Abstract
Residual force enhancement has been observed following active stretch of skeletal muscles and single fibres. However, there has been intense debate whether force enhancement is a sarcomeric property, or is associated with sarcomere length instability and the associated development of non-uniformities. Here, we studied force enhancement for the first time in isolated myofibrils (n=18) that, owing to the strict in series arrangement, allowed for evaluation of this property in individual sarcomeres (n=79). We found consistent force enhancement following stretch in all myofibrils and each sarcomere, and forces in the enhanced state typically exceeded the isometric forces on the plateau of the force–length relationship. Measurements were made on the plateau and the descending limb of the force–length relationship and revealed gross sarcomere length non-uniformities prior to and following active myofibril stretching, but in contrast to previous accounts, revealed that sarcomere lengths were perfectly stable under these experimental conditions. We conclude that force enhancement is a sarcomeric property that does not depend on sarcomere length instability, that force enhancement varies greatly for different sarcomeres within the same myofibril and that sarcomeres with vastly different amounts of actin–myosin overlap produce the same isometric steady-state forces. This last finding was not explained by differences in the amount of contractile proteins within sarcomeres, vastly different passive properties of individual sarcomeres or (half-) sarcomere length instabilities, suggesting that the basic mechanical properties of muscles, such as force enhancement, force depression and creep, which have traditionally been associated with sarcomere instabilities and the corresponding dynamic redistribution of sarcomere lengths, are not caused by such instabilities, but rather seem to be inherent properties of the mechanisms of contraction.
Keywords: skeletal muscle, mechanism of contraction, sarcomere length instability, cross-bridge theory, sliding filament theory
1. Introduction
It has been known for a long time that when an activated muscle is stretched its force increases, and although the precise nature of this increase remains a matter of debate (e.g. Pinniger et al. 2006), it was conceptually incorporated into the general behaviour of muscle contraction through the force–velocity relationship (e.g. Hill 1938) and is accounted for in the cross-bridge theory (Huxley 1957). Similarly, when a muscle is actively stretched, and then held at the stretched length long enough for all transient force response to disappear, the isometric steady-state force at the stretched length is greater than the steady-state isometric force at that same length for a purely isometric contraction (figure 1). This observation has first been described systematically by Abbott & Aubert (1952), and has been referred to as residual force enhancement (Edman et al. 1982). In contrast to the force increase during stretch, the residual force enhancement following stretch has not been accounted for in the cross-bridge theory (Huxley 1957, 1969; Huxley & Simmons 1971; Rayment et al. 1993). In fact, it can be shown that cross-bridge models are unable to predict residual force enhancement, except if one allows for muscle stretch to change the kinetics of the cross-bridge cycle permanently (Walcott & Herzog 2006).
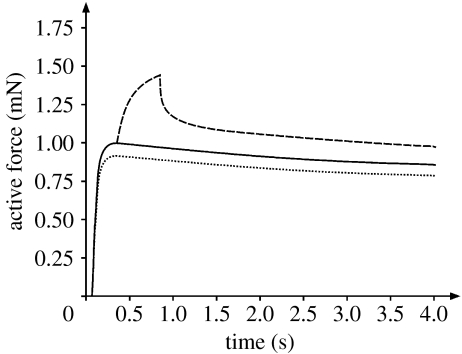
Figure 1.
Force–time histories obtained from a single fibre of frog tibialis anterior for two isometric contractions at optimal (solid line) and final (dotted line) lengths, and for a contraction in which the preparation was stretched from the optimal to the final length (dashed line) (Rassier et al. 2003b). The stretch magnitude corresponded to 10% of the fibre length and was performed at a speed of approximately 20% fibre length s−1. Following stretch, the fibre was held long enough for all force transients to disappear, so that the steady-state forces could be compared. The increase in isometric force following the stretch compared with the isometric force at the final length is defined as the residual force enhancement. The increase in isometric force following the stretch compared with the isometric force obtained at the optimal sarcomere length indicates the increase in force above the plateau of the force–length relationship.
Residual force enhancement is known to increase with the magnitude of stretch (Abbott & Aubert 1952; Edman et al. 1978; Herzog & Leonard 2002, 2005), at least to a certain threshold value (Bullimore et al. 2007), appears to be independent of the speed of stretch (Edman et al. 1982) and is sensitive to the initial length of the muscle (Edman et al. 1978, 1982). Residual force enhancement is long lasting (more than 20 s in cat soleus; Herzog & Rassier 2002), but can be abolished by deactivating the muscle long enough for force to drop to zero (Abbott & Aubert 1952; Morgan et al. 2000). It occurs in human muscles activated electrically (De Ruiter et al. 2000) and voluntarily (Lee & Herzog 2002), isolated muscle preparations (e.g. Abbott & Aubert 1952; Herzog & Leonard 2002) and single fibre or fibre bundle preparations (Edman et al. 1978, 1982; Sugi & Tsuchiya 1988; Bagni et al. 2002, 2004). Despite an abundance of experimental observations, the mechanisms underlying residual force enhancement remain a matter of debate (Herzog & Leonard 2006; Herzog et al. 2006; Morgan & Proske 2006).
It has been suggested that residual force enhancement has a passive and an active component (Edman et al. 1978; De Ruiter et al. 2000; Herzog & Leonard 2002; Herzog et al. 2006). The passive component has been associated with the molecular spring titin and its interaction with calcium upon activation (Bagni et al. 2002, 2004; Labeit et al. 2003; Joumaa et al. 2007). The active component is associated with the so-called sarcomere length non-uniformity theory (Morgan 1990, 1994; Morgan et al. 2000). This theory is based on the idea that sarcomeres on the descending limb of the force–length relationship are unstable as suggested by Hill (1953), and when stretched, this instability will cause differential elongation of sarcomeres: some might hardly be stretched at all, while others are stretched beyond actin–myosin filament overlap and are only held in force equilibrium with the ‘active’ sarcomeres by their passive forces (e.g. Morgan et al. 2000; Morgan & Proske 2006). However, recently, we demonstrated that sarcomeres can be perfectly stable on the descending limb of the force–length relationship in a single myofibril preparation (Rassier et al. 2003a), but we were unable to measure force and sarcomere length simultaneously. Therefore, force enhancement could not be determined. Others have measured force and sarcomere length simultaneously in myofibril preparations following stretch (Telley et al. 2006), but they did not wait long enough (1s) for force to reach steady-state values and sarcomeres to finish the transient length changes associated with myofibril stretching; thus no insight into force enhancement was obtained.
The determination of force enhancement in single myofibrils is crucial for many reasons: first, if there was force enhancement in myofibrils, it would be possible to eliminate extra-sarcomeric structures as a cause; second, if there was force enhancement in the presence of steady sarcomere lengths, we could eliminate sarcomere length instability as a mechanism; third, since sarcomeres are mechanically arranged in series within a myofibril, measuring the force at the end of a myofibril gives the instantaneous force in each sarcomere; and finally, by measuring individual sarcomere lengths, it would be possible to calculate force enhancement for individual sarcomeres, thus providing novel insight into how force enhancement may affect the basic contractile unit of muscle.
2. Material and methods
(a) Extraction of single myofibrils and experimental set-up
Strips of rabbit psoas were dissected and tied to small wooden sticks. These samples were stored in a rigor/glycerol (50:50) solution at −20°C. On the day of the experiments, the muscle strips were cut into pieces of approximately 2 mm length using a razor blade and subsequently blended using previously described protocols (Rassier et al. 2003a). The blended muscle was then put into a chamber whose bottom was a glass cover-slip placed on top of an inverted microscope (Zeiss, Axiovert 200M, Germany). After a sufficient time for stabilization (5–10 min), the rigor solution was replaced with a relaxing solution, and myofibrils in suspension were washed away leaving those attached to the bottom of the experimental chamber. A myofibril with a good striation pattern was then selected and attached to a glass needle and motor at one end, and to a pair of nano-levers (Bartoo et al. 1993) that allowed for myofibril force measurements at the other end.
The image of the attached myofibril was projected onto a high-density linear photodiode array (Schafter and Kirschoff Model SK10680DJR, Hamburg, Germany, resolution: 6 nm) to give tracings of the myofibrillar striation pattern for identification of the A- and I-bands and the Z-lines. Sarcomere lengths were calculated from Z-line to Z-line, or when these could not be identified reliably, from the centroids of adjacent A-bands. Half-sarcomere lengths were calculated from the Z-line to the centroid of the corresponding A-bands.
(b) Protocol
Once a myofibril was ready for mechanical testing, and a clear striation pattern could be observed, a 10 min rest was given and then the relaxing solution was replaced by the activating solution causing contraction of the myofibril. Six myofibrils were tested isometrically at an average sarcomere length of 2.4 and 3.4 μm and the active and passive forces were determined. A further 12 myofibrils were activated isometrically at a short length, then stretched while activated and then held isometrically for another 30 s until force transients had disappeared. Six of these myofibrils were stretched from a nominal average sarcomere length of 2.4 to 3.4 μm, while the remaining six myofibrils underwent a series of stretches, starting at different average sarcomere lengths and being subjected to variable stretch magnitudes (between 12 and 38% of the initial sarcomere length). For all myofibrils, the mid-diameter was measured using a calibrated eyepiece, and myofibril cross-sectional areas were calculated assuming a cylindrical myofibril shape. Forces were normalized relative to the cross-sectional areas to provide myofibril stress, which allowed for comparison of differently sized preparations.
(c) Solutions
The rigor, relaxing and activating solutions were identical to those described previously in our studies (e.g. Rassier et al. 2003a).
(d) Definition of force enhancement
Force enhancement for the myofibril preparation was defined as the difference in the steady-state isometric force following the stretch (test) contraction, and the purely isometric (reference) contraction at the corresponding length (i.e. length at the end of the stretch), or as the difference in steady-state isometric force obtained prior to stretch and the corresponding force after stretch corrected for the average sarcomere lengths in the myofibril prior to and following stretch and accounting for the loss of myofilament overlap during stretch in accordance with Gordon et al. (1966) but scaled to the actin–myosin lengths of rabbit skeletal muscle (Page & Huxley 1963; Herzog et al. 1992).
Force enhancement for individual sarcomeres was obtained in the same way as defined for the whole myofibril with the exception that the individual sarcomere lengths and the corresponding filament overlap (Page & Huxley 1963; Herzog et al. 1992) and expected force for the reference and test contractions were accounted for individually. This had to be done as sarcomere length in the reference and test contractions could not be assumed to be the same, despite the same myofibril length, and because sarcomere lengths increased for all sarcomeres in each myofibril during stretching.
3. Results
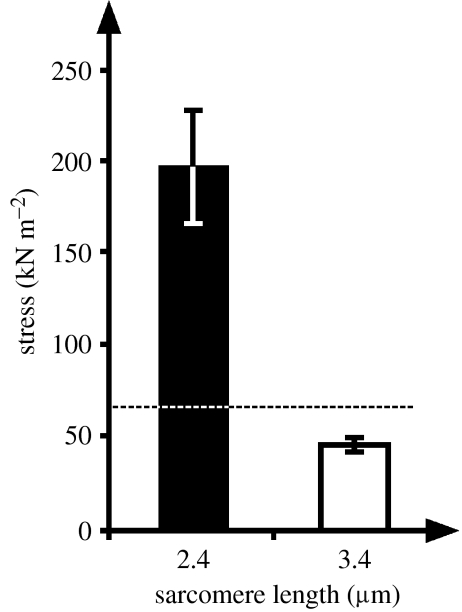
Myofilament lengths in rabbit skeletal muscles are 1.65 μm for myosin and between 1.07 and 1.09 μm for actin (Page & Huxley 1963; Herzog et al. 1992). Therefore, assuming an actin length of 1.08 μm, the plateau of the sarcomere force–length relationship occurs between 2.26 μm (twice the actin filament length plus the width of the Z-line, 0.1 μm) and 2.43 μm (2.26 μm plus the width of the bare zone in the middle of myosin—0.17 μm; Herzog et al. 1992). Similarly, the end of the descending limb of the force–length relationship occurs at 3.91 μm (twice the actin length, plus the length of myosin, plus the width of the Z-line). Isometric reference measurements at mean sarcomere lengths of 2.4 and 3.4 μm gave average steady-state stresses of 194 and 43 kN m−2, respectively (figure 2), thereby confirming expected maximal values at optimal length reported for mammalian skeletal muscles, when due account is taken of the temperature (21°C) at which the experiments were performed (Ranatunga & Wylie 1983), and approximating the expected linear decrease in force with loss of myofilament overlap at increasing sarcomere lengths (Gordon et al. 1966).
Figure 2.
Stresses (total force/cross-sectional area) of single myofibrils (n=6) at average sarcomere lengths of 2.4 and 3.4 μm. The dashed line represents the predicted force for a sarcomere length of 3.4 μm based on the force-length relationship and the actual forces observed at 2.4 μm. Note that the stress was much greater at 2.4 than 3.4 μm, thereby indicating that all stretch experiments were performed on the descending part of the force–sarcomere length relationship. Single myofibrils were obtained from rabbit psoas and all experiments were performed at a temperature of 21°C.
All myofibrils showed residual force enhancement (figure 3) averaging (means±s.d.) 328±250 kN m−2 or 386±251% of the isometric reference forces. Similarly, all individual sarcomeres of all myofibrils showed force enhancement (figure 4), but sarcomeric force enhancement within the same myofibril differed substantially owing to the different lengths of sarcomeres prior to and following stretch (figure 3). For 11 out of the 12 myofibrils, stretching resulted in residual force enhancement whose forces were greater than the isometric reference forces at optimal sarcomere length. The average force enhancement above plateau for the 12 myofibrils was 106±85%, or essentially a doubling of the maximal isometric forces obtained for the purely isometric reference contractions, for an average stretch of 35±15% of the initial sarcomere length.
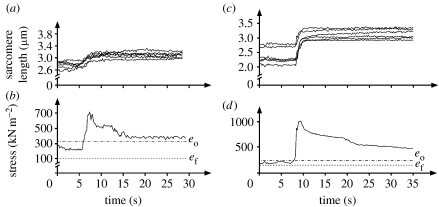
Figure 3.
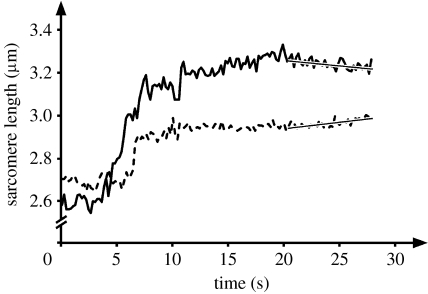
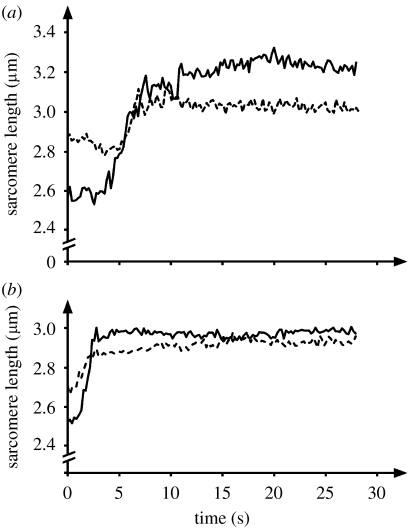
(a,b) Sarcomere length and stress (force/cross-sectional area) as a function of time for two exemplar myofibrils stretched from an initial sarcomere length on the descending limb or (c,d) near the plateau of the force–length relationship. Sarcomere lengths are non-uniform for the isometric contraction prior to stretch and remain non-uniform following stretch. Stresses increase during stretch dramatically and remain greater after stretch (for at least 25 s) than the forces prior to stretch, and greater than the expected forces (based on myofilament overlap for the average sarcomere length) at optimal length (eo) and final length achieved after the stretch (ef).
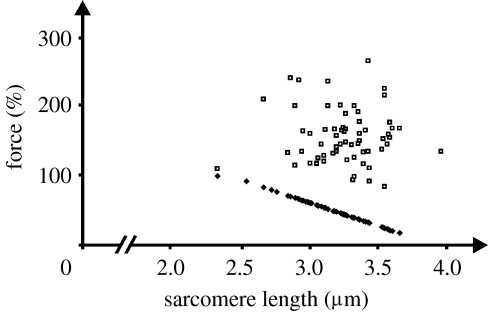
Figure 4.
Expected forces for single sarcomeres based on the myofilament overlap theory and the actin and myosin filament lengths for rabbit skeletal muscle (filled squares), and the corresponding sarcomere forces achieved following myofibril stretching (open squares).
Sarcomere lengths prior to and following stretch were non-uniform, and for the stretch magnitudes used here, all sarcomeres elongated during myofibril stretch, albeit not necessarily by the same amount (figure 3). Statistical analysis of the slopes of the sarcomere lengths versus time graphs for the last 10 s of ‘steady state’ revealed that 55 sarcomeres had a zero slope, 18 sarcomeres had a small positive slope and 6 had a small negative slope. However, all positive slopes were so small that it would have taken minutes to pull these sarcomeres beyond myofilament overlap (3.91 μm). In order to investigate whether the observed sarcomere length changes were small, random fluctuations or systematic instabilities in the sense of Hill (1953) and Morgan et al. (2000), the shortest and longest sarcomeres in each myofibril were considered further. The instability theory states that long sarcomeres are weak and short are strong on the descending limb of the force–length relationship (Hill 1953), and predicts the short (strong) sarcomeres to shorten and the long (weak) to be stretched and pulled beyond overlap (Morgan et al. 2000; Morgan & Proske 2006). However, this expected pattern was never observed in any of the 12 myofibrils, while the opposite, the shortest sarcomere being stretched and the longest shortening, was observed in one myofibril (figure 5). Therefore, any small remnant shortening or stretch of individual sarcomeres seemed to be random and not associated with sarcomere length instabilities as predicted by Hill (1953).
Figure 5.
Sarcomere lengths as a function of time for two sarcomeres from the same myofibril. The two sarcomeres are those that end up being the longest and shortest following active myofibril stretching. Note that the stretch magnitude is substantially different for the two sarcomeres and that the shorter sarcomere prior to stretch becomes the longer sarcomere following stretch. Note further that the long sarcomere after stretch is not pulled beyond myofilament overlap by the short sarcomere. Rather, the long sarcomere is slightly shortening towards the end of the contraction at the expense of the short sarcomere that is slowly elongating.
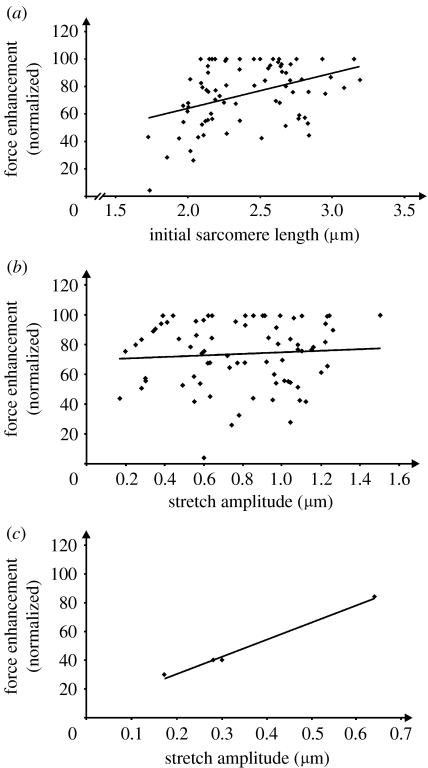
Force enhancement has been shown to increase with increasing stretch magnitudes and increasing initial sarcomere lengths (Abbott & Aubert 1952; Edman et al. 1982). In this study, sarcomeric force enhancement was positively correlated with initial sarcomere lengths (figure 6a), but not stretch amplitude (figure 6b) as one might have expected, except for single sarcomeres of myofibrils for which the stretch magnitude was small (figure 6c; four sarcomeres from a single myofibril that was stretched by 12% of its initial length).
Figure 6.
Normalized force enhancement as a function of (a) initial sarcomere length (the average sarcomere length of the myofibril prior to stretching) and (b,c) stretch amplitude. (a) Force enhancement was normalized relative to the maximal force enhancement observed in each of the 12 myofibrils. There is a small but statistically significant relationship (R2=0.20, p<0.05) between force enhancement and initial sarcomere length. (b) Normalized force enhancement as a function of sarcomere stretch magnitude for all sarcomeres from the 12 myofibril preparations. There was no statistically significant relationship between these two variables for the conditions of this test. (c) Force enhancement as a function of sarcomere stretch amplitude in a given myofibril, where the total stretch magnitude was small (12% of the initial sarcomere length). For this scenario, there is a positive correlation between sarcomere stretch and force enhancement (r2=0.98, p<0.05).
Since sarcomere length stability was observed in all myofibril preparations, but sarcomere lengths prior to and following stretch were not uniform, sarcomeres at different lengths on the descending limb of the force–length relationship, and thus presumably different actin–myosin overlap, supported the same amount of force. One might argue that this is caused by differences in the contractile materials within neighbouring sarcomeres. However, if that is the case, sarcomere lengths should remain at a constant ratio on the descending limb of the force–length relationship, but that is not necessarily what happens (figures 5, 7a and 8a,b). Furthermore, corresponding half-sarcomeres that share the central myosin filament, and thus would be expected to have identical contractile material on each side of the half-sarcomere, also do not necessarily support the same amount of force on the descending limb of the force–length relationship (figure 9).
Figure 7.
(a) Sarcomere lengths versus time for all six sarcomeres of a single myofibril, and (b) the corresponding force–length relationship for single sarcomeres based on the myofilament overlap theory and the actin and myosin filament lengths for rabbit skeletal muscle (open squares connected by solid lines), and the corresponding sarcomere forces achieved following myofibril stretching (filled squares). For the longest sarcomere following stretch (approx. 3.25 μm), the expected force is 58% lower than the maximal isometric force at the plateau of the force–length relationship, while the actual force was 44% above the maximal force. The vertical line connecting the open and filled squares represents the force enhancement for each single sarcomere. Note that the shortest sarcomere (following stretch) should be approximately twice as strong as the longest sarcomere based on myofilament overlap.
Figure 8.
(a,b) Sarcomere length as a function of time for two pairs of sarcomeres from two myofibrils stretched on the descending limb of the force–length relationship. Note that in both cases, the short sarcomere prior to stretch becomes the long sarcomere after stretch. In (a) the stretch magnitudes are different by a factor of 3 and in (b) the difference is approximately a factor of 2.
Figure 9.
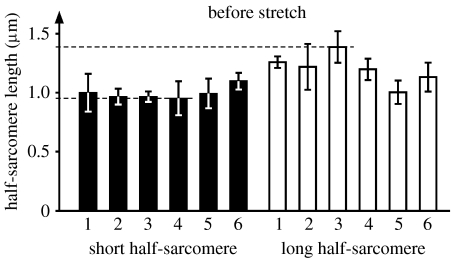
Short and long half-sarcomere lengths from an exemplar myofibril tested in this study. The shortest and longest half-sarcomeres are approximately 0.95 and 1.4 μm, while some half-sarcomeres are at optimal length for maximal force production based on the myofilament overlap theory (1.13–1.22 μm). Despite these differences that correspond to an expected force difference from the strongest to weakest half-sarcomere of approximately 30%, all half-sarcomeres were perfectly stable while supporting identical forces.
4. Discussion
Here, we provide first direct evidence of residual force enhancement in single myofibrils and individual sarcomeres, and further show that the isometric forces obtained at average sarcomere lengths corresponding to optimal actin–myosin overlap can be more than doubled if the isometric steady-state conditions are preceded by an appropriate stretch (35±15% of the initial sarcomere length). These findings are qualitatively consistent with earlier observations for in situ muscles (e.g. De Ruiter et al. 2000; Lee & Herzog 2002), isolated muscles (e.g. Abbott & Aubert 1952; Herzog & Leonard 2002) and single fibres or fibre bundles (Edman et al. 1978, 1982; Sugi & Tsuchiya 1988), although the magnitudes achieved are somewhat greater in single myofibrils than those in any previously investigated preparation.
Recently, Telley et al. (2006) measured simultaneous force and sarcomere lengths in single myofibril preparations with the aim to assess sarcomere and half-sarcomere dynamics during stretch. They observed myofibrils for just 1s following stretch when force was still in its transient decay and sarcomere lengths were not constant; therefore, residual force enhancement could not be determined. However, their data for the stretch and initial transient phase are similar to ours, and there is reason to believe that they would have found residual force enhancement in their preparations as we did here. Their measurements were typically made at shorter sarcomere lengths than ours, thereby suggesting that our findings on the descending limb would probably also hold for the plateau region of the sarcomere force–length relationship.
The residual force enhancement, calculated for individual sarcomeres according to the force–length relationship, varied within a given myofibril. This is explained by the fact that sarcomere lengths prior to and following myofibril stretch were non-uniform and that the stretch magnitudes for the individual sarcomeres were not necessarily the same (figures 3, 5 and 8). Sarcomere forces in the enhanced state often exceeded the forces obtained at optimal sarcomere lengths. It has been found that the magnitude of stretch and the length of a muscle or fibre prior to stretch are related to the magnitude of the force enhancement (Abbott & Aubert 1952; Edman et al. 1978, 1982; Herzog & Leonard 2002). However, in this study only the initial sarcomere lengths were loosely but statistically significantly correlated with the amount of force enhancement (figure 6a), while the magnitude of sarcomere stretch was not (figure 6b). The lack of correlation between stretch magnitude and sarcomeric force enhancement was surprising and is inconsistent with most previously published observations. However, Bullimore et al. (2007) showed that although force enhancement and stretch magnitude were well correlated for stretches of approximately 25% of the optimal fibre length, once stretch magnitudes were beyond 25%, force enhancement remained approximately constant or even decreased slightly. The stretch magnitudes used in our preparations for 8 out of the 12 myofibrils exceeded 25%; therefore, stretch magnitude and force enhancement might not have been correlated in this study owing to the great stretch magnitudes used in two-thirds of the tests. This hypothesis is strengthened by the correlation obtained between the force enhancement and the amplitude of the stretch within a myofibril where the average stretch magnitude was only 12% (figure 6c).
Residual force enhancement in muscles and single fibres typically does not exceed 50%. However, here we measured enhanced forces for single myofibrils and sarcomeres, which often were more than twice those measured on the plateau. Comparable force enhancement magnitudes have been observed in isolated fibre preparation treated with great amounts of 2,3-butanedione monoxime (BDM; Bagni et al. 2002, 2004; Lee et al. 2007), or for fibres stretched to great final sarcomere lengths (Edman et al. 1982). BDM limits cross-bridge attachment in the strongly bound state by inhibiting phosphate release (Herrmann et al. 1992), thereby biasing the ratio of weakly to strongly bound cross-bridges towards the weakly bound state. This bias is associated with a great decrease in force, but only a small decrease in stiffness, as weakly bound cross-bridges are not expected to contribute (much) to force, but they contribute to the stiffness of fibres (Herrmann et al. 1992; Regnier et al. 1995). The great magnitudes of force enhancement in BDM-treated preparations might be explained if we assume that there is a stretch-induced change in the ratio of weakly to strongly bound cross-bridges.
However, the single myofibrils were not treated with BDM. At this point, it is not clear why force enhancement in the myofibrils and sarcomeres is very much greater than that in the single fibre or muscle preparations. However, several possibilities exist. For example, all experiments were performed at less than 22°C, which is cold for mammalian muscles such as the rabbit psoas used in this study. Cold temperatures have been associated with a bias of the weakly to strongly bound cross-bridges towards the weakly bound state (Decostre et al. 2005; Linari et al. 2005), similar to what has been observed for BDM-treated preparations. Therefore, the results observed here might be associated with temperature.
Another possibility for the great force enhancement could be the lengths at which the final force enhancement measurements were made for many of the sarcomeres (greater than 3.4 μm). For example, Edman et al. (1982) obtained force enhancement in excess of 60% in single fibre preparations at sarcomere lengths of approximately 2.8 μm (estimated from their fig. 3A). They further demonstrated a consistent increase in force enhancement with increasing stretch magnitudes and final sarcomere lengths, thereby suggesting that had they increased stretch magnitude or made measurements at sarcomere lengths greater than 2.8 μm, force enhancement could easily have reached values in excess of 100%. In our case, many of the final sarcomere lengths were greater than 3.4 μm, which gives reference forces of approximately 34% of the maximal isometric force at the plateau of the force–length relationship. For the case of Edman et al. (1982), a 60% force enhancement at 2.8 μm would have resulted in a 244% force enhancement at 3.4 μm in their preparation, assuming that the absolute force enhancement remained about the same, a conservative estimate, as they showed increasing absolute force enhancements with increasing final sarcomere lengths. Thus, a probable explanation for the vast force enhancements observed in this study is the fact that final sarcomere lengths were often very long and the associated isometric references forces were small. However, comparison with the literature is not possible at this time, as consistent force enhancements at sarcomere lengths of 3.4 μm or more have not been made.
Myofibrils are ideal preparations to determine the mechanical properties of individual sarcomeres, as sarcomeres are arranged strictly in series. That means, at any given time, the force transmitted by one sarcomere has to be the same as that of any other sarcomere, which has to be the same as the force measured at the end of the myofibril. Therefore, and in accordance with the sliding filament and cross-bridge theories, one would expect all sarcomeres to be of the same length on the descending limb of the force–length relationship so that myofilament overlap, and therefore steady-state force, would be the same for all sarcomeres. However, this was not the case. For example, as shown in figure 7, the shortest sarcomere after stretch (approx. 2.7 μm) would be expected to produce approximately 80% of its maximal isometric force, while the longest sarcomere (approx. 3.3 μm) would be expected to produce approximately 40% of its maximal isometric force, a difference of 100% in force production ability between these two sarcomeres. Both sarcomeres were isometric when the measurements were made (figure 7a), and this difference is not consistent with the myofilament overlap theory (Gordon et al. 1966).
One might explain the difference in sarcomere lengths (for the same isometric force) with a difference in the number of contractile proteins in one sarcomere compared with the other. For example, if one sarcomere had 20% more contractile proteins, it should be able to produce the same amount of force on the descending limb of the force–length relationship as another sarcomere even if its length was 20% greater than that of the other sarcomere. However, if this was the case, then length increases during myofibril stretching should be proportional, so that this explanation holds for the entire range of the descending limb of the force–length relationship. However, there are numerous examples where this is not the case; one such example is shown in figure 5 and another two in figure 8. In each case shown (figures 5 and 8), the short sarcomere prior to stretch becomes the long sarcomere after stretch. Since all sarcomeres are always on the descending limb of the force–length relationship (i.e. starting sarcomere lengths are greater than 2.43 μm), the results observed here cannot be explained by differences in contractile proteins between sarcomeres. This conclusion is further supported by pairs of sarcomeres starting at the same length prior to stretch and ending up at different lengths after stretch, or sarcomeres of different lengths prior to stretch ending up at the same length after stretch, observations that were made in this study (not shown) and observations made by us previously (Rassier et al. 2003a; their figures 3a and 4a, respectively).
Another possibility could be that differences in sarcomeric passive forces might account for the observed results. However, if this were the case, one would not expect the length reversal during stretch of the pairs of sarcomeres shown in figures 5 and 8. Furthermore, the average passive forces at sarcomere lengths of 3.0–3.2 μm have been reported to be approximately 5–10% of the maximal active isometric force at optimal sarcomere lengths (Bartoo et al. 1997). Following active stretching from 2.4 to 3.4 μm, as was done in this study, the passive forces (including the passive force enhancement; Herzog & Leonard 2002) were 18% of the maximum isometric forces at the plateau of the force–length relationship (Joumaa et al. 2007). If it was assumed that a short sarcomere had no passive forces while a long one had the full 18%, an unlikely scenario, it still could not explain the force difference (up to 68% of the maximum isometric force) found here based on the observed sarcomere length non-uniformities.
Finally, one could argue that active force production just depends on one half of the sarcomere, while the other half produces much of its force passively. However, if that was the case, then, in accordance with the myofilament overlap theory, all short (and therefore strong) half-sarcomere lengths should be the same; however they are not as shown in figure 9. Furthermore, all long (and therefore weak) half-sarcomeres should be pulled beyond myofilament overlap, and would be expected to be at similar lengths, which they are not. In fact, for the six sarcomeres from a single myofibril shown in figure 9, the biggest difference in half-sarcomere lengths is observed in sarcomere 3 and amounts to approximately 0.4 μm. Some of the half-sarcomeres shown in figure 9 correspond to optimal myofilament overlap (e.g. short half-sarcomere 6) and they should be at a length of maximal isometric force capabilities, while other sarcomeres are much shorter (short half-sarcomere 4) or longer (long half-sarcomere 3), and their force capabilities based on the myofilament overlap theory (Gordon et al. 1966) should be approximately 69 and 75% of maximum. Therefore, we conclude that not only sarcomeres but also half-sarcomeres have force behaviour that cannot be explained with the theory of myofilament overlap (Huxley & Hanson 1954; Huxley & Niedergerke 1954).
In order to further characterize the mechanical behaviour of half-sarcomeres, we determined the modulus of elasticity for 12 half-sarcomeres in a myofibril with a clearly discernible Z-line pattern. We found that the modulus of elasticity, calculated as the ratio of the change in stress and half-sarcomere strain, was greater in the long compared with the short half-sarcomeres (table 1). Thus, differences in half-sarcomere lengths decreased after the stretch, and long half-sarcomeres seem to be endowed with a higher stiffness than short sarcomeres. This result is consistent with the observations of Edman et al. (1982) in single fibres and Telley et al. (2006) in isolated myofibrils who suggested that stretch has a stabilizing effect on sarcomere dynamics on the descending limb of the force–length relationship. As a consequence, long half-sarcomeres resist stretch more than short half-sarcomeres, and thus they cannot be pulled easily beyond myofilament overlap as proposed by the sarcomere length instability theory (Morgan 1990).
Table 1.
Half-sarcomere lengths before and after stretch in a myofibril of six sarcomeres. Note that half-sarcomeres before stretch were non-uniform. The difference in length between long (L) and short (S) half-sarcomeres in a sarcomere is reduced after stretch. In most half-sarcomeres, the elasticity modulus (E) of long half-sarcomeres is higher than that of the short ones. This might be explained by differences in the proportion of two coexisting titin isoforms in half-sarcomeres.
| sarcomere 1 | sarcomere 2 | sarcomere 3 | sarcomere 4 | sarcomere 5 | sarcomere 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | S | L | S | L | S | L | S | L | S | L | S | |
| before stretch | 1.26 | 1.00 | 1.22 | 0.97 | 1.39 | 0.97 | 1.20 | 0.95 | 1.01 | 0.99 | 1.13 | 1.10 |
| after stretch | 1.68 | 1.58 | 1.69 | 1.57 | 1.74 | 1.61 | 1.72 | 1.61 | 1.70 | 1.65 | 1.72 | 1.71 |
| E (kN m−2) | 233.6 | 134.7 | 204.6 | 124.1 | 310.7 | 116.0 | 180.6 | 112.7 | 113.8 | 118.2 | 151.9 | 140.6 |
Stiffness in active myofibrils can be attributed to the proportion of attached cross-bridges, and passive structures, most notably in myofibrillar preparations, the titin filaments. The proportion of attached cross-bridges would be expected to be greater at short compared with long half-sarcomere length on the plateau and descending part of the force–length relationship, and thus is unlikely to explain the differences in stiffness, except if we assume that increased length favours cross-bridge attachment, an intriguing, but as yet, unproven idea. Alternatively, the differences in stiffness could be caused by passive structural proteins. Rabbit psoas muscle expresses two isoforms of titin, which are also observed in single psoas fibres (Neagoe et al. 2003). Furthermore, Trombitas et al. (2001) showed that a half-sarcomere could coexpress different titin isoforms; thus differences in half-sarcomere stiffness could be caused by changes in the ratio of coexpressed titin isoforms, an idea that needs independent evaluation.
5. Conclusion
From the results of this study, we conclude that there is force enhancement in single myofibrils and forces in the enhanced state are more than twice those at the plateau of the force–length relationship. Furthermore, there is residual force enhancement in all individual sarcomeres in a myofibril following active stretch; this force enhancement is derived from the sarcomere force–length relationship (Gordon et al. 1966) and varies substantially between sarcomeres, and is not associated with the magnitude of sarcomere stretch (except for sarcomeres in a given myofibril for which stretch magnitudes were small), but is significantly related to the sarcomere length prior to stretch. Finally, we confirm previous results that sarcomeres in a myofibril preparation are highly non-uniform but perfectly stable on the descending limb of the force–length relationship, and that sarcomeres and half-sarcomeres, at different lengths (and therefore different actin–myosin overlap), produce the same amount of steady-state isometric force. The expected sarcomere force differences on the descending limb of the force–length relationship (Huxley 1957; Gordon et al. 1966) do not exist, and this result cannot be explained at present with differences in the number of contractile proteins across sarcomeres, passive forces or systematic changes in the half-sarcomere dynamics.
Acknowledgments
The support of the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the Canada Research Chair Programme for Molecular and Cellular Biomechanics is gratefully acknowledged.
Footnotes
The first two authors contributed equally to this paper.
References
- Abbott B.C, Aubert X.M. The force exerted by active striated muscle during and after change of length. J. Physiol. (Lond.) 1952;117:77–86. [PMC free article] [PubMed] [Google Scholar]
- Bagni M.A, Cecchi G, Colombini B, Colomo F. A non-cross-bridge stiffness in activated frog muscle fibers. Biophys. J. 2002;82:3118–3127. doi: 10.1016/S0006-3495(02)75653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni M.A, Colombini B, Geiger P, Berlinguer P.R, Cecchi G. Non-cross-bridge calcium-dependent stiffness in frog muscle fibers. Am. J. Physiol. Cell Physiol. 2004;286:C1353–C1357. doi: 10.1152/ajpcell.00493.2003. doi:10.1152/ajpcell.00493.2003 [DOI] [PubMed] [Google Scholar]
- Bartoo M.L, Popov V.I, Fearn L.A, Pollack G.H. Active tension generation in isolated skeletal myofibrils. J. Muscle Res. Cell Motil. 1993;14:498–510. doi: 10.1007/BF00297212. doi:10.1007/BF00297212 [DOI] [PubMed] [Google Scholar]
- Bartoo M.L, Linke W.A, Pollack G.H. Basis of passive tension and stiffness in isolated rabbit myofibrils. Am. J. Physiol. 1997;273:C266–C276. doi: 10.1152/ajpcell.1997.273.1.C266. [DOI] [PubMed] [Google Scholar]
- Bullimore S.R, Leonard T.R, Rassier D.E, Herzog W. History-dependence of isometric muscle force: effect of prior stretch or shortening amplitude. J. Biomech. 2007;40:1518–1524. doi: 10.1016/j.jbiomech.2006.06.014. doi:10.1016/j.jbiomech.2006.06.014 [DOI] [PubMed] [Google Scholar]
- Decostre V, Bianco P, Lombardi V, Piazzesi G. Effect of temperature on the working stroke of muscle myosin. Proc. Natl Acad. Sci. USA. 2005;102:13 927–13 932. doi: 10.1073/pnas.0506795102. doi:10.1073/pnas.0506795102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ruiter C.J, Didden W.J.M, Jones D.A, de Haan A. The force–velocity relationship of human adductor pollicis muscle during stretch and the effects of fatigue. J. Physiol. (Lond.) 2000;526:671–681. doi: 10.1111/j.1469-7793.2000.00671.x. doi:10.1111/j.1469-7793.2000.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K.A.P, Elzinga G, Noble M.I.M. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J. Physiol. (Lond.) 1978;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K.A.P, Elzinga G, Noble M.I.M. Residual force enhancement after stretch of contracting frog single muscle fibers. J. Gen. Physiol. 1982;80:769–784. doi: 10.1085/jgp.80.5.769. doi:10.1085/jgp.80.5.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A.M, Huxley A.F, Julian F.J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. (Lond.) 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C, Wray J, Travers F, Barman T. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry. 1992;31:12 227–12 232. doi: 10.1021/bi00163a036. doi:10.1021/bi00163a036 [DOI] [PubMed] [Google Scholar]
- Herzog W, Leonard T.R. Force enhancement following stretching of skeletal muscle: a new mechanism. J. Exp. Biol. 2002;205:1275–1283. doi: 10.1242/jeb.205.9.1275. [DOI] [PubMed] [Google Scholar]
- Herzog W, Leonard T.R. The role of passive structures in force enhancement of skeletal muscles following active stretch. J. Biomech. 2005;38:409–415. doi: 10.1016/j.jbiomech.2004.05.001. doi:10.1016/j.jbiomech.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Herzog W, Leonard T.R. Response to the (Morgan and Proske) letter to the editor by Walter Herzog (on behalf of the authors) and Tim Leonard. J. Physiol (Lond.) 2006;578:617–620. doi:10.1113/jphysiol.2006.125443 [Google Scholar]
- Herzog W, Rassier D.E. History dependence of skeletal muscle force production: a forgotten property. J. Mech. Med. Biol. 2002;2:347–358. doi:10.1142/S0219519402000447 [Google Scholar]
- Herzog W, Kamal S, Clarke H.D. Myofilament lengths of cat skeletal muscle: theoretical considerations and functional implications. J. Biomech. 1992;25:945–948. doi: 10.1016/0021-9290(92)90235-s. doi:10.1016/0021-9290(92)90235-S [DOI] [PubMed] [Google Scholar]
- Herzog W, Lee E.J, Rassier D.E. Residual force enhancement in skeletal muscle. J. Physiol. (Lond.) 2006;574:635–642. doi: 10.1113/jphysiol.2006.107748. doi:10.1113/jphysiol.2006.107748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. B. 1938;126:136–195. doi:10.1098/rspb.1938.0050 [Google Scholar]
- Hill A.V. The mechanics of active muscle. Proc. R. Soc. B. 1953;141:104–117. doi: 10.1098/rspb.1953.0027. doi:10.1098/rspb.1953.0027 [DOI] [PubMed] [Google Scholar]
- Huxley A.F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley H.E. The mechanism of muscular contraction. Science. 1969;164:1356–1366. doi:10.1126/science.164.3886.1356 [PubMed] [Google Scholar]
- Huxley H.E, Hanson J. Changes in cross-striations of muscle during contraction and stretch and their structural implications. Nature. 1954;173:973–976. doi: 10.1038/173973a0. doi:10.1038/173973a0 [DOI] [PubMed] [Google Scholar]
- Huxley A.F, Niedergerke R. Structural changes in muscle during contraction. Interference microscopy of living muscle fibres. Nature. 1954;173:971–973. doi: 10.1038/173971a0. doi:10.1038/173971a0 [DOI] [PubMed] [Google Scholar]
- Huxley A.F, Simmons R.M. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. doi:10.1038/233533a0 [DOI] [PubMed] [Google Scholar]
- Joumaa V, Rassier D.E, Leonard T.R, Herzog W. Passive force enhancement in single myofibrils. Pflugers Arch. 2007;455:367–371. doi: 10.1007/s00424-007-0287-2. doi:10.1007/s00424-007-0287-2 [DOI] [PubMed] [Google Scholar]
- Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H.L. Calcium-dependent molecular spring elements in the giant protein titin. Proc. Natl Acad. Sci. USA. 2003;100:13 716–13 721. doi: 10.1073/pnas.2235652100. doi:10.1073/pnas.2235652100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.D, Herzog W. Force enhancement following muscle stretch of electrically and voluntarily activated human adductor pollicis. J. Physiol. (Lond.) 2002;545:321–330. doi: 10.1113/jphysiol.2002.018010. doi:10.1113/jphysiol.2002.018010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.J, Joumaa V, Herzog W. New insights into the passive force enhancement in skeletal muscles. J. Biomech. 2007;40:719–727. doi: 10.1016/j.jbiomech.2006.10.009. doi:10.1016/j.jbiomech.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Linari M, Brunello E, Reconditi M, Sun Y.B, Panine P, Narayanan T, Piazzesi G, Lombardi V, Irving M. The structural basis of the increase in isometric force production with temperature in frog skeletal muscle. J. Physiol. (Lond.) 2005;567(Pt 2):459–469. doi: 10.1113/jphysiol.2005.089672. doi:10.1113/jphysiol.2005.089672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.L. New insights into the behavior of muscle during active lengthening. Biophys. J. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.L. An explanation for residual increased tension in striated muscle after stretch during contraction. Exp. Physiol. 1994;79:831–838. doi: 10.1113/expphysiol.1994.sp003811. [DOI] [PubMed] [Google Scholar]
- Morgan D.L, Proske U. Can all residual force enhancement be explained by sarcomere non-uniformities? J. Physiol. (Lond.) 2006;578:613–615. doi: 10.1113/jphysiol.2006.125039. doi:10.1113/jphysiol.2006.125039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.L, Whitehead N.P, Wise A.K, Gregory J.E, Proske U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J. Physiol. (Lond.) 2000;522:503–513. doi: 10.1111/j.1469-7793.2000.t01-2-00503.x. doi:10.1111/j.1469-7793.2000.t01-2-00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neagoe C, Opitz C.A, Makarenko I, Linke W.A. Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J. Muscle Res. Cell Motil. 2003;24:175–189. doi: 10.1023/a:1026053530766. doi:10.1023/A:1026053530766 [DOI] [PubMed] [Google Scholar]
- Page S.G, Huxley H.E. Filament lengths in striated muscle. J. Cell Biol. 1963;19:369–390. doi: 10.1083/jcb.19.2.369. doi:10.1083/jcb.19.2.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinniger G.J, Ranatunga K.W, Offer G.W. Crossbridge and non-crossbridge contributions to tension in lengthening rat muscle: force-induced reversal of the power stroke. J. Physiol. (Lond.) 2006;573(Pt 3):627–643. doi: 10.1113/jphysiol.2005.095448. doi:10.1113/jphysiol.2005.095448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K.W, Wylie S.R. Temperature-dependent transitions in isometric contractions of rat muscle. J. Physiol. (Lond.) 1983;339:87–95. doi: 10.1113/jphysiol.1983.sp014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassier D.E, Herzog W, Pollack G.H. Dynamics of individual sarcomeres during and after stretch in activated single myofibrils. Proc. R. Soc. B. 2003a;270:1735–1740. doi: 10.1098/rspb.2003.2418. doi:10.1098/rspb.2003.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassier D.E, Herzog W, Wakeling J, Syme D.A. Stretch-induced, steady-state force enhancement in single skeletal muscle fibers exceeds the isometric force at optimum fiber length. J. Biomech. 2003b;36:1309–1316. doi: 10.1016/s0021-9290(03)00155-6. doi:10.1016/S0021-9290(03)00155-6 [DOI] [PubMed] [Google Scholar]
- Rayment I, Holden H.M, Whittaker M, Yohn C.B, Lorenz M, Holmes K.C, Milligan R.A. Structure of the actin–myosin complex and its implications for muscle contraction. Science. 1993;261:58–65. doi: 10.1126/science.8316858. doi:10.1126/science.8316858 [DOI] [PubMed] [Google Scholar]
- Regnier M, Morris C, Homsher E. Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. Am. J. Physiol. 1995;269(Pt 1):C1532–C1539. doi: 10.1152/ajpcell.1995.269.6.C1532. [DOI] [PubMed] [Google Scholar]
- Sugi H, Tsuchiya T. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J. Physiol. (Lond.) 1988;407:215–229. doi: 10.1113/jphysiol.1988.sp017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telley I.A, Stehle R, Ranatunga K.W, Pfitzer G, Stussi E, Denoth J. Dynamic behaviour of half-sarcomeres during and after stretch in activated rabbit psoas myofibrils: sarcomere asymmetry but no ‘sarcomere popping’. J. Physiol. (Lond.) 2006;573(Pt 1):173–185. doi: 10.1113/jphysiol.2006.105809. doi:10.1113/jphysiol.2006.105809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombitas K, Wu Y, Labeit D, Labeit S, Granzier H. Cardiac titin isoforms are coexpressed in the half-sarcomere and extend independently. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1793–H1799. doi: 10.1152/ajpheart.2001.281.4.H1793. [DOI] [PubMed] [Google Scholar]
- Walcott, S. & Herzog, W. 2006 Can traditional cross-bridge models explain force-enhancement? In Proc. Biophysical Society 50th Annual Meeting, 18–22 February, Salt Lake City, Utah, p. 1517-Plat.