurid crocodylomorph. This is the only Brazilian locality from where the remains of these marine reptiles were obtained (Kellner 1998; Kellner & Campos 1999). One possible aquatic crocodylomorph was previously found in Brazil, but is not a dyrosaurid and comes from much older deposits (Kellner 1987).
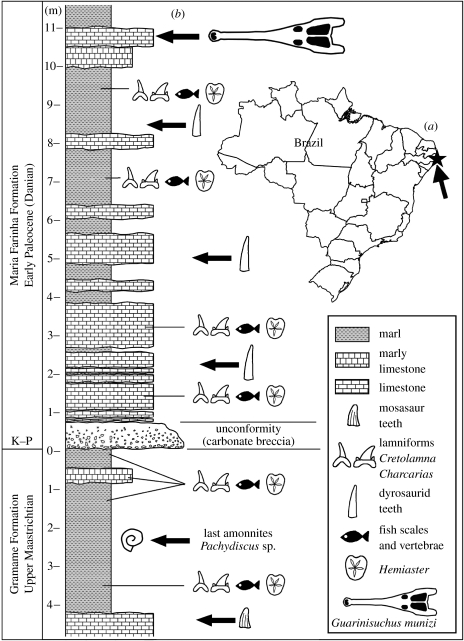
The new specimen was collected from the deposits of the Maria Farinha Formation, Danian, Paraíba Basin (figure 1a). The Poty Quarry is of particular interest, since it shows the most complete exposed marine section of the Cretaceous–Palaeogene transition in South America, and the associated faunal turnover across the K–P section has been well studied (Albertão et al. 1994; Stinnesbeck & Keller 1995; Keller 2001; Fauth et al. 2005; figure 1b).
Figure 1.
(a) Location of Poty Quarry, Paraíba Basin, northeastern Brazil. (b) Stratigraphic section of the K–P transition at the Poty Quarry with the location where G. munizi was collected is indicated.
2. Systematic palaeontology
Crocodylomorpha Walker, 1970.
Neosuchia Benton & Clark, 1988.
Dyrosauridae de Stefano, 1903.
Guarinisuchus munizi gen. et sp. nov.
Etymology. Guarini from the Tupi language meaning warrior+suchus from the Greek language meaning crocodile; munizi, in honour of Geraldo da Costa Barros Muniz owing to his pioneer palaeontological studies in the Paraíba Basin.
Holotype. Skull and mandible, ulna, cervical and caudal vertebrae, ribs, dermal scutes and isolated teeth (DG-CTG-UFPE 5723). Casts at the Museu Nacional (MN-7063-V), Rio de Janeiro and Universidade Estadual Vale do Acaraú, Sobral, Ceará (MDJ R 001). Specimens were collected by J. A. Barbosa and M. S. S. Viana in 2003.
Type locality, horizon and age. The Poty Quarry is located in the region of Paulista (7°54′47″ S,34°51′00″ W), north of Recife, Pernambuco State; Maria Farinha Formation, Paraíba Basin; Early Palaeocene (Late Danian; Albertão et al. 1994; Stinnesbeck & Keller 1995; Fauth et al. 2005).
Diagnosis. Comparatively small dyrosaurid crocodylomorph (approx. 3 m) with the postorbital region of the skull elongated; posterior margin of parietal slightly concave; elongated basioccipital with a distinct depression on the ventral surface; and a marked ‘V’-shaped basioccipital in occipital view.
3. Description and comparison
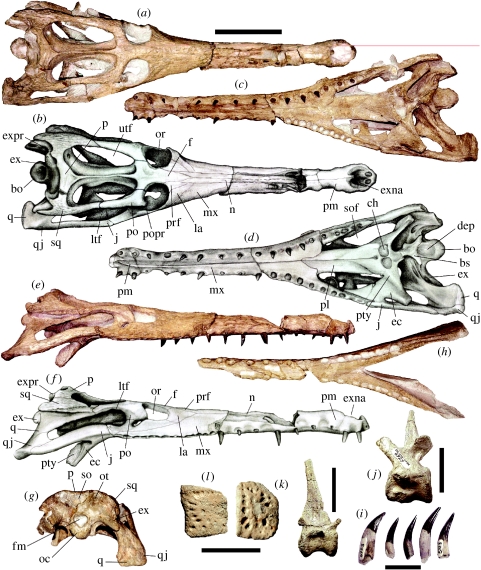
All bones were found in one limestone block and were not articulated, suggesting that some transportation had taken place before the animal's final burial. The material is well preserved, although some signs of dorsoventral compression in the skull are evident (figure 2a–f).
Figure 2.
Material of G. munizi (DG-CTG-UFPE 5723). Skull in (a,b) dorsal, (c,d) ventral, (e,f) right lateral and (g) occipital views; (h) lower jaw in dorsal view; (i) isolated teeth in labial view; (j) cervical vertebra in right lateral view; (k) posterior caudal vertebra in right lateral view and (l) dermal scutes in dorsal view. Scale bars: (a)–(h) 10 cm, (i) 2 cm, (j,k) 5 cm, (l) 5 cm. bo, basioccipital; bs, basisphenoid; dep, depression; ch, choanae; ec, ectopterygoid; ex, exoccipital; exna, external narina; expr, exoccipital processes; f, frontal; fm, foramen magnum; j, jugal; la, lacrymal; ltf, lower temporal fenestra; mx, maxilla; n, nasal; oc, occipital condyle; or, orbit; ot, occipital tuberosity; p, parietal; pl, palatine; pm, premaxilla; po, postorbital; popr, postorbital process; prf, prefrontal; pty, pterygoid; q, quadrate; qj, quadratojugal; so, supraocciptal; sof, suborbital fenestra; sq, squamosal; utf, upper temporal fenestra.
Guarinisuchus munizi can be referred to the Dyrosauridae, which is defined as the most common ancestor of Chenanisuchus lateroculi and Rhabdognathus keiniensis and all their descendants (see the electronic supplementary material), based on the presence of exoccipital processes and the elongated upper temporal fenestra, which in this clade is always larger than the orbit (Brochu et al. 2002; Jouve et al. 2005a). The skull is almost complete and lacks part of the right quadrate and quadratojugal (figure 2a–g). The bone surface of almost all elements is ornamented with pits, shallow grooves and ridges, but not as strongly as in most dyrosaurid taxa (e.g. Chenanisuchus, Sokotosuchus ianwilsoni and Hyposaurus rogersii). The dorsal surface near the contact area between the premaxilla and the maxilla is partially broken. The skull is approximately 525 mm long and its maximum width (over the quadratojugals) is 160 mm, indicating that the total length of this individual was approximately 3 m, estimated from dyrosaurid body×skull chart proportions presented by Jouve et al. (2005b). On the cervical vertebra, the suture of the centrum and the neural arch is fused while in all of the preserved caudals the sutures are open, showing that this specimen represents a sub-adult individual at the time of death. In crocodylians and relatives, the vertebrae fuse from the tail forward (Brochu 1996). Nonetheless, G. munizi appears to be smaller than most dyrosaurid taxa known so far except for H. rogersii from the Maastrichtian of the USA (Denton et al. 1997).
The skull has an elongated tubular rostrum, which is a typical dyrosaurid feature. The preorbital region is narrow, differing from more primitive members of this group (e.g. Chenanisuchus, Sokotosuchus) and is shorter than in R. keiniensis, Arambourgisuchus khouribgaensis and Dyrosaurus phosphaticus (Buffetaut 1979; Jouve 2005; Jouve et al. 2005a,b). Unlike other dyrosaurids, the postorbital portion of the skull in G. munizi is proportionally more elongated (figure 2a,c).
The orbit is placed dorsolaterally and has a rather circular outline. Its posterolateral corner is cut by a well-developed anterolateral preorbital process (figure 2a,c). The upper temporal fenestra is elongated anteroposteriorly, which is a typical feature of dyrosaurids. Nonetheless, there is some variation in the elongation of this opening as indicated by the width–length ratio: Guarinisuchus and Rhabdognathus have the longest upper temporal fenestrae of all dyrosaurids (ratio lower than 0.4; see the electronic supplementary material). The bony bar that separates the upper temporal fenestrae is formed by the parietal and frontal. This element is not broad as in Chenanisuchus or ‘T’ shaped as in Dyrosaurus (Jouve et al. 2005b; figure 2a,c), but is very thin, unsculpted and forms a sagittal crest, similar to the condition observed in Arambourgisuchus and Rhabdognathus and differs from other dyrosaurids, including those with a thin interfenestral bony bar (e.g. Hyposaurus). The lower temporal fenestra is very elongated anteroposteriorly, with a concave ventral margin, less pronounced than in Rhabdognathus aslerensis (Brochu et al. 2002). Even taking into account the dorsoventral compression to which the skull was subjected to, this cranial opening appears to be more elongated than in most other dyrosaurids.
The premaxilla is slightly expanded at the distal end laterally, with the tip that bears the first premaxillary teeth slightly projected, similar to A. khouribgaensis and Congosaurus bequaerti (Jouve & Schwarz 2004), but differs from the rounded and triangular termination of R. keiniensis and C. lateroculi, respectively (figure 2a–f). The maxilla is long (approx. two and a half times the length of jugal) and forms most of the lateral margin of the skull. There are 12–13 rounded alveoli in each maxilla, some with the teeth in situ, particularly on the left side (figure 2c–f). One of the more striking features are the deep occlusal pits, present particularly in the posterior region of the maxillae that get less pronounced anteriorly. These pits indicate that the teeth from the upper and lower jaws alternate, forming a strong interlocking system when the jaws were closed (figure 2c–f). Similar deep occlusal pits were reported in R. aslerensis (Brochu et al. 2002), but in G. munizi they are better developed, being almost the same size as the adjacent alveoli. The preorbital bears a well-developed anterolateral process, preserved only on the right side in the present material, which is also regarded as a dyrosaurid feature (Buffetaut 1979). This process is less developed than in D. phosphaticus and R. keiniensis, and less broad than in D. phosphaticus (figure 2a,b,e,f).
In dorsal view, the posterior margin of the parietal is slightly concave, differing from all other dyrosaurids (figure 2a,b), although within this clade of marine crocodylomorphs this feature is variable. The primitive condition is a straight posterior parietal margin present in C. lateroculi, D. phosphaticus, Phosphatosaurus gavialoides, R. aslerensis and also in Terminonaris (Wu et al. 2001) that is regarded as the sister group of Dyrosauridae (Jouve et al. 2005a; see the electronic supplementary material). Hyposaurus rogersii displays a distinct V-shaped condition, in R. keiniensis this region presents a strong concavity and in A. khouribgaensis the parietals taper posteriorly showing a pointed end (Jouve et al. 2005a), all features that are regarded here as autapomorphies of the respective species. The squamosal forms the ventroposterior dorsal corner of the skull (figure 2a,b,e,f). The posterior margin of this bone lateral to the occipital processes is concave, a feature shared with Rhabdognathus (R. keiniensis and R. aslerensis) and A. khouribgaensis. The exoccipital processes (or tuberosities) are well developed, being larger than in Chenanisuchus and S. ianwilsoni, but smaller than in Rhabdognathus (Jouve et al. 2005b; figure 2a–g). These tuberosities are rounded and not flattened as reported in H. rogersii and A. khouribgaensis (Jouve et al. 2005a). The occipital region between the exoccipital processes is concave. The basioccipital is elongated posteriorly and shows a distinct depression on the ventral surface; in occipital view it is V shaped, features that are unique to this new taxon. The quadratojugal is laterally expanded, as in most dyrosaurids, differing from the anteroposteriorly expanded condition observed in R. aslerensis (Brochu et al. 2002; figure 2a–g).
The lower jaw is incomplete, lacking the distal end and the retroarticular process. As in the upper jaw, alveoli are rounded and deep occlusal pits are present on the posterior region. The mandibular symphysis is more wide than deep, differing from C. bequaerti, D. phosphaticus and R. keiniensis (figure 2h). Although not complete, we estimate the total number of the teeth present in the lower jaw similar to the upper jaw (see below), with the symphysis starting at around the 14th alveolus.
The total number of alveoli on each side of the upper jaw is 17–18, 14 out of which are confined to the preorbital region (figure 2h). This is less than the number of teeth reported in R. keiniensis (27–28, 25 preorbital), D. phosphaticus (25, 21 preorbital), and C. bequaerti (minimum 20, with 17 preorbital; Jouve & Schwarz 2004) and slightly less than A. khouribgaensis (21, with 16 preorbital) but more than Hyposaurus that has only 15 teeth (Jouve & Schwarz 2004). The teeth are long, show strong striations and unserrated anterior and posterior carinae (figure 2i). They lack the particular medially ‘twisted’ anterior carina reported in Hyposaurus (Denton et al. 1997). The anterior-most teeth are arched, curving posteriorly. They show a rounded cross-section and the posterior ones are not labiolingually compressed as are the ones reported in C. bequaerti (Jouve & Schwarz 2004).
The postcranial elements recovered so far agree with the general description of dyrosaurid postcranials (Schwarz et al. 2006), with the caudal vertebrae displaying a comparatively high neural spine (figure 2j,k). The dermal scutes recovered are flat, with the dorsal (external) surface ornamented by pits of different depth and the ventral surface smooth (figure 2l).
Until recently, only fragmentary dyrosaurid remains were reported from Brazil and Bolivia in South America. The specimens from Bolivia consist of one isolated vertebra from the El Molino Formation (Gayet et al. 1991), two isolated teeth, one dorsal vertebra and dermal scutes from the region of Lake Titicaca, placed in the Palaeocene (Argollo et al. 1987), and fragmentary material first regarded as closely related to Sokotosuchus aff. ianwilsoni from the Santa Lucía Formation, Palaeocene (Buffetaut 1991), which is presently regarded as an indeterminate dyrosaurid (Jouve 2007).
From Brazil, isolated vertebrae and teeth referred to Dyrosauridae have been reported from the Maria Farinha Formation, which is the same unit where G. munizi came from (Carvalho & Azevedo 1997; Gallo et al. 2001). Cope (1886) previously described fragmentary elements, including a lower jaw, as ‘Hyposaurus derbianus’ which he regarded as coming from the Cretaceous strata of Pernambuco. However, he did not figure this specimen and no precise location was provided; so it is possible that this material might be from the Maria Farinha Formation. Furthermore, the type material of H. derbianus is scattered in different collections (Longbottom 1988), some of which could not be located during the preparation of this paper. In any case, the comparisons presented above shows that G. munizi is quite distinct from Hyposaurus (see the electronic supplementary material).
4. Discussion and conclusions
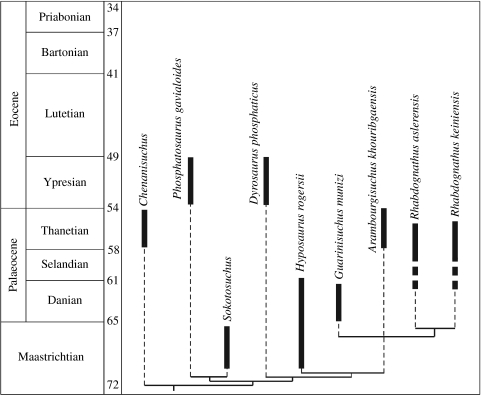
In order to access the phylogenetic position of G. munizi, we performed a phylogenetic analysis using a modified version of the dataset published by Jouve et al. (2005a; see the electronic supplementary material). The results indicate that the Brazilian taxon is closely related to the African forms A. khouribgaensis from the Palaeocene deposits of Morocco (Jouve et al. 2005a) and Rhabdognathus from the Palaeocene strata of Mali (Jouve 2007), although we could not precisely estimate to which of the two it is more closely related (figure 3).
Figure 3.
Temporally calibrated phylogeny of dyrosaurid crocodylomorphs based on the strict consensus tree resulting from the phylogenetic analysis (see the electronic supplementary material). Solid bars indicate known temporal ranges and dashed lines missing ranges. Guarinisuchus munizi from the Early Palaeocene fills the gap between the Late Cretaceous and the Late Palaeocene dyrosaurids.
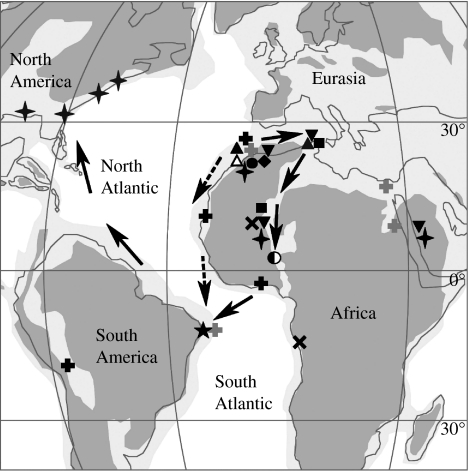
The phylogenetic analysis and the precise stratigraphical position of G. munizi within the Maastrichtian–Palaeocene transition zone gives relevant information regarding the group's evolutionary history, and raises some possibilities regarding dyrosaurid dispersal routes. Based on the known record of dyrosaurids and their phylogenetic relationships presented here, there are two general possibilities that can account for their distribution; both take into account the origin of this group in northern Africa (Jouve et al. 2005a; figure 4). The first hypothesis advocates a dispersal route going eastwards. From there they could have reached Asia and the Benue Trough, an inland sea that stretched across the present Sahara. Before the end of the Cretaceous, dyrosaurids could have crossed the Atlantic Ocean from the western coast of Africa to the area presently corresponding to Pernambuco in northeastern Brazil, where the distance between Africa and South America was (and still is) shorter. From there they could have dispersed northwards along the coast reaching North America and other areas of the South American continent using epicontinental seas that existed at that time, including the regions of Colombia and Bolivia. The alternative hypothesis differs by taking into account the possibility that dyrosaurids could also have dispersed westwards and then south along the marginal basins of the African coast, crossing the Atlantic Ocean from there. Those two hypotheses do not necessarily exclude each other, but more information on the dyrosaurids from the western coast of Africa is needed in order to test the second one. Theoretically, one could also argue for a northern dispersal route through which, after their origin, dyrosaurids reached Europe and then crossed the North Atlantic Ocean to North America, reaching South America from there. The present study, however, did not find any evidence for this alternative scenario, particularly since Guarinisuchus is more closely related to the African forms than the North American dyrosaurids. Based on the phylogenetic analysis presented here, this dispersal event must have occurred before the Maastrichtian–Palaeogene boundary.
Figure 4.
Palaeogeography of North America, South America, Europe and Africa during the Upper Maastrichtian–Palaeocene, with the indication of two possible dispersal routes for the Dyrosauridae. In both hypotheses, dyrosaurids have originated in northern Africa, with the first dispersal route (solid arrow) going eastwards, reaching Asia, and crossing the inland sea that stretched across the present Sahara. The second hypothesis (broken arrow) suggests that dyrosaurids could also have moved westwards and then south along marginal basins of the African coast. Before the end of the Cretaceous, they had crossed the Atlantic Ocean reaching South America in the present area of Pernambuco, dispersing from there northwards along the coast reaching North America and other areas of the South American continent. Occurrences of taxa are indicated as follows: four-point stars, Hyposaurus; left-filled circle, Sokotosuchus; star, Guarinisuchus; diamond, Chenanisuchus; circle, Arambourgisuchus; open triangle, Atlantosuchus; filled down triangles, Rhabdognathus; crosses, Congosaurus; squares, Phosphatosaurus; filled upright triangles, Dyrosaurus; light grey pluses, Dyrosauridae indet from the Cretaceous deposits; black pluses, Dyrosauridae indet from the Palaeocene deposits.
A detailed stratigraphic work shows the presence of the K–P boundary in the Poty Quarry (Albertão et al. 1994; Stinnesbeck & Keller 1995; Fauth et al. 2005). The lower portion of the sedimentary sequence is formed by the Maastrichtian Gramame Formation that is overlain by the Early Palaeocene Maria Farinha Formation. Guarinisuchus munizi was found 11 m above the K–P boundary in layers whose age, established based on microfossils, is regarded as the P2 biozone of the Late Danian (Fauth et al. 2005), and indicates an age of 62–61 Myr ago (figure 1). Mosasaurs remains have been collected in the uppermost layers of the Gramame Formation in the Poty Quarry and four distinct clades were recognized (Mosasaurini, Globodensini, Plioplatecarpini and Prognathodontini; Price 1957; Carvalho & Azevedo 1998). Despite intensive collection, they have not been found in the Maria Farinha Formation, corroborating the general notion that those large marine reptiles did not survive the mass extinction event of the K–P boundary.
Based on the studies of the Poty Quarry, and the collected material described here, we can suggest that at least in this region of the northeastern Brazilian margin, dyrosaurids occupied the niche of major predators left by mosasaurs after the K–P transition, becoming the major predator in shallow marine and near-shore habitats (figure 1). In the coastal deposits of New Jersey, which present an important K–P record, although dyrosaurids coexisted with mosasaurs during the Late Maastrichtian, their remains are rare, but after the K–P boundary they become more numerous, and marine crocodyliforms such as the dyrosaurids Hyposaurus and Thoracosaurus, together with lamnid sharks, became the major predators (Gallagher 2005). Also, some large sharks that are present in the Maastrichtian of the Paraíba Basin disappeared during the K–P event, but two smaller genera of lamnid sharks crossed the K–P boundary (Gallo et al. 2001) and coexisted with G. munizi. It is also interesting to note that dyrosaurids diversified considerably during the Palaeocene, in contrast to the rarity of their remains in the Maastrichtian (Buffetaut 1990; Bardet 1994; Bardet et al. 2005). More studies, including detailed stratigraphic work in other basins, particularly in Africa where the Cretaceous–Palaeogene boundary is present as well, might shed more light on the question if the replacement of mosasaurs by dyrosaurids after the K–P transition was a local event or a global trend.
Acknowledgments
We thank Gerta Keller (Princeton University) for helping with the determination of the age of the specimen, and Marcia Silva and Jessica Pontes for the preparation. This study was partially funded by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Rio de Janeiro (FAPERJ no. E-26/152.885/2006) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq no. 304965/2006-5) grants to A.W.A.K.
Supplementary Material
Phylogenetic analysis
References
- Albertão G.A, Koutsoukos E.A.M, Regali M.P.S, Attrep M, Jr, Martins P.P., Jr The Cretaceous–Tertiary boundary in southern low-latitude regions: preliminary study in Pernambuco, north-eastern Brazil. Terra Nova. 1994;6:366–375. doi:10.1111/j.1365-3121.1994.tb00509.x [Google Scholar]
- Argollo J, Buffetaut E, Cappetta H, Fornari M, Herail G, Laubacher G, Sigé B, Vizcarra G. Découverte de Vertébrés aquatiques présumés Paléocènes dans les Andes septentrionales des Bolivie (Rio Suches, synclinorium de Puntina) Geobios. 1987;20:123–127. doi:10.1016/S0016-6995(87)80061-X [Google Scholar]
- Bardet N. Extinction events among Mesozoic marine reptiles. Hist. Biol. 1994;7:313–324. [Google Scholar]
- Bardet N, Superbiola X.P, Iarochene M, Bouya B, Amaghzaz M. A new species of Halisaurus from the Late Cretaceous phosphates of Morocco, and the phylogenetical relationships of the Halisaurinae (Squamata: Mosasauridae) Zool. J. Linn. Soc. 2005;143:447–472. doi:10.1111/j.1096-3642.2005.00152.x [Google Scholar]
- Brochu C.A. Closure of neurocentral sutures during crocodylian ontogeny: implications for maturity assessment in fossil archosaurs. J. Vert. Paleontol. 1996;16:49–62. [Google Scholar]
- Brochu C.A, Bouaré M.L, Sissoko F, Roberts E.M, O'Leary M.A. A dyrosaurid crocodyliform braincase from Mali. J. Paleontol. 2002;76:1060–1071. doi:10.1666/0022-3360(2002)076<1060:ADCBFM>2.0.CO;2 [Google Scholar]
- Buffetaut E. Les Dyrosauridae (Crocodylia, Mesosuchia) des phosphates de l'Eocéne inférieur de Tunisie: Dyrosaurus, Rhabdognathus, Phosphatosaurus. Géologie Méditerranéenne. 1978a;5:237–256. [Google Scholar]
- Buffetaut E. Crocodilian remains from the Eocene of Pakistan. Neues Jahrb. Geol. Paläontol. Abh. 1978b;156:262–283. [Google Scholar]
- Buffetaut, E. 1979 Sokotosuchus ianwilsoni and the evolution of the dyrosaurid crocodilians The Nigerian Field Monograph, no. 1, pp. 31–41.
- Buffetaut E. Radiation évolutive, paléoécologie et biogéographie des crocodiliens Mésosuchiens. Mém. Soc. Géol. France. 1981;60:1–88. [Google Scholar]
- Buffetaut E. Vertebrate extinction and survival across the Cretaceous–Tertiary boundary. Tectonophysics. 1990;171:337–345. doi:10.1016/0040-1951(90)90108-K [Google Scholar]
- Buffetaut E. Fossil crocodilians from Tiupampa, (Santa Lucia Formation, Early Paleocene) Bolivia: a preliminary report. Revista Técnica de YPFB. 1991;12:541–544. [Google Scholar]
- Carvalho L.B, Azevedo S.A.K. Um crocodilo marinho (Mesosuchia: Dyrosauridae) no Paleoceno da Bacia Pernambuco-Paraíba, Brasil. Ameghiniana. 1997;34:532. [Google Scholar]
- Carvalho L.B, Azevedo S.A.K. Proposta taxônomica para os répteis marinhos (Lepidosauria, Mosasauridae) do Neocretáceo da Bacia Pernambuco-Paraíba. Boletins do Museu Nacional. 1998;43:1–9. [Google Scholar]
- Cope E.D. A contribution to the vertebrate paleontology of Brazil. Proc. Am. Philos. Soc. 1886;23:1–20. [Google Scholar]
- Denton R.K.J, Bobie J.L, Parris D.C. The marine crocodilian Hyposaurus in North America. In: Callaway J.M, Nicholls E.L, editors. Ancient marine reptiles. Academic Press; London, UK: 1997. pp. 375–397. [Google Scholar]
- Fauth G, Colin J, Koutsoukos E.A.M, Bengtson P. Cretaceous–Tertiary boundary ostracodes from the Poty quarry, Pernambuco, Northeastern Brazil. J. South Am. Earth Sci. 2005;19:285–305. doi:10.1016/j.jsames.2005.01.007 [Google Scholar]
- Gallagher W.B. Recent mosasaur discoveries from New Jersey and Delaware, USA: stratigraphy, taphonomy and implications for mosasaur extinction. Geologie en Mijnbouw. 2005;84:241–245. [Google Scholar]
- Gallo V, Figueiredo F.J, Carvalho L.B, Azevedo S.A.K. Vertebrate assemblage from the Maria Farinha formation after the K–T boundary. Neues Jahrb. Geol. Paläontol. Abh. 2001;219:261–284. [Google Scholar]
- Gayet M, Marshall L.G, Sempere T. The Mesozoic and Paleocene vertebrates of Bolivia and their stratigraphic context: a review. Revista Técnica de YPBF. 1991;12:393–433. [Google Scholar]
- Halstead L.B. Sokotosuchus ianwilsoni n.g., n. sp., a new teleosaur crocodile from the Upper Cretaceous of Nigeria. J. Mining Geol. 1975;11:101–103. [Google Scholar]
- Hastings, A. & Bloch, J. 2007 New short-snouted Dyrosaurid (Crocodylomorpha) from the Paleocene of Northern Colombia. J. Vert. Paleontol 27(Suppl. 67th Ann. Meet. Abstracts), 87A.
- Jouve S. A new description of Dyrosaurus phosphaticus (Thomas, 1893) (Mesoeucrocodylia: Dyrosauridae) from the Lower Eocene of North Africa. Can. J. Earth Sci. 2005;42:323–337. doi:10.1139/E05-008 [Google Scholar]
- Jouve S. Taxonomic revision of the dyrosaurid assemblage (crocodyliformes: mesoeucrocodylia) from the Paleocene of the Iullemmeden Basin, West Africa. J. Paleontol. 2007;81:163–175. doi:10.1666/0022-3360(2007)81[163:TROTDA]2.0.CO;2 [Google Scholar]
- Jouve S, Schwarz D. Congosaurus bequaerti, a Paleocene dyrosaurid (Crocodyliformes; Mesoeucrocodylia) from Landana (Angola) Bulletin de l'Institut Royal des Sciences Naturelles de Belgique. 2004;74:129–146. [Google Scholar]
- Jouve S, Iarochène M, Bouya B, Amaghzaz M. A new crocodyliform dyrosaurid from the Palaeocene of Morocco and a phylogenetic analysis of Dyrosauridae. Acta Palaeontol. Polonica. 2005a;50:581–594. [Google Scholar]
- Jouve S, Bouya B, Amaghzaz M. A short-snouted dyrosaurid (Crocodyliformes, Mesoeucrocodylia), from the Palaeocene of Morocco. Palaeontology. 2005b;48:359–369. doi:10.1111/j.1475-4983.2005.00442.x [Google Scholar]
- Keller G. The end-Cretaceous mass extinction in the marine realm: year 2000 assessment. Planet. Space Sci. 2001;49:817–830. doi:10.1016/S0032-633(01)00032-0 [Google Scholar]
- Kellner A.W.A. Ocorrência de um novo crocodiliano no Cretáceo Inferior da Bacia do Araripe, Nordeste do Brasil. An. Acad. Bras. Ciênc. 1987;59:219–232. [Google Scholar]
- Kellner A.W.A. Panorama e perspectiva do estudo de répteis fósseis no Brasil. An. Acad. Bras. Ciênc. 1998;70:647–676. [Google Scholar]
- Kellner A.W.A, Campos D.A. Vertebrate paleontology in Brazil—a review. Episodes. 1999;22:238–251. [Google Scholar]
- Kellner A.W.A, Mader B.J. Archosaur teeth from the Cretaceous of Morocco. J. Paleontol. 1997;71:525–527. [Google Scholar]
- Longbottom A.E. A note on the location of the type specimens of vertebrates from Brazil described by Cope in 1886. J. Paleontol. 1988;62:828–832. [Google Scholar]
- Owen R.D. Notes on remains of fossil reptiles discovered by Prof. Henry Rodgers of Pennsylvania, U.S., in Greensand formations of New Jersey. Q. J. Geol. Soc. Lond. 1849;5:380–383. [Google Scholar]
- Price L.I. Restos de mosasáurios de Pernambuco e considerações sobre a presença destes répteis na Bacia Amazônica do Brasil. Bol. Dep. Nac. Prod. Min. Brasil. 1957;169:1–29. [Google Scholar]
- Schwarz D, Frey E, Martin T. The postcranial skeleton of the Hyposaurinae (Dyrosauridae; Crocodyliforms) Palaeontol. 2006;49:695–718. doi:10.1111/j.1475-4983.2006.00563.x [Google Scholar]
- Stinnesbeck W, Keller G. Environmental changes across the Cretaceous–Tertiary boundary in northeastern Brazil. In: MacLeod N, Keller G, editors. Cretaceous–Tertiary mass extinctions: biotic and environmental changes. Norton; New York, NY: 1995. pp. 451–469. [Google Scholar]
- Storrs G.W. A dyrosaurid crocodile (Crocodylia, Mesosuchia) from the Paleocene of Pakistan. Postilla. 1986;197:1–16. [Google Scholar]
- Swinton W.E. On Congosaurus bequaerti Dollo. Annales du Musée du Congo Belge. 1950;4:1–37. [Google Scholar]
- Wu X.C, Russell A.P, Cumbaa S.L. Terminonaris (Archosauria: Crocodyliformes): new material from Saskatchewan, Canada, and comments on its phylogenetic relationships. J. Vert. Paleontol. 2001;21:492–514. doi:10.1671/0272-4634(2001)021[0492:TACNMF]2.0.CO;2 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic analysis