Abstract
Metabolic rate is traditionally assumed to scale with body mass to the 3/4-power, but significant deviations from the ‘3/4-power law’ have been observed for several different taxa of animals and plants, and for different physiological states. The recently proposed ‘metabolic-level boundaries hypothesis’ represents one of the attempts to explain this variation. It predicts that the power (log–log slope) of metabolic scaling relationships should vary between 2/3 and 1, in a systematic way with metabolic level. Here, this hypothesis is tested using data from birds and mammals. As predicted, in both of these independently evolved endothermic taxa, the scaling slope approaches 1 at the lowest and highest metabolic levels (as observed during torpor and strenuous exercise, respectively), whereas it is near 2/3 at intermediate resting and cold-induced metabolic levels. Remarkably, both taxa show similar, approximately U-shaped relationships between the scaling slope and the metabolic (activity) level. These predictable patterns strongly support the view that variation of the scaling slope is not merely noise obscuring the signal of a universal scaling law, but rather is the result of multiple physical constraints whose relative influence depends on the metabolic state of the organisms being analysed.
Keywords: allometry, birds, body mass, constraints, mammals, metabolic scaling
1. Introduction
All biological activities depend on metabolic energy, and thus understanding why rates of metabolism vary is of fundamental importance. A major factor affecting metabolic rate is body size. Respiratory metabolic rate (R) typically scales with body mass (M) according to the power function R=aMb, where a is a normalization constant (antilog of the intercept in a log–log plot) and b is the scaling exponent (slope in a log–log plot). Rubner (1883) observed that the scaling exponent b was 2/3 in dogs of different size, which he explained using the theory of Sarrus & Rameaux (1839: cited in McNab 2002). According to this theory, to maintain a constant body temperature, endothermic animals must metabolically produce enough body heat to exactly balance the amount of heat lost through their body surface. Therefore, since body surface scales as M2/3, so should metabolic rate. However, in broader comparisons of different species of mammals, Kleiber (1932) found that b was closer to 3/4 than 2/3. Since that time, it has been commonly assumed that b is typically 3/4, a generalization known as ‘Kleiber's law’ or the ‘3/4-power law’ (Brody 1945; Hemmingsen 1960; Kleiber 1961; Peters 1983; Calder 1984; Schmidt-Nielsen 1984). Recently, it has even been claimed that scaling based on multiples of a 1/4-power is universal, or nearly so, not only for metabolic rate, but also for the rates and durations of other biological processes dependent on metabolic energy (Brown et al. 2004; Savage et al. 2004; West & Brown 2005). In addition, influential theoretical models have been proposed to explain quarter-power scaling, thus further reifying the 3/4-power law (West et al. 1997, 1999; Banavar et al. 1999, 2002).
However, the universal status of the 3/4-power law has been seriously weakened by recent empirical and theoretical work. First, several rigorous empirical analyses, involving body sizes spanning several orders of magnitude, have shown that b often deviates substantially from 3/4, varying significantly among different taxonomic groups of animals and plants (Glazier 2005; Reich et al. 2006; White et al. 2006, 2007), and among different physiological states (Glazier 2005; Niven & Scharlemann 2005; White & Seymour 2005; Makarieva et al. 2005a, 2006b). Second, the models supporting the so-called 3/4-power law appear to have flawed assumptions and serious mathematical inconsistencies that have not yet been resolved, despite much debate (Dodds et al. 2001; Kozlowski & Konarzewski 2004, 2005; Brown et al. 2005; Makarieva et al. 2005b, 2006a; Painter 2005b,c; West & Brown 2005; Banavar et al. 2006; Chaui-Berlinck 2006, 2007; Etienne et al. 2006; Savage et al. 2007).
As a result, Glazier (2005) proposed a new model, the metabolic-level boundaries (MLB) hypothesis, to help explain the extensive variation in metabolic scaling that has been observed (other models are also reviewed in Glazier 2005). According to the MLB hypothesis, the scaling slope b should vary between two extreme boundary limits: 2/3 as a result of surface-related constraints on fluxes of resources, wastes and heat, and 1 as a result of mass (volume) constraints on energy use or power production (cf. Kooijman 2000). Variation in b is mediated by the overall metabolic level of the organisms being considered, which determines the relative influences of surface area and volume on the scaling of metabolic rate. In resting organisms, b should be negatively related to metabolic level because when maintenance costs are high, metabolic scaling should be chiefly limited by fluxes of resources, wastes and (or) heat across surfaces (scaling as M2/3; see also §4), whereas when they are low and amply met by surface-dependent processes, metabolic scaling should be more related to the energy demand required to sustain the tissues, which is directly proportional to tissue mass or volume (scaling as M1). This negative relationship between b and metabolic level should extend to dormant or ectothermic organisms with very low metabolic levels, where b should be near 1, and to cold-exposed endothermic animals with relatively high metabolic rates, where b should be near 2/3. However, in active animals, b should be positively related to metabolic level because as activity increases metabolic rate is increasingly affected by the energy demand of muscular tissue, which scales in direct proportion to muscle mass, which in turn scales as M1 (Calder 1984; Weibel et al. 2004; Glazier 2005). During bursts of maximal activity, b should approach 1 because metabolic rate is chiefly driven by the resource demand of metabolizing tissues, rather than by surface-dependent resource supply or waste removal (cf. Hammond & Diamond 1997; Weibel & Hoppeler 2005). This is briefly made possible by stored oxygen and energy in the muscle tissues and their temporary tolerance to accumulation of wastes (e.g. lactic acid). Overall, the MLB hypothesis predicts a U-shaped (or V-shaped) relationship between the metabolic scaling slope b and metabolic level.
Here, the MLB hypothesis is tested in birds and mammals, the only taxa for which sufficient data were available on metabolic scaling in all of the several physiological states mentioned above.
2. Material and methods
The MLB hypothesis was tested by examining metabolic scaling relationships that were based on the largest, most taxonomically comprehensive datasets available, and those which represented a diversity of statistical methods. Most scaling relationships included 10 or more species (table 1), as well as body mass ranges exceeding two orders of magnitude (figure 1), except for torpid birds (N=8; body mass range=1.37 orders of magnitude). Minimal metabolic rates were represented by torpid animals at the lowest body temperatures for which sufficient data were available (5°C for mammals and 15–16°C for birds). Resting metabolic rates (RMRs) were measured under basal conditions (White et al. 2006). Field metabolic rates were estimated in free-living animals using the doubly labelled water method (Nagy et al. 1999). Maximal metabolic rates (MMRs) of thermoregulation were estimated by exposure to cold in a He–O2 atmosphere (Rezende et al. 2002; White & Seymour 2005), whereas that of locomotion were estimated in strenuously running mammals and flying or running birds (Bishop 1999; Weibel et al. 2004). The datasets featured in figure 1 were taken from the most rigorous and comprehensive studies available, though similar patterns are observed for other datasets as well (see table 1; and White et al. 2007). Most of the scaling relationships were calculated using conventional least-squares regression (LSR). Scaling slopes determined by other statistical procedures were almost always very close to that determined by LSR (table 1; see also §4). Relative metabolic levels of the scaling relationships for different physiological states were calculated for animals with 50 g wet body mass, because this intermediate value is well within the body mass ranges of all the scaling relationships analysed here. This method was deemed adequate because it yielded similar results to those based on other methods of estimating metabolic level (e.g. the intercept at 1 g mass; and the mass-specific metabolic rate at the midpoint of each regression line; see also §4).
Table 1.
Scaling slopes (b±95% confidence limits) and antilog intercepts (a) of log metabolic rate (ml O2 h−1) versus log wet body mass (g) of birds and mammals in various physiological states. (The b values (p<0.05 in all cases) were determined by conventional LSR analysis with each species regarded as an independent data point, except where indicated (PIC, phylogenetically independent contrasts; RMA, reduced major axis analysis; MA, major axis analysis; RRA, robust regression analysis; BBM, binned body mass classes). N is the sample size, which is the number of species, or number of estimates with number of species in parentheses. The b values in italics are those used in figure 1.)
| physiological state | taxon | b | a | N | source |
|---|---|---|---|---|---|
| low metabolic level | |||||
| hibernation (5°C) | mammals | 0.941±0.086 | 0.047 | 29 | Geiser (1988) |
| hibernation (20°C) | mammals | 0.794±0.064 | 0.373 | 36 | Geiser (1988) |
| hibernation (4.3–10.1°C) | mammals | 0.879±0.082 | 0.044 | 27 (16) | Weiner (1989) |
| torpor (15–16°C) | birds | 1.028±0.210 | 0.155 | 8 | Lasiewski (1963), Lasiewski & Lasiewski (1967) and Lasiewski et al. (1967) |
| resting metabolic level | mammals | 0.676±0.013 | 4.98 | 456 | White et al. (2006) |
| mammals (RMA) | 0.69±0.013 | 5.10 | 456 | White et al. (2006) | |
| mammals (BBM) | 0.737±0.026 | 3.25 | 52 (626) | Savage et al. (2004) | |
| mammals | 0.690 | 4.11 | 487 | Lovegrove (2000) | |
| mammals (RRA) | 0.678±0.014 | 391 | Heusner (1991) | ||
| mammals | 0.693±0.022 | 4.36 | 293 | Hayssen & Lacy (1985) | |
| birds | 0.64±0.03 | 6.07 | 83 | White et al. (2006) | |
| birds (RMA) | 0.66±0.03 | 5.36 | 83 | White et al. (2006) | |
| birds | 0.669±0.030 | 6.17 | 126 | McKechnie & Wolf (2004) | |
| birds (PIC) | 0.677 | 4.67 | 126 | McKechnie & Wolf (2004) | |
| birds | 0.635±0.043 | 8.51 | 37 | Rezende et al. (2002) | |
| birds (PIC) | 0.721±0.087 | 5.70 | 37 | Rezende et al. (2002) | |
| birds | 0.68±0.06 | 8.92 | 45 | Frappell et al. (2001) | |
| birds (PIC) | 0.68±0.10 | 8.43 | 44 | Frappell et al. (2001) | |
| birds | 0.638±0.028 | 7.75 | 82 | Tieleman & Williams (2000) | |
| birds (PIC) | 0.677±0.054 | 5.38 | 82 | Tieleman & Williams (2000) | |
| birds | 0.677 | 6.98 | 263 | Daan et al. (1991) | |
| birds (MA) | 0.67±0.03 | 4.86 | 399 | Bennett & Harvey (1987) | |
| field metabolic level | mammals | 0.734±0.038 | 9.94 | 79 | Nagy et al. (1999) |
| mammals (RMA) | 0.75±0.04 | 4.53 | 79 | White & Seymour (2005) | |
| mammals (BBM) | 0.749±0.054 | 9.31 | 35 (79) | Savage et al. (2004) | |
| mammals | 0.72 | 1.40 | 111 (86) | Anderson & Jetz (2005) | |
| mammals (PIC) | 0.73 | 1.40 | 110 (86) | Anderson & Jetz (2005) | |
| birds | 0.681±0.036 | 21.66 | 95 | Nagy et al. (1999) | |
| birds | 0.68 | 2.10 | 132 (96) | Anderson & Jetz (2005) | |
| birds (PIC) | 0.67 | 2.10 | 131 (96) | Anderson & Jetz (2005) | |
| birds | 0.703±0.042 | 19.84 | 81 | Tieleman & Williams (2000) | |
| birds (PIC) | 0.671±0.065 | 16.65 | 81 | Tieleman & Williams (2000) | |
| high metabolic level cold-exposed | mammals | 0.65±0.05 | 31.56 | 70 | White & Seymour (2005) |
| mammals (RMA) | 0.68±0.05 | 28.3 | 70 | White & Seymour (2005) | |
| mammals | 0.668±0.060 | 30.34 | 56 (28) | Weiner (1989) | |
| birds | 0.600±0.033 | 56.10 | 47 | Rezende et al. (2002) | |
| birds (PIC) | 0.651±0.088 | 44.36 | 47 | Rezende et al. (2002) | |
| high metabolic level strenuous activity | mammals | 0.872±0.059 | 17.17 | 34 | Weibel et al. (2004) |
| mammals (BBM) | 0.828±0.070 | 26.06 | 21 (28) | Savage et al. (2004) | |
| athletic | |||||
| mammals | 0.942±0.050 | 17.11 | 10 | Weibel et al. (2004) | |
| mammals | 0.857±0.040 | 18.00 | 67 (45) | Weiner (1989) | |
| mammals | 0.841±0.045 | 22.93 | 18 | Koteja (1987) | |
| birds & mammals | 0.879±0.020 | 35.02 | 15 | Bishop (1999) | |
| birds | 0.837±0.107 | 33.65 | 39 (35) | Norberg (1996) |
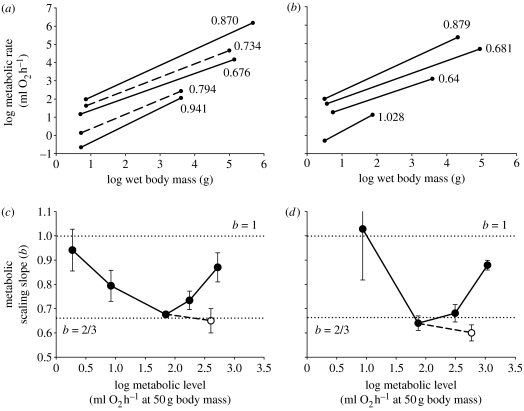
Figure 1.
Scaling of metabolic (respiration) rate in relation to wet body mass in (a,c) mammals and (b,d) birds exhibiting various physiological states (data from sources in table 1). (a,b) The dots at the ends of each log–log least-squares regression line denote the minimum and maximum body masses for each sample. The scaling slopes of solid lines are significantly different from 3/4, whereas the slopes of dashed lines are not significantly different from 3/4. The physiological states for the scaling lines are in ascending order for mammals: hibernating at 5°C, torpid at 20°C, resting, field active and maximally active; and for birds: torpid at 15–16°C, resting, field active and maximally active. (c,d) Scaling slopes (b±95% confidence limits) versus metabolic level at 50 g body mass. Filled circles denote various levels of activity from minimal to maximal, whereas open circles indicate cold-exposed metabolic rates. For the different levels of activity, note the approximately U-shaped relationship shown for both mammals and birds with extreme values of the scaling slopes near 2/3 and 1 (indicated by dotted lines), as predicted by the MLB hypothesis.
3. Results
As predicted by the MLB hypothesis, the metabolic scaling slopes (b) of mammals and birds in various states of activity vary mostly between 2/3 and 1, and with an approximately U-shaped relationship with metabolic level (figure 1c,d, table 1). In both taxa, b approaches 1 at the lowest and highest metabolic levels, and is near 2/3 for resting and cold-exposed animals, as predicted.
4. Discussion
The relationships between the scaling slope (b) and metabolic level are very similar in mammals and birds, and thus represent a remarkable case of convergent evolution between these independently evolved endothermic taxa. These patterns are robust as they are seen regardless of what statistical method is used to estimate the scaling parameters (see table 1; and also below).
The relationships between b and metabolic level are also seen regardless of the body mass used for comparison, or the method used to estimate metabolic level. This is because the relative elevation of the scaling lines observed at various activity levels are maintained over the entire ranges of body masses analysed (figure 1a,b). Therefore, for this and other reasons, the relationships observed between b and metabolic level are not statistical artefacts, as might be expected for closely proximate regression lines that intersect one another (Glazier 2005).
Although in most cases the statistical method used appears to have little effect on the present results, one deviation deserves special discussion. By using a ‘binning’ procedure that gives equal weight to all body size intervals, Savage et al. (2004) showed that the RMR of mammals scales with a slope of 0.737, which is significantly different from 2/3, unlike that shown by most other recent analyses (table 1). However, this deviant result appears to be a statistical artefact because, since the larger size intervals contain far fewer species than the smaller size intervals, the binning procedure actually gives greater weight to each large mammal species, which collectively are known to have a steeper scaling slope than smaller mammals (Hayssen & Lacy 1985; Heusner 1991; Lovegrove 2000; Dodds et al. 2001; Glazier 2005; Kozlowski & Konarzewski 2005).
In mammals, the dependence of b on body size interval may be a function, at least in part, of including large herbivores in the scaling analysis. Since large herbivores process their food for long periods of time, it is probable that short-term fasting does not completely remove the heat increment of feeding (as required for basal conditions), thus artificially elevating their metabolic rate, which in turn causes b to be biased upward (White & Seymour 2005). Removing large herbivores from the analysis results in the overall scaling exponent of mammals being indistinguishable from 2/3 (White & Seymour 2005).
Alternatively, since fur insulation in large mammals is more effective in preventing heat loss (Heldmaier 1989), the effect of surface area constraints (proportional to M2/3) on their metabolic scaling may be reduced relative to that for small mammals, thus causing b to be more related to tissue demand (proportional to M1). This interpretation nicely fits into the framework of the MLB hypothesis, and is consistent with the observation that b in the largest mammals appears to approach 1 (Makarieva et al. 2003; Painter 2005a).
An additional noteworthy result of this study is that in both birds and mammals, b increases significantly with increases in locomotor activity, but does not with increases in thermoregulatory demands resulting from exposure to cold. As predicted by the MLB hypothesis, MMRs during locomotion are more related to (muscle) tissue demand (proportional to M1), whereas that during thermoregulation are more related to surface-dependent loss of heat (proportional to M2/3). These differences may help explain why some studies have found that MMR is a constant multiple of RMR, whereas others have found that the ratio of MMR to RMR varies with body size. To facilitate understanding, the effects of locomotor and thermoregulatory demands on metabolic rate should be kept separate, which has not always been done in the past.
The observation that resting and cold-induced metabolic rates in both mammals and birds tend to scale as M∼2/3 further suggests that, during these physiological states, external surface area constraints on heat loss (scaling as M2/3: Rubner 1883; Calder 1984; Reynolds 1997; White & Seymour 2005) predominate over internal surface area constraints, which are expected to scale as M3/4 according to theory (West et al. 1997, 1999). The ability of birds and mammals to use a variety of behavioural and physiological mechanisms to regulate heat loss does not abolish the influence of surface area, as claimed by West & Brown (2005). The effect of surface area may still be prominent, especially if these mechanisms are employed equally (or nearly so) among species of different body size. This is expected to be true for the highly controlled thermal conditions at which the basal and cold-induced metabolic rates of birds and mammals are measured.
It is also unlikely that the relatively high b of MMR in running mammals (and other active animals) is a simple result of a positive correlation between body temperature and mass, as claimed by Gillooly & Allen (2007). First, as Gillooly & Allen (2007) themselves admit, their postulated effect of temperature on metabolic rate can explain only approximately 50% or less of the difference in b between the resting and maximally active conditions, if the most current and comprehensive datasets on mammals are examined (resting b=0.68; active b=0.87: see table 1). This is true even if the athletic species are removed from the sample (the scaling exponent for MMR is still relatively high: 0.85; Weibel et al. 2004).
Second, and more importantly, data on the relative timing of changes in metabolic rate and body temperature in running horses strongly suggest that muscle activity increases metabolic rate (heat production), which then elevates body temperature, the reverse of the causation emphasized by Gillooly & Allen (2007; Mukai et al. in press; J. H. Jones, H. Hoppeler & E. R. Weibel 2007, personal observations). These data show that once running starts, metabolic rate increases to peak levels almost immediately, whereas an increase in muscle temperature lags behind and does not peak until after running has ceased. Furthermore, once a peak metabolic rate is reached, it is unaffected by the later increase in muscle temperature.
Third, steep scaling of MMR is also observed in ectothermic vertebrates, despite their showing little change in body temperature during activity (Glazier 2005).
Other patterns of metabolic scaling in mammals and birds also support the MLB hypothesis. As predicted, heterotherms, desert dwellers, and ‘ectothermic’ African mole rats and newly born or hatched young, all of which have a relatively low RMR, tend to show relatively steep scaling slopes (Glazier 2005). The higher b for MMR in athletic versus non-athletic mammals (0. 94 versus 0.85; Weibel et al. 2004) also conforms to the MLB hypothesis, because the relative effect of muscle resource demand (scaling as M1) on b is predicted to be higher in relatively muscular athletic mammals.
The effect of metabolic level on b is even seen within species. For example, in the laboratory rat, an increase of activity level results in a significant increase in b, whereas cold exposure does not change b (Refinetti 1989), just as observed at the interspecific level. Also in humans, increases in activity cause significant increases in b, with values approaching 1 during maximal exercise (Rogers et al. 1995; Batterham & Jackson 2003).
This and other evidence provided by Glazier (2005), Makarieva et al. (2005a), Niven & Scharlemann (2005), Reich et al. (2006) and White et al. (2006, 2007) strongly support the MLB hypothesis, but is inconsistent with the 3/4-power law and models proposed to explain it. In fact, of the 37 b values in table 1 with calculated 95% confidence limits, 78% (29) are significantly different from 3/4. Therefore, metabolism does not scale with body mass according to a single universal law, but rather appears to depend on multiple physical constraints, whose relative influence depends on the metabolic state of the organisms being analysed. In essence, the scaling of metabolism with body mass itself scales with overall metabolic (activity) level.
Acknowledgments
I thank David Atkinson, Hans Hoppeler, Ewald Weibel and Craig White for useful discussions about metabolic scaling; James Jones, Hans Hoppeler and Ewald Weibel for access to their unpublished data on metabolic rate and body temperature in running horses; and Anastassia Makarieva and an anonymous referee for helpful comments on the manuscript.
References
- Anderson K.J, Jetz W. The broad-scale ecology of energy expenditure of endotherms. Ecol. Lett. 2005;8:310–318. doi:10.1111/j.1461-0248.2005.00723.x [Google Scholar]
- Banavar J.R, Maritan A, Rinaldo A. Size and form in efficient transport networks. Nature. 1999;399:130–134. doi: 10.1038/20144. doi:10.1038/20144 [DOI] [PubMed] [Google Scholar]
- Banavar J.R, Damuth J, Maritan A, Rinaldo A. Supply–demand balance and metabolic scaling. Proc. Natl Acad. Sci. USA. 2002;99:10 506–10 509. doi: 10.1073/pnas.162216899. doi:10.1073/pnas.162216899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banavar J.R, Damuth J, Maritan A, Rinaldo A. Comment on “revising the distributive networks models of West, Brown and Enquist (1997) and Banavar, Maritan and Rinaldo (1999): metabolic inequity of living tissues provides clues for the observed allometric scaling rules” by Makarieva, Gorshkov and Li. J. Theor. Biol. 2006;239:391–393. doi: 10.1016/j.jtbi.2005.08.023. doi:10.1016/j.jtbi.2005.08.023 [DOI] [PubMed] [Google Scholar]
- Batterham A.M, Jackson A.S. Validity of the allometric cascade model at submaximal and maximal metabolic rates in exercising men. Respir. Physiol. Neurobiol. 2003;135:103–106. doi: 10.1016/s1569-9048(03)00027-2. doi:10.1016/S1569-9048(03)00027-2 [DOI] [PubMed] [Google Scholar]
- Bennett P.M, Harvey P.H. Active and resting metabolism in birds: allometry, phylogeny and ecology. J. Zool. 1987;213:327–363. [Google Scholar]
- Bishop C.M. The maximum oxygen consumption and aerobic scope of birds and mammals: getting to the heart of the matter. Proc. R. Soc. B. 1999;266:2275–2281. doi: 10.1098/rspb.1999.0919. doi:10.1098/rspb.1999.0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. Reinhold; New York, NY: 1945. Bioenergetics and growth. [Google Scholar]
- Brown J.H, Gillooly J.F, Allen A.P, Savage V.M, West G.B. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. doi:10.1890/03-9000 [Google Scholar]
- Brown J.H, West G.B, Enquist B.J. Yes, West, Brown and Enquist's model of allometric scaling is both mathematically correct and biologically relevant. Funct. Ecol. 2005;19:735–738. doi:10.1111/j.1365-2435.2005.01022.x [Google Scholar]
- Calder W.A. Harvard University Press; Cambridge, MA: 1984. Size, function, and life history. [Google Scholar]
- Chaui-Berlinck J.G. A critical understanding of the fractal model of metabolic scaling. J. Exp. Biol. 2006;209:3045–3054. doi: 10.1242/jeb.02362. doi:10.1242/jeb.02362 [DOI] [PubMed] [Google Scholar]
- Chaui-Berlinck J.G. Response to ‘comment on “a critical understanding of the fractal model of metabolic scaling”’. J. Exp. Biol. 2007;210:3875–3876. doi: 10.1242/jeb.02362. doi:10.1242/jeb.006858 [DOI] [PubMed] [Google Scholar]
- Daan S, Masman D, Strijkstra A.M, Kenagy G.J. Daily energy turnover during reproduction in birds and mammals: its relationship to basal metabolic rate. Int. Ornithol. Congr. 1991;20:1976–1987. [Google Scholar]
- Dodds P.S, Rothman D.H, Weitz J.S. Re-examination of the “3/4-law” of metabolism. J. Theor. Biol. 2001;209:9–27. doi: 10.1006/jtbi.2000.2238. doi:10.1006/jtbi.2000.2238 [DOI] [PubMed] [Google Scholar]
- Etienne R.S, Apol M.E.F, Olff H. Demystifying the West, Brown and Enquist model of the allometry of metabolism. Funct. Ecol. 2006;20:394–399. doi:10.1111/j.1365-2435.2006.01136.x [Google Scholar]
- Frappell P.B, Hinds D.S, Boggs D.F. Scaling of respiratory variables and the breathing pattern in birds: an allometric and phylogenetic approach. Physiol. Biochem. Zool. 2001;74:75–89. doi: 10.1086/319300. doi:10.1086/319300 [DOI] [PubMed] [Google Scholar]
- Geiser F. Reduction of metabolism during hibernation and daily torpor in mammals and birds: temperature effect or physiological inhibition? J. Comp. Physiol. B. 1988;158:25–37. doi: 10.1007/BF00692726. doi:10.1007/BF00692726 [DOI] [PubMed] [Google Scholar]
- Gillooly J.F, Allen A.P. Changes in body temperature influence the scaling of and aerobic scope in mammals. Biol. Lett. 2007;3:99–102. doi: 10.1098/rsbl.2006.0576. doi:10.1098/rsbl.2006.0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier D.S. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. 2005;80:611–662. doi: 10.1017/S1464793105006834. doi:10.1017/S1464793105006834 [DOI] [PubMed] [Google Scholar]
- Hammond K.A, Diamond J.M. Maximal sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. doi:10.1038/386457a0 [DOI] [PubMed] [Google Scholar]
- Hayssen V, Lacy R.C. Basal metabolic rates in mammals: taxonomic differences in the allometry of BMR and body mass. Comp. Biochem. Physiol. A. 1985;81:741–754. doi: 10.1016/0300-9629(85)90904-1. doi:10.1016/0300-9629(85)90904-1 [DOI] [PubMed] [Google Scholar]
- Heldmaier G. Seasonal acclimatization of energy requirements in mammals: functional significance of body weight control, hypothermia, torpor and hibernation. In: Wieser W, Gnaiger E, editors. Energy transformation in cells and organisms. Georg Thieme Verlag; New York, NY: 1989. pp. 130–139. [Google Scholar]
- Hemmingsen A.M. Energy metabolism as related to body size and respiratory surfaces, and its evolution. Rep. Steno Memorial Hospital Nordisk Insulin Laboratorium. 1960;9:1–110. [Google Scholar]
- Heusner A.A. Size and power in mammals. J. Exp. Biol. 1991;160:25–54. doi: 10.1242/jeb.160.1.25. [DOI] [PubMed] [Google Scholar]
- Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–353. [Google Scholar]
- Kleiber M. Wiley; New York, NY: 1961. The fire of life. [Google Scholar]
- Kooijman S.A.L.M. Cambridge University Press; Cambridge, UK: 2000. Dynamic energy and mass budgets in biological systems. [Google Scholar]
- Koteja P. On the relation between basal and maximum metabolic rate in mammals. Comp. Biochem. Physiol. A. 1987;87:205–208. doi: 10.1016/0300-9629(87)90447-6. doi:10.1016/0300-9629(87)90447-6 [DOI] [PubMed] [Google Scholar]
- Kozlowski J, Konarzewski M. Is West, Brown and Enquist's model of allometric scaling mathematically correct and biologically relevant? Funct. Ecol. 2004;18:283–289. doi:10.1111/j.0269-8463.2004.00830.x [Google Scholar]
- Kozlowski J, Konarzewski M. West, Brown and Enquist's model of allometric scaling again: the same questions remain. Funct. Ecol. 2005;19:739–743. doi:10.1111/j.1365-2435.2005.01021.x [Google Scholar]
- Lasiewski R.C. Oxygen consumption of torpid, resting, active, and flying hummingbirds. Physiol. Zool. 1963;36:122–140. [Google Scholar]
- Lasiewski R.C, Lasiewski R.J. Physiological responses of the blue-throated and Rivoli's hummingbirds. Auk. 1967;84:34–48. [Google Scholar]
- Lasiewski R.C, Weathers W.W, Bernstein M.H. Physiological responses of the giant hummingbird, Patagonia gigas. Comp. Biochem. Physiol. 1967;23:797–813. doi: 10.1016/0010-406x(67)90342-8. doi:10.1016/0010-406X(67)90342-8 [DOI] [PubMed] [Google Scholar]
- Lovegrove B.G. The zoogeography of mammalian basal metabolic rate. Am. Nat. 2000;156:201–219. doi: 10.1086/303383. doi:10.1086/303383 [DOI] [PubMed] [Google Scholar]
- Makarieva A.M, Gorshkov V.G, Li B.-L. A note on metabolic rate dependence on body size in plants and animals. J. Theor. Biol. 2003;221:301–307. doi: 10.1006/jtbi.2003.3185. doi:10.1006/jtbi.2003.3185 [DOI] [PubMed] [Google Scholar]
- Makarieva A.M, Gorshkov V.G, Li B.-L. Energetics of the smallest: do bacteria breathe at the same rate as whales? Proc. R. Soc. B. 2005a;272:2219–2224. doi: 10.1098/rspb.2005.3225. doi:10.1098/rspb.2005.3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarieva A.M, Gorshkov V.G, Li B.-L. Revising the distributive networks models of West, Brown and Enquist (1997) and Banavar, Maritan and Rinaldo (1999): metabolic inequity of living tissues provides clues for the observed allometric scaling rules. J. Theor. Biol. 2005b;237:291–307. doi: 10.1016/j.jtbi.2005.04.016. doi:10.1016/j.jtbi.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Makarieva A.M, Gorshkov V.G, Li B.-L. Distributive network model of Banavar, Damuth, Maritan and Rinaldo (2002): critique and perspective. J. Theor. Biol. 2006a;239:394–397. doi: 10.1016/j.jtbi.2005.08.018. doi:10.1016/j.jtbi.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Makarieva A.M, Gorshkov V.G, Li B.-L, Chown S.L. Size- and temperature-independence of minimum life-supporting metabolic rates. Funct. Ecol. 2006b;20:83–96. doi:10.1111/j.1365-2435.2006.01070.x [Google Scholar]
- McKechnie A.E, Wolf B.O. The allometry of avian basal metabolic rate: good predictions need good data. Physiol. Biochem. Zool. 2004;77:502–521. doi: 10.1086/383511. doi:10.1086/383511 [DOI] [PubMed] [Google Scholar]
- McNab B.K. Cornell University Press; Ithaca, NY: 2002. The physiological ecology of vertebrates: a view from energetics. [Google Scholar]
- Mukai, K. et al In press. Warm-up intensity affects oxygen transport during supramaximal exercise in thoroughbred horses. Am. J. Vet. Res.69 [DOI] [PubMed]
- Nagy K.A, Girard I.A, Brown T.K. Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 1999;19:247–277. doi: 10.1146/annurev.nutr.19.1.247. doi:10.1146/annurev.nutr.19.1.247 [DOI] [PubMed] [Google Scholar]
- Niven J.E, Scharlemann J.P. Do insect metabolic rates at rest and during flight scale with body mass? Biol. Lett. 2005;1:346–349. doi: 10.1098/rsbl.2005.0311. doi:10.1098/rsbl.2005.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg U.M. Energetics of flight. In: Carey C, editor. Avian energetics and nutritional ecology. Chapman and Hall; New York, NY: 1996. pp. 199–249. [Google Scholar]
- Painter P.R. Data from necropsy studies and in vitro tissue studies lead to a model for allometric scaling of basal metabolic rate. Theor. Biol. Med. Model. 2005a;2:39. doi: 10.1186/1742-4682-2-39. doi:10.1186/1742-4682-2-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter P.R. Supply–demand balance in outward-directed networks and Kleiber's law. Theor. Biol. Med. Model. 2005b;2:45. doi: 10.1186/1742-4682-2-45. doi:10.1186/1742-4682-2-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter P.R. The fractal geometry of nutrient exchange surfaces does not provide an explanation for 3/4-power metabolic scaling. Theor. Biol. Med. Model. 2005c;3:31. doi: 10.1186/1742-4682-2-30. doi:10.1186/1742-4682-3-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R.H. Cambridge University Press; New York, NY: 1983. The ecological implications of body size. [Google Scholar]
- Refinetti R. Body size and metabolic rate in the laboratory rat. Exp. Biol. 1989;48:291–294. [PubMed] [Google Scholar]
- Reich P.B, Tjoelker M.G, Machado J.-L, Oleksyn J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature. 2006;439:457–461. doi: 10.1038/nature04282. doi:10.1038/nature04282 [DOI] [PubMed] [Google Scholar]
- Reynolds P.S. Phylogenetic analysis of surface areas of mammals. J. Mammal. 1997;78:859–868. doi:10.2307/1382944 [Google Scholar]
- Rezende E.L, Swanson D.L, Novoa F.F, Bozinovic F. Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics. J. Exp. Biol. 2002;205:101–107. doi: 10.1242/jeb.205.1.101. [DOI] [PubMed] [Google Scholar]
- Rogers D.M, Olson B.L, Wilmore J.H. Scaling for the VO2-to-body size relationship among children and adults. J. Appl. Physiol. 1995;79:958–967. doi: 10.1152/jappl.1995.79.3.958. [DOI] [PubMed] [Google Scholar]
- Rubner M. Über den Einfluss der Körpergrösse auf Stoff- und Kraftwechsel. Z. Biol. 1883;19:535–562. [Google Scholar]
- Savage V.M, Gillooly J.F, Woodruff W.H, West G.B, Allen A.P, Enquist B.J, Brown J.H. The predominance of quarter-power scaling in biology. Funct. Ecol. 2004;18:257–282. doi:10.1111/j.0269-8463.2004.00856.x [Google Scholar]
- Savage V.M, Enquist B.J, West G.B. Comment on “a critical understanding of the fractal model of metabolic scaling”. J. Exp. Biol. 2007;210:3873–3874. doi: 10.1242/jeb.006734. doi:10.1242/jeb.006734 [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; New York, NY: 1984. Scaling: why is animal size so important? [Google Scholar]
- Tieleman B.I, Williams J.B. The adjustment of avian metabolic rates and water fluxes to desert environments. Physiol. Biochem. Zool. 2000;73:461–479. doi: 10.1086/317740. doi:10.1086/317740 [DOI] [PubMed] [Google Scholar]
- Weibel E.R, Hoppeler H. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J. Exp. Biol. 2005;208:1635–1644. doi: 10.1242/jeb.01548. doi:10.1242/jeb.01548 [DOI] [PubMed] [Google Scholar]
- Weibel E.R, Bacigalupe L.D, Schmitt B, Hoppeler H. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respir. Physiol. Neurobiol. 2004;140:115–132. doi: 10.1016/j.resp.2004.01.006. doi:10.1016/j.resp.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Weiner J. Metabolic constraints to mammalian energy budgets. Acta Theriol. 1989;34:3–35. [Google Scholar]
- West G.B, Brown J.H. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 2005;208:1575–1592. doi: 10.1242/jeb.01589. doi:10.1242/jeb.01589 [DOI] [PubMed] [Google Scholar]
- West G.B, Brown J.H, Enquist B.J. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. doi:10.1126/science.276.5309.122 [DOI] [PubMed] [Google Scholar]
- West G.B, Brown J.H, Enquist B.J. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–1679. doi: 10.1126/science.284.5420.1677. doi:10.1126/science.284.5420.1677 [DOI] [PubMed] [Google Scholar]
- White C.R, Seymour R.S. Allometric scaling of mammalian metabolism. J. Exp. Biol. 2005;208:1611–1619. doi: 10.1242/jeb.01501. doi:10.1242/jeb.01501 [DOI] [PubMed] [Google Scholar]
- White C.R, Phillips N.F, Seymour R.S. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2006;2:125–127. doi: 10.1098/rsbl.2005.0378. doi:10.1098/rsbl.2005.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C.R, Cassey P, Blackburn T.M. Allometric exponents do not support a universal metabolic allometry. Ecology. 2007;88:315–323. doi: 10.1890/05-1883. doi:10.1890/05-1883 [DOI] [PubMed] [Google Scholar]