Abstract
Recent work has shown that captive rooks, like chimpanzees and other primates, develop cooperative alliances with their conspecifics. Furthermore, the pressures hypothesized to have favoured social intelligence in primates also apply to corvids. We tested cooperative problem-solving in rooks to compare their performance and cognition with primates. Without training, eight rooks quickly solved a problem in which two individuals had to pull both ends of a string simultaneously in order to pull in a food platform. Similar to chimpanzees and capuchin monkeys, performance was better when within-dyad tolerance levels were higher. In contrast to chimpanzees, rooks did not delay acting on the apparatus while their partner gained access to the test room. Furthermore, given a choice between an apparatus that could be operated individually over one that required the action of two individuals, four out of six individuals showed no preference. These results may indicate that cooperation in chimpanzees is underpinned by more complex cognitive processes than that in rooks. Such a difference may arise from the fact that while both chimpanzees and rooks form cooperative alliances, chimpanzees, but not rooks, live in a variable social network made up of competitive and cooperative relationships.
Keywords: corvids, rooks, chimpanzees, cooperation, cognition, tolerance
1. Introduction
Animal cooperation is a topic that has fascinated researchers from many disciplines (Noe 2006), perhaps in part because cooperation is a defining feature of the social behaviour of our own species (Moll & Tomasello 2007). For comparative psychologists, the most pressing question is whether or not cooperation in animals is underpinned by the complex cognitive abilities that characterize cooperation in humans, such as an understanding of the role and intentions of the collaborative partner.
Research addressing this question has focused on primates, such as chimpanzees, whose cooperative hunting behaviour in the wild has led field researchers to refer to cognitive sophistication. Boesch & Boesch (1989) describe the hunting behaviour of chimpanzees in the Tai National Forest as being truly collaborative, with different individuals playing different roles. However, research in the laboratory, motivated by a desire to test this proposal under controlled conditions, has revealed a limited tendency among chimpanzees and other primates when it comes to solving a task requiring the actions of two individuals. In several studies, chimpanzees needed training or extensive experience before they could successfully cooperate (Crawford 1937; Chalmeau 1994; Hirata 2003).
Similarly, while studies of monkey species have found that they can learn to solve a problem involving simultaneous effort by two individuals, their seemingly cooperative behaviour may come from a mutual attraction to the apparatus and the food, resulting in fortuitous co-production rather than behavioural coordination or an understanding of the role of the partner (Petit et al. 1992; Chalmeau et al. 1997; Visalberghi et al. 2000). The subjects in these studies were no more likely to pull when their partner was near to the other handle. However, Mendres & de Waal (2000) argue that capuchin monkeys (i) understand when cooperation is necessary, because the capuchins in their study glanced at their partners more often when the efforts of two monkeys were required for success and (ii) display behavioural coordination, because the monkeys pulled more often when their partners were in the vicinity of the apparatus, and putting an opaque barrier between the subjects resulted in significantly poorer performance. Similarly, Cronin et al. (2005) report that cotton-top tamarins, Saguinus oedipus, solve a problem requiring two individuals to pull simultaneously, and that they pull significantly more often when their partner is present than when they are alone. However, it should be noted that in both studies the monkeys still pulled the handle (albeit less frequently) when the partner was out of the testing room, a finding that questions the notion that they had a robust understanding that a partner was required.
Recent work by Melis et al. (2006a) investigated whether social constraints might explain the limited success of chimpanzees in the previous studies of cooperation. Pairs of chimpanzees were tested for their tendency to share food before being tested on a task requiring the simultaneous pulling of two ropes to bring in a platform containing food. The tolerant pairs that would feed together were able to spontaneously find a solution to the cooperative task, in contrast to the performance of the pair originally tested by Hirata & Fuwa using the pulling task, which did not cooperate in the initial trials of the experiment (Hirata & Fuwa 2007). Melis et al. (2006a) found that the three intolerant pairs (in which one individual monopolized access to the food) did not bring in the platform in 60 trials. Further work with another 16 dyads showed that the proportion of successful trials on the cooperation task correlated significantly with the ease with which food was shared. The authors propose that this finding highlights the importance of the role of temperament when considering the evolution of cooperation. In support of this notion is the fact that bonobos, Pan paniscus, which are far more egalitarian than chimpanzees in their social structure, cooperate at higher levels than chimpanzees for food that can be easily monopolized (Hare et al. 2007). The difference in the temperament between chimpanzees and humans might reflect an important evolutionary step, providing a platform upon which our cooperative culture and sophisticated cognition could have evolved (Hare & Tomasello 2005; Tomasello et al. 2005; Melis et al. 2006a; Moll & Tomasello 2007).
Melis et al. (2006b) went on to assess the cognitive underpinnings of the cooperative behaviour. They found evidence that the chimpanzees were able to (i) coordinate their actions with those of their collaborator by delaying the pulling of the rope until the other subject was permitted to enter the testing room, (ii) understand when cooperation was necessary, because when focal subjects could control the access of their partners to the test room, they allowed them to enter more often on trials when two subjects were needed to solve the task than on trials when one subject could prevail and (iii) use information about the effectiveness of different collaborators, because when given a choice between two potential partners, they gave access to the more efficient partner more frequently than the less efficient one, after some experience of cooperating with each of them.
The authors interpret these results in the light of what they mean for the evolution of cooperation in our own lineage. However, are such abilities unique to primates? Emery & Clayton (2004) suggested that many of the cognitive feats described for great apes may have a parallel in corvids, a group of large-brained birds. Indeed, several studies of social cognition have described abilities in corvids that rival those found in apes. A series of experiments carried out with western scrub-jays and ravens have shown that the strategies used by food-storing corvids to protect their caches are based not just on simple rules of thumb (e.g. by only hiding food when there is no competitor in sight), but are instead highly flexible (e.g. dependent on who is watching when Dally et al. 2006). This flexibility may depend on an ability to take the visual perspective and even the knowledge state of the competitor into account (reviewed in Clayton et al. 2007).
Corvids have also displayed primate-like social skills in competitive foraging paradigms. Jackdaws steal food more quickly from a human competitor who is either glancing away or has their eyes closed than one who is looking directly at the food (von Bayern & Emery submitted). Ravens lead conspecifics away from boxes baited with food (Bugnyar & Kotrschal 2004), follow the gaze of humans around objects (Bugnyar et al. 2004) and rush to recover a piece of food that a dominant has seen being hidden, but delay their approach if the dominant has not seen the baiting process (Bugnyar & Heinrich 2005).
However, the social cognition of corvids in the context of a cooperative instrumental task is as yet untested. Recent research with a colonial living species of corvid, the rook, has provided some evidence that cooperation, as well as competition, is an important feature of their social lives. Captive rooks, like chimpanzees, develop alliances with individuals in their social group and maintain these cooperative relationships throughout the year through affiliative behaviours. During the development of these relationships, rooks affiliate with several different partners, notably showing high levels of active food sharing of preferred foods. After these first few months of instability, the rooks develop long-term alliances with one or two individuals (Emery et al. 2007). The members of a partnership show high levels of social tolerance, exchange affiliative behaviour such as preening and food sharing, aid one another in agonistic encounters and engage in post-conflict affiliation (Seed et al. 2007).
In the wild, rooks live in large groups, and forage, roost and nest in close proximity to hundreds of conspecifics. In comparison with territorial species of corvids, therefore, rooks have a high level of social tolerance, which has been argued to provide a platform upon which cooperative action could evolve (Hare & Tomasello 2005; Tomasello et al. 2005; Melis et al. 2006a; Moll & Tomasello 2007). There is some evidence that rooks engage in cooperative behaviours in the wild. For example, Coombs (1961) describes group defence by rooks nesting in the same tree, against a newcomer attempting to establish a territory.
Interestingly, rook pairs synchronize their movements and vocalizations tightly when displaying in aggressive situations, as well as synchronizing individual body movements such as head orientation and bill wiping with their social partner (Emery et al. 2007; Seed et al. 2007). Rook pairs also engage in various joint behaviours, such as dual caching and dual object manipulation, which seem to involve some degree of coordination.
In this study, we aimed to compare the performance of rooks and chimpanzees on a cooperative problem by employing the apparatus designed by Hirata & Fuwa (2007) and used by Melis et al. (2006a,b). If the rooks were found capable of cooperation, our aims were twofold. First, we aimed to investigate the effect of social tolerance and shareability of the food rewards on their performance, according to Melis et al. (2006a; experiment 1), and second, the cognitive underpinnings of their solution, specifically, whether or not the rooks were sensitive to the requirements of the apparatus and the need for a partner (experiment 2).
2. Experiment 1: can rooks cooperate to solve a problem?
Eight rooks were tested on the cooperative paradigm developed by Hirata (2003), and used by Melis et al. (2006a,b), to test chimpanzees.
(a) Methods
(i) Study animals
Eight rooks (two males; Connelly and Cook, and six females; Monroe, Fry, Guillem, Callas, Selvino and Cooper) were tested between September 2006 and March 2007, at an age of 3 years. They were members of a group that was collected under English Nature Permit 20030108 from two colonies in Cambridge on 16 and 17 April 2003 and hand-raised. The rooks were housed in an outdoor aviary approximately 20×8 m2. This aviary contains four testing rooms, 2×1 m2, which can be visually isolated from one another. A holding run, 6×1 m2, leads to each testing room where subjects waiting to be tested can sit in visual contact with the rest of the group. Tests were conducted in accordance with Home Office and University of Cambridge guidelines for animal use, and subjects could choose whether or not to participate at all times.
(ii) Apparatus
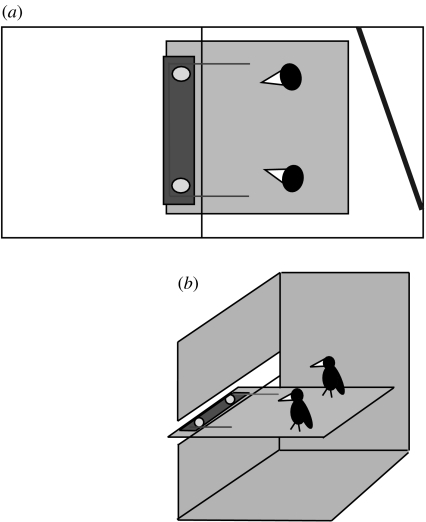
The apparatus consisted of a flat rectangular wooden platform (0.6×0.1 m2), which was placed outside the subjects' test room, on a shelf at a height of 1.5 m above the ground (figure 1). This shelf extended from the subjects' testing room into the adjoining room. Above the shelf there was a 5 cm high gap in the wire mesh of the room, which extended across the length of the shelf, through which the platform could be pulled across the shelf into the subjects' room (figure 1). Food was placed in circular plastic dishes (lids from jars of ‘Vegemite’, 6 cm diameter) attached to the ends of the food platform. A piece of string was threaded through metal loops on top of the platform, so that both ends of the string extended from the platform, under the 5 cm gap, into the subjects' test room. Pulling from only one end of the string was ineffectual because the string would become unthreaded without moving the platform. Only by pulling both ends of the string simultaneously could the subjects move the platform.
Figure 1.
Experiment 1: cooperation test. The experimental set-up is shown in (a) plan and (b) isometric views. The perch at the back of the test room seen in the plan view is not portrayed in the isometric view, for clarity.
(iii) Procedure
2.1.3.1 Familiarization
Before participating in the cooperation test, each subject was individually introduced to the cooperation apparatus. Rooks are highly neophobic, and so the first step was to present individuals with the apparatus baited with food (egg yolk and mealworms) until they were happy to feed from it as soon as it was pushed into their room. The next stage of familiarization was designed to allow the subjects to learn that both ends of the string must be pulled in order to move the platform. The food platform was placed outside the test room and baited with food. The string was placed in one of three positions, which differed in how far the two ends of the string were from each other: (i) overlapping, (ii) 1 cm apart, and (iii) 6 cm apart. It was always possible for a single subject to pick up both pieces of the string without pulling the string from the loops. In the overlapping condition, the subjects usually picked up both pieces of the string with one action. In the 1 cm condition, subjects could either (i) sweep the strings together with the mandibles of the beak, and then pick them up with one action, or (ii) pick up one end, hold it in the beak and then pick up the other before pulling. In the 6 cm condition, the subject had to pick up one end, hold it in its beak and then pick up the other before pulling, in order to be successful.
If the subject did not succeed in retrieving the food platform, the trial ended when either the string had been pulled out of the loops or the platform had not been pulled in after 5 min from the start of the trial. The subjects were given the three conditions in the order of difficulty, starting with the easiest (overlapping), until they had performed three successful trials in a row. They then proceeded to the next condition, and, after three consecutive failures, returned to the previous condition. They received between 10 and 20 trials a day. The subjects took between 3 and 10 days before they succeeded in the 6 cm condition on three consecutive trials, at which point familiarization ended.
2.1.3.2 Food-sharing tests
Each pair was first tested for their ability to share food, in order to assess inter-individual tolerance of the dyads. The food platform was baited in one of the following three ways, according to Melis et al. (2006a): (i) dispersed-divisible—both food dishes were each baited with five mealworms and five small pieces of egg yolk, (ii) clumped-divisible—one food dish was baited with 10 mealworms and 10 small pieces of egg yolk, and (iii) dispersed-solid—each food dish was baited with one egg yolk quarter. The rationale behind these different trial types was that they may reveal fine-grained differences in tolerance; members of a highly tolerant dyad may feed together irrespective of the trial type, whereas birds of intermediate tolerance may feed together only if the food is dispersed. Once the platform was baited, it was pushed into the subjects' room so that they could retrieve the food. Each dyad was given three trials (one of each type) at the start and the end of the cooperation test.
2.1.3.3 Cooperation test
After the individual introduction to the apparatus, and the first food-sharing test, each dyad participated in the cooperation test. The food platform was placed outside the subjects' test room, out of reach of the subjects and again baited in one of the three ways described above. The two ends of the string were now 60 cm apart. The length of each end of the string extending into the subjects' room was 12 cm, which was the maximum length that would not allow a single subject to bring both ends of the string together and pull in the platform alone. The trial started with the dyad in the test room, and the experimenter holding up both ends of the string outside the test room for 5 s, before placing them simultaneously into the room. If the dyad was unsuccessful, the trial ended either when the string had been pulled out of the loops or after 5 min from the start of the trial if the platform had not been pulled in. Subjects received six trials a day, two of each of the three baiting conditions, for a total of 10 days, resulting in a total of 60 trials. They were then re-paired with a different partner and again received six food-sharing (three before and three after the cooperation test) and 60 cooperation tests. The food-sharing and cooperation tests conducted on partnerships 1 and 2 are referred to as phases I and II, respectively.
(iv) Scoring and data analysis
A tolerance score was calculated from the food-sharing tests. In each trial, a dyad received a point when both birds (i) ate, (ii) ate simultaneously, rather than alternating turns at the tray, (iii) ate simultaneously from the same dish and (iv) actively shared food according to the definitions used in the studies of corvid food sharing (de Kort et al. 2006; von Bayern et al. 2007), i.e. if one bird inserted a piece of food into the beak or throat of the other. A point was deducted if there was any aggression, giving a maximum score of 4 and a minimum score of −1 for each of the sharing tests.
Dyads with a mean score between −1 and 0 would be regarded as intolerant, those with a mean score between 0 and 1 as merely tolerant; and those with a mean score above 1 as attracted. A mean score was calculated for the three trials before the cooperation test and the three trials after it. For the cooperation tests, the trials were scored as successful (the birds pulled in the platform) or unsuccessful (one of the birds pulled the string out of the loops), and a mean score out of 6 was calculated for each dyad from the 10 sessions.
All statistical tests reported are non-parametric and two tailed. We had only eight birds available for testing, and therefore investigated the effect of tolerance by retesting each individual with another partner from among the eight, because we could not look at a larger number of tolerant and intolerant dyads. Because the eight dyads that we investigated were made up from eight birds, paired in two different combinations, our data do not completely satisfy the conditions of independence. Despite this, in the correlation analyses, we treat dyads as independent units, because the individual birds performed differently when paired with different partners.
(b) Results and discussion
All pairs in the first phase of the experiment (table 1) were able to spontaneously solve the cooperation problem: two pairs in their first session of six trials (Cook & Fry, Selvino & Cooper); Guillem & Callas were successful in their second session; and Connelly & Monroe in their fourth. In this respect, their performance was similar to the tolerant chimpanzee dyads in the Melis et al. (2006a) study, which solved the task within their first session.
Table 1.
Dyad identities, tolerance scores and performances. (The four dyads in phase I (1a–d) and the four reshuffled dyads in phase II (2a–d) are shown alongside their tolerance scores before and after the cooperation tests, and their performance (percentage of trials in which they successfully obtained the food platform) in the cooperation test.)
| dyad no. | subject names | tolerance before | tolerance after | performance |
|---|---|---|---|---|
| 1a | Connelly & Monroe | 2.67 | 2.33 | 41.67 |
| 1b | Cook & Fry | 2.67 | 3.67 | 63.33 |
| 1c | Guillem & Callas | 2.67 | 2 | 43.33 |
| 1d | Selvino & Cooper | 0.33 | 2.33 | 41.67 |
| 2a | Connelly & Cook | −0.33 | 1.67 | 20 |
| 2b | Monroe & Fry | 0.67 | 1.67 | 15 |
| 2c | Guillem & Selvino | 2.67 | 1.67 | 73.33 |
| 2d | Callas & Cooper | 0.33 | 1.67 | 30 |
Unlike chimpanzees, and similar to bonobos (Hare et al. 2007), a single rook rarely monopolized all of the food in the food-sharing tests; instead, all of the rook pairs in phase I were able to feed within at least two of the three trials. However, there were differences in their levels of tolerance; whereas the pair that started with a sharing score of less than 1 (table 1) alternated feeding at the platform, displaying some aggression, those with a score of more than 1 fed simultaneously and from the same food dish. In addition, these pairs displayed some instances of active food sharing. Indeed, three of the four pairs were classified as ‘attracted’, having a score of more than 2 in the initial food-sharing test; only one pair had a score of less than 1. The tolerance scores are shown in the first four rows of table 1.
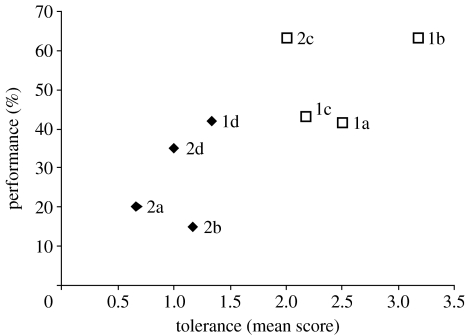
The data from phase I suggested that, as for chimpanzees, the level of tolerance between members of a dyad might affect their performance: for example, Cook & Fry, the most tolerant pair, was successful in the highest percentage of trials (table 1; figure 2). In phase II, we therefore reshuffled the pairs in a way that observations of their social group (Seed 2007) suggested would put each individual in the opposite type of relationship (table 1). Once again, both subjects in all of the new pairs were able to obtain food within at least two of their three food-sharing trials, but the other measures of tolerance revealed differences between the dyads. The initial food-sharing test revealed that there was now only one attracted pair; the other three pairs had a mean score of less than 1, and one of these, the male–male dyad, had a score of less than 0 and was therefore classified as intolerant (table 1).
Figure 2.
Relationship between tolerance and performance. Points are labelled with the dyad number (table 1). Squares are dyads with an ‘attracted’ starting score of more than 2 and diamonds are dyads with a starting score of less than 1. Performance is the percentage of successful trials.
The mean tolerance score (an average of the before and after scores) of the eight pairs correlated significantly with the percentage of trials in which they successfully obtained the food in the cooperation phase (Spearman's correlation test: ρ=0.752, p<0.05, n=8; figure 2).
There was no effect of the ease of monopolization of the food rewards. Wilcoxon signed-rank tests revealed no significant differences between the three types of baiting conditions (mean number of successful trials out of two for dispersed food in one dish=0.64, mean for dispersed food in two dishes=0.925, mean for clumped food in two dishes=0.9; p>0.05 for pairwise comparisons between all conditions).
The results of this experiment show that, like chimpanzees, rooks are capable of finding the solution to a problem that involves the cooperative action of two individuals and, more importantly, they can do so without training. Similar to the chimpanzees in Melis et al.'s study (2006a), tolerant dyads were able to solve the problem within the first session of testing, despite never having been explicitly rewarded for pulling just one string or coordinating their actions. The performance of the dyads correlated significantly with their food-sharing score. The importance of inter-individual tolerance between members of a dyad, over and above any individual differences, is made apparent by the fact that the eight dyads consisted of the same eight birds, all but one of which were paired in both an attracted dyad with a high food-sharing score and in a dyad with a sharing score of less than 1.
3. Experiment 2: cognition underpinning cooperation
While there is evidence that both rooks and chimpanzees form valuable cooperative alliances, their social groups differ in terms of their organization and stability. Chimpanzees live in dynamic social networks in which both long-term alliances and short-term coalitions are subject to change both over time (de Waal 1982) and with respect to the make-up of the group, which changes owing to their fission–fusion social structure (Mitani 2007). By contrast, the captive rooks studied to date have been shown to maintain long-term alliances with one or two individuals, making for a more stable society (Emery et al. 2007).
These differences in social system derive mainly from differences in mating system. Chimpanzees, like most mammals, are polygamous, while rooks are long-term monogamous and usually pair for life. Oviparous birds can often benefit from greater chick survival rates if two parents care for the young, and so social monogamy is favoured. However, the reproductive biology of mammals puts the burden of care on the female (gestation and lactation), and it often pays the male to look for further mating opportunities and leave the female to raise the offspring, meaning that polygyny is favoured in the majority of species. This may produce a corresponding difference in the potential advantages to be had from maintaining a network of relationships in the two groups. Numerous species of primates are reported to maintain a changing network of valuable relationships: between males, for competition for alpha status and therefore mating rights and between females, for protecting offspring from infanticidal males. In promiscuous chimpanzees, all non-related adult members of the opposite sex have value as potential mates. These mating-system-related advantages of a network of relationships do not apply to a monogamous species. For further discussion, see Seed et al. (2007).
The coexistence of both a qualitative similarity and a quantitative difference, between cooperative alliance formation in the two species provides a framework for making predictions with regard to the use of complex cognitive mechanisms for cooperation. Two hypotheses are conceivable, emphasizing the importance of either (i) the existence of social relationships, regardless of how many are there within a group or how stable are they over time or (ii) the existence of a network of relationships that vary both with regard to the identity of the group members and over time.
Social relationship hypothesis. The existence of cooperative relationships, which might be with just one or two individuals, selects for the evolution of complex cognitive abilities in order to improve the quality and effectiveness of the relationship.
Social variability hypothesis. The existence of a changing network of potentially cooperative and competitive relationships with a range of different individuals, or in other words a biological marketplace, provides the selective pressure for the evolution of complex cognitive abilities, in order to cooperate efficiently and in a way that optimizes personal gain, namely only when necessary, and only with effective partners that will share the profits of cooperative action, and/or reciprocate cooperative assistance.
Hypothesis (i) predicts that rooks, like chimpanzees, would be under selective pressure to evolve complex cognition in the cooperative context, as both form cooperative relationships. However, hypothesis (ii) predicts that while rooks might be able to cooperate to solve a task, their solution would not be as cognitively complex as that of chimpanzees, because promiscuous chimpanzees, but not monogamous rooks, live in a variable social market place in which one individual might cooperate with another on one day (e.g. two males might form an alliance to overthrow the alpha male) and compete with the same individual on another day (e.g. for access to a female).
We investigated whether or not the rooks had an understanding of the need of a partner for effective cooperation by seeing whether they could delay acting on the apparatus while their partner gained access to the test room (after Melis et al. 2006b). We also tested the subjects' understanding of the requirements of the apparatus, by giving them a choice between one that they could operate singly over one that required the actions of two individuals.
(a) Methods
(i) Procedure and apparatus
3.1.1.1 Delay test
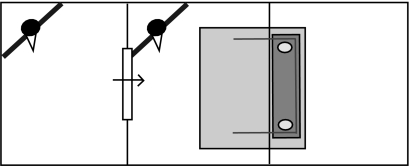
After completing phases I and II of the cooperation test, the subjects were tested to see whether they could delay pulling the string for the period of time it took their partner (the partner from phase I) to gain access to the test room from the adjoining one, through a one-way flap with some resistance. The experimental set-up is shown in figure 3. Before testing, all of the birds had an experience of opening the flap in order to get access to the test room, which was baited with food, until it took them all less than 10 s to do so. During a trial, the apparatus was baited, the focal subject allowed into the room and the flap unlocked so that the partner could push the flap to enter the test room. If the subjects were not successful, the trial ended either when the string had been pulled out of the loops or after 5 min from the start of the trial if the platform had not been pulled in. The subjects were given six trials a day, three as the focal bird and three as the partner bird in the adjoining room, for a total of 5 days, resulting in a total of 15 trials per subject.
Figure 3.
Experiment 2: delay test. The experimental set-up for the delay test shown in plan view. The one-way flap (depicted as a window with an arrow through it) was released by the experimenter once the birds were in the positions shown in the figure.
3.1.1.2 Choice test
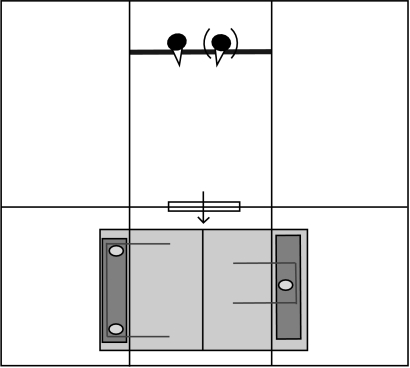
The subjects were given a choice between (i) a platform with the strings 6 cm apart, which could be pulled in by a single bird (single platform) and (ii) the platform used in the cooperation test, with the strings 60 cm apart (double platform). They were tested either alone or together with their partner from phase I. Figure 4 shows the experimental set-up. The single platform had one plastic dish, baited with four mealworms and one small piece of egg yolk. The double platform had two plastic dishes as before, each containing eight mealworms and two small pieces of egg yolk. When tested alone, the birds should have preferred to pull the single apparatus because it was not possible for them to get food from the double apparatus. When tested with their partner, they should have attempted to pull in the double apparatus because twice as much food was available per subject if they did so. The subjects were given four trials a day, for 10 days, in one of the following two conditions: individually or with their partner from phase I. The condition alternated day by day, so that by the end of the 10 days each subject had received 20 trials in each condition. The subject(s) began the trial in a holding run that was separated from the test room by an opaque wall (figure 4). The experimenter therefore baited both platforms out of sight of the subject(s). The side on which each platform was placed was randomized and counterbalanced, with the limitation that each platform appeared twice to the left and twice to the right. At the start of the trial, the experimenter opened a flap that allowed the birds to enter the test room. As soon as a subject pulled a string on one platform, the other platform was removed.
Figure 4.
Experiment 2: choice test. The experimental set-up for the choice test shown in plan view. The flap (depicted as a window) was opened by the experimenter at the start of the trial.
(b) Results and discussion
(i) Delay test
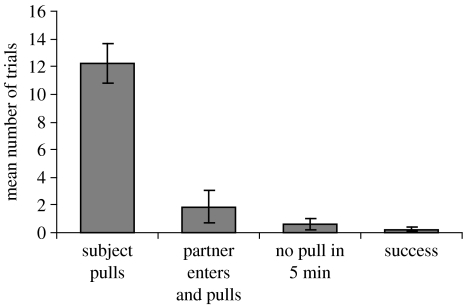
All of the subjects pulled the string without waiting for their partner to enter the room on the majority of trials (figure 5). On some occasions, the partner entered the room and pulled the string without the focal bird, while on others, neither the subject nor the partner pulled the string and on just two occasions, the pair was successful. However, not all birds were motivated to enter the test room quickly when they were not the focal subject; although two of the subjects entered the test room within 1 min on the majority of trials (Cook Guillem: 11 and 14 trials out of 15, respectively), the other six did not (Selvino, Monroe, Connelly, Fry, Callas and Cooper: 0, 5, 3, 3, 3 and 3 trials out of 15, respectively).
Figure 5.
Results of experiment 2: delay test. The mean number of trials (out of 15) in which each of the possible outcomes occurred. Error bars show standard errors.
(ii) Choice test
One of the pairs was not tested because Callas was not motivated to leave the holding run (from which the rest of the group can be seen) and enter the testing room. Connelly and Monroe had started breeding and were sitting on their nest, and so completed only 12 trials in each condition.
None of the pairs successfully pulled the strings in the double apparatus when tested together. When tested alone, four of the six birds showed no significant preference for either platform. In the first 10 trials, Cook and Selvino showed no significant preference for the single platform (Cook, 3 out of 10; Selvino, 7 out of 10); however, in the second half, binomial tests revealed that Selvino displayed a significant preference for the single platform (10 out of 10, p<0.05) and Cook had a non-significant tendency to prefer the single platform (8 out of 10, p=0.057).
The inability of the rooks to coordinate their actions in the delay test might reflect an inability to understand that the task required the actions of two individuals. However, the results of this test must be interpreted with caution, because six of the birds did not enter the test room within 1 min on the majority of trials when they were playing the role of the partner. A further limitation of the waiting test is the fact that it may confound understanding with inhibition. It is well documented that many animals find it very difficult to inhibit a learned response for even short periods of time in order to get food, and this may be an important limiting factor for the evolution of cooperative action (Stevens & Hauser 2004). The chimpanzees in the Melis et al. (2006b) study were able to wait for their partner, but the experimenters were able to control how long they had to wait, and rather than being successful at a long delay from the start, the subjects were given a number of shaping trials, beginning with a 5 s delay and building up through 10 and 20 s to a 30 s delay. Nevertheless, the chimpanzees made comparatively few errors. Two test subjects made no mistakes, two subjects made 3–4 mistakes while the other four subjects made between 12 and 28 mistakes before completing two consecutive successful trials at all of the delays.
However, the choice test did not require the birds to inhibit a learned response. The results from the paired condition of this test provided more evidence to support the idea that the birds were not able to coordinate their actions over a longer temporal and spatial distance, as none of the three dyads tested were able to fly in from the holding run and pull in the double platform together. Furthermore, in the individual test, four of the six subjects tested showed no preference for the single apparatus (which they could pull in by themselves) over the double apparatus (which required the actions of two individuals) when tested alone. Two birds did prefer to pull the single apparatus, but in both the cases they only had a significant preference in the second 10 trials of the test. It is therefore possible that they learned to pull in the single platform over the course of the experiment in response to the feedback they received (the single platform was removed after they had touched the double platform). This would be impressive, given that Selvino only made three errors, but corvids are known to be capable of rapid learning, from tests of physical cognition (Seed et al. 2006; Tebbich et al. 2007) to tests that require them to learn about perishability and pilfering (Clayton & Dickinson 1998). It therefore seems unlikely that the birds had an understanding of when cooperation was necessary to solve the task, unlike the chimpanzees tested in Melis et al. (2006b).
The results of this experiment suggest that, despite the striking similarities in the ability of rooks and chimpanzees to solve this cooperative task, as well as the effect of inter-individual tolerance, there may be fundamental differences between the cognitive mechanisms underpinning cooperative action in the two species. While chimpanzees have displayed an understanding of when cooperation is necessary (Melis et al. 2006b), there was no evidence that the rooks in this test had any such understanding. Overall, of the two hypotheses outlined in the introduction to experiment 2, the results of this experiment support the social variability hypothesis. However, differences in their ecology may also play a role. Chimpanzees display a high level of food competition for clumped high-quality food resources such as fruit and meat, while rooks derive the majority of their nutritional requirements from dispersed foods such as grains and insects.
However, the results of an experiment must always be interpreted with caution, and it will be important to test rooks with different paradigms before any firm conclusions can be made concerning the cognitive mechanisms underpinning cooperation in rooks.
4. General discussion
Rooks, like chimpanzees, are capable of solving a cooperative problem without training. Similar to chimpanzees, there was a correlation between performance on the cooperation test and food-sharing tendency; more tolerant dyads performed better on the task. However, unlike chimpanzees, the ease with which the food could be shared had no effect on the rooks' performance. This makes sense in the light of the difference in the overall tolerance levels between rook dyads when compared with chimpanzee dyads; while dominant chimpanzees would monopolize a single food dish, all of the rook pairs were able to feed in the food-sharing tests and the more tolerant dyads would do so from the same dish.
In contrast to the findings for chimpanzees (Melis et al. 2006b), we found no evidence in experiment 2 that rooks understood the requirements of the task, as they did not wait for their partner to gain access to the test room before pulling the string, and the majority of the subjects, when tested alone, did not choose an apparatus that they could operate singly over one that required the joint action of two individuals. This was perhaps surprising, given that both rooks and chimpanzees form tolerant relationships, which have been stressed as an important prerequisite for the evolution of cognition associated with cooperation (Hare & Tomasello 2005; Melis et al. 2006a; Hare et al. 2007). Perversely, it may be the competitive dimension of chimpanzee social living that favoured the evolution of complex cognitive mechanisms underpinning cooperation. For example, an understanding of when it is and is not necessary to cooperate may evolve in response to the pressure to behave optimally in a social environment that is made up of several different multifaceted relationships. Furthermore, an ability to chose not only when, but also with whom, to cooperate would allow chimpanzees to foster only those cooperative relationships that result in maximal personal gain. This biological marketplace may provide further selective pressure as ineffective collaborators lose out.
We therefore argue that while increasing levels of social tolerance may have been an important factor in the evolution of cognitively complex cooperation, the comparative perspective afforded by the current study highlights that competition and social variability may also play a crucial role. Further comparative work examining the cognition associated with cooperation in species living in different social and ecological environments is needed to test this notion. The abilities of bonobos, which have been found to cooperate in this paradigm (Hare et al. 2007), but which have not been tested in the recruitment tests of Melis et al. (2006b), would be particularly interesting, given that they have high levels of social tolerance, but low levels of food competition, compared with chimpanzees (Hare et al. 2007). Similarly, parrot species such as keas, which live in harsh environments and feed on scarce clumped food resources such as fruit and carrion (Diamond & Bond 1999), would be another interesting group. Keas have been shown to succeed on a cooperative task, although their success was the result of coercion by the dominant individual (Tebbich et al. 1996). The study of the proximate mechanisms underpinning cooperation in animals is in its infancy, due in part to the poor performances of animals such as chimpanzees in tests that did not take factors such as inter-individual tolerance into account. More work is needed to uncover the extents and limits of cooperative ability in rooks and chimpanzees, as well as in other species.
Acknowledgments
Tests were conducted in accordance with Home Office and University of Cambridge guidelines for animal use.
The research was supported by the BBSRC and the University of Cambridge. A.M.S. was supported by a BBSRC Postgraduate Studentship and N.J.E. by a Royal Society University Research Fellowship. We would like to thank Charmaine Donovan for her care of the rooks, Ian Millar for building the apparatus, and Anne Helme, Bill McGrew and Juan Carlos Gomez for their helpful comments on an earlier version of the manuscript.
References
- Boesch C, Boesch H. Hunting behaviour of wild chimpanzees in the Tai Forest national park. Am. J. Phys. Anthropol. 1989;78:547–573. doi: 10.1002/ajpa.1330780410. doi:10.1002/ajpa.1330780410 [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Heinrich B. Ravens, Corvus corax, differentiate between knowledgable and ignorant competitors. Proc. R. Soc. B. 2005;272:1641–1646. doi: 10.1098/rspb.2005.3144. doi:10.1098/rspb.2005.3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T, Kotrschal K. Leading a conspecific away from food in ravens (Corvus corax)? Anim. Cogn. 2004;7:69–76. doi: 10.1007/s10071-003-0189-4. doi:10.1007/s10071-003-0189-4 [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Stowe M, Heinrich B. Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc. R. Soc. B. 2004;271:1331–1336. doi: 10.1098/rspb.2004.2738. doi:10.1098/rspb.2004.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmeau R. Do chimpanzees cooperate in a learning task? Primates. 1994;35:385–392. doi:10.1007/BF02382735 [Google Scholar]
- Chalmeau R, Visalberghi E, Gallo A. Capuchin monkeys, Cebus apella, fail to understand a cooperative task. Anim. Behav. 1997;54:1215–1225. doi: 10.1006/anbe.1997.0517. doi:10.1006/anbe.1997.0517 [DOI] [PubMed] [Google Scholar]
- Clayton N.S, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. doi:10.1038/26216 [DOI] [PubMed] [Google Scholar]
- Clayton N.S, Dally J.M, Emery N.J. Social cognition by food-caching corvids. The western scrub-jay as a natural psychologist. Phil. Trans. R. Soc. B. 2007;362:507–522. doi: 10.1098/rstb.2006.1992. doi:10.1098/rstb.2006.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs C.J.F. Rookeries and roosts of the rook and jackdaw in south-west Cornwall. Part I. Population distribution and rookeries. Bird Study. 1961;8:32–37. [Google Scholar]
- Crawford M.P. The cooperative solving of problems by young chimpanzees. Comp. Psychol. Monogr. 1937;14:1–88. [Google Scholar]
- Cronin K.A, Kurian A.V, Snowdon C.T. Cooperative problem solving in a cooperatively breeding primate (Saguinus oedipus) Anim. Behav. 2005;69:133–142. doi: 10.1016/j.anbehav.2004.02.024. doi:10.1016/j.anbehav.2004.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dally J.M, Emery N.J, Clayton N.S. Food-caching western scrub-jays keep track of who was watching when. Science. 2006;312:1662–1665. doi: 10.1126/science.1126539. doi:10.1126/science.1126539 [DOI] [PubMed] [Google Scholar]
- de Kort S.R, Emery N.J, Clayton N.S. Food sharing in jackdaws, Corvus monedula: what, why and with whom? Anim. Behav. 2006;72:297–304. doi:10.1016/j.anbehav.2005.10.016 [Google Scholar]
- de Waal F.B. Johnathon Cape; London, UK: 1982. Chimpanzee politics. [Google Scholar]
- Diamond J, Bond A. University of California Press; Berkely, CA: 1999. Kea, bird of paradox: the evolution and behaviour of a New Zealand parrot. [Google Scholar]
- Emery N.J, Clayton N.S. The mentality of crows: convergent evolution of intelligence in corvids and apes. Science. 2004;306:1903–1907. doi: 10.1126/science.1098410. doi:10.1126/science.1098410 [DOI] [PubMed] [Google Scholar]
- Emery N.J, Seed A.M, von Bayern A.M, Clayton N.S. Cognitive adaptations of social bonding in birds. Phil. Trans. R. Soc. B. 2007;362:489–505. doi: 10.1098/rstb.2006.1991. doi:10.1098/rstb.2006.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B, Tomasello M. Human-like social skills in dogs? Trends Cogn. Sci. 2005;9:439–444. doi: 10.1016/j.tics.2005.07.003. doi:10.1016/j.tics.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Hare B, Melis A.P, Woods V, Hastings S, Wrangham R.W. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. doi:10.1016/j.cub.2007.02.040 [DOI] [PubMed] [Google Scholar]
- Hirata S. Cooperation in chimpanzees. Hattatsu. 2003;95:103–111. [Google Scholar]
- Hirata S, Fuwa K. Chimpanzees (Pan troglodytes) learn to act with other individuals in a cooperative task. Primates. 2007;48:13–21. doi: 10.1007/s10329-006-0022-1. doi:10.1007/s10329-006-0022-1 [DOI] [PubMed] [Google Scholar]
- Melis A.P, Hare B, Tomasello M. Engineering cooperation in chimpanzees: tolerance constraints on cooperation. Anim. Behav. 2006a;72:275–286. doi:10.1016/j.anbehav.2005.09.018 [Google Scholar]
- Melis A.P, Hare B, Tomasello M. Chimpanzees recruit the best collaborators. Science. 2006b;311:1297–1300. doi: 10.1126/science.1123007. doi:10.1126/science.1123007 [DOI] [PubMed] [Google Scholar]
- Mendres K.A, de Waal F.B.M. Capuchins do cooperate: the advantage of an intuitive task. Anim. Behav. 2000;60:523–529. doi: 10.1006/anbe.2000.1512. doi:10.1006/anbe.2000.1512 [DOI] [PubMed] [Google Scholar]
- Mitani, J. C. 2007 Chimpanzee minds in nature: lessons from Ngogo. In Int. Conf. on The mind of the chimpanzee Chicago, Illinois.
- Moll H, Tomasello M. Cooperation and human cognition: the Vygotskian intelligence hypothesis. Phil. Trans. R. Soc. B. 2007;362:639–648. doi: 10.1098/rstb.2006.2000. doi:10.1098/rstb.2006.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe R. Cooperation experiments: coordination through communication versus acting apart together. Anim. Behav. 2006;71:1–18. doi:10.1016/j.anbehav.2005.03.037 [Google Scholar]
- Petit O, Desportes C, Thierry B. Differential probability of coproduction in two species of macaque (Macaca tonkeana, M. mulatta) Ethology. 1992;90:107–120. [Google Scholar]
- Seed, A. M. 2007 Cognition in rooks and chimpanzees: a case of convergent evolution? In Department of Experimental Psychology PhD thesis, p. 233. University of Cambridge, Cambridge.
- Seed A.M, Tebbich S, Emery N.J, Clayton N.S. Investigating physical cognition in rooks, Corvus frugilegus. Curr. Biol. 2006;16:697–701. doi: 10.1016/j.cub.2006.02.066. doi:10.1016/j.cub.2006.02.066 [DOI] [PubMed] [Google Scholar]
- Seed A.M, Clayton N.S, Emery N.J. Third-party postconflict affiliation in rooks, Corvus frugilegus. Curr. Biol. 2007;17:152–158. doi: 10.1016/j.cub.2006.11.025. doi:10.1016/j.cub.2006.11.025 [DOI] [PubMed] [Google Scholar]
- Stevens J.R, Hauser M.D. Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn. Sci. 2004;8:60–65. doi: 10.1016/j.tics.2003.12.003. doi:10.1016/j.tics.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Tebbich S, Taborsky M, Winkler H. Social manipulation causes cooperation in keas. Anim. Behav. 1996;52:1–10. doi:10.1006/anbe.1996.0147 [Google Scholar]
- Tebbich S, Seed A.M, Emery N.J, Clayton N.S. Non-tool-using rooks (Corvus frugilegus) solve the trap-tube task. Anim. Cogn. 2007;10:225–231. doi: 10.1007/s10071-006-0061-4. doi:10.1007/s10071-006-0061-4 [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain Sci. 2005;28:675–735. doi: 10.1017/S0140525X05000129. doi:10.1017/S0140525X05000129 [DOI] [PubMed] [Google Scholar]
- Visalberghi E, Quarantotti B.P, Tranchida F. Solving a cooperation task without taking into account the partner's behavior: the case of capuchin monkeys (Cebus apella) J. Comp. Psychol. 2000;114:297–301. doi: 10.1037/0735-7036.114.3.297. doi:10.1037/0735-7036.114.3.297 [DOI] [PubMed] [Google Scholar]
- von Bayern, A. M. P. & Emery, N. J. Submitted. Hand-reared jackdaws recognise human attentional and communicative states. [DOI] [PubMed]
- von Bayern A.M.P, de Kort S.R, Clayton N.S, Emery N.J. The role of food- and object- sharing in the development of social bonds in juvenile jackdaws (Corvus monedula) Behaviour. 2007;144:711–733. doi:10.1163/156853907781347826 [Google Scholar]