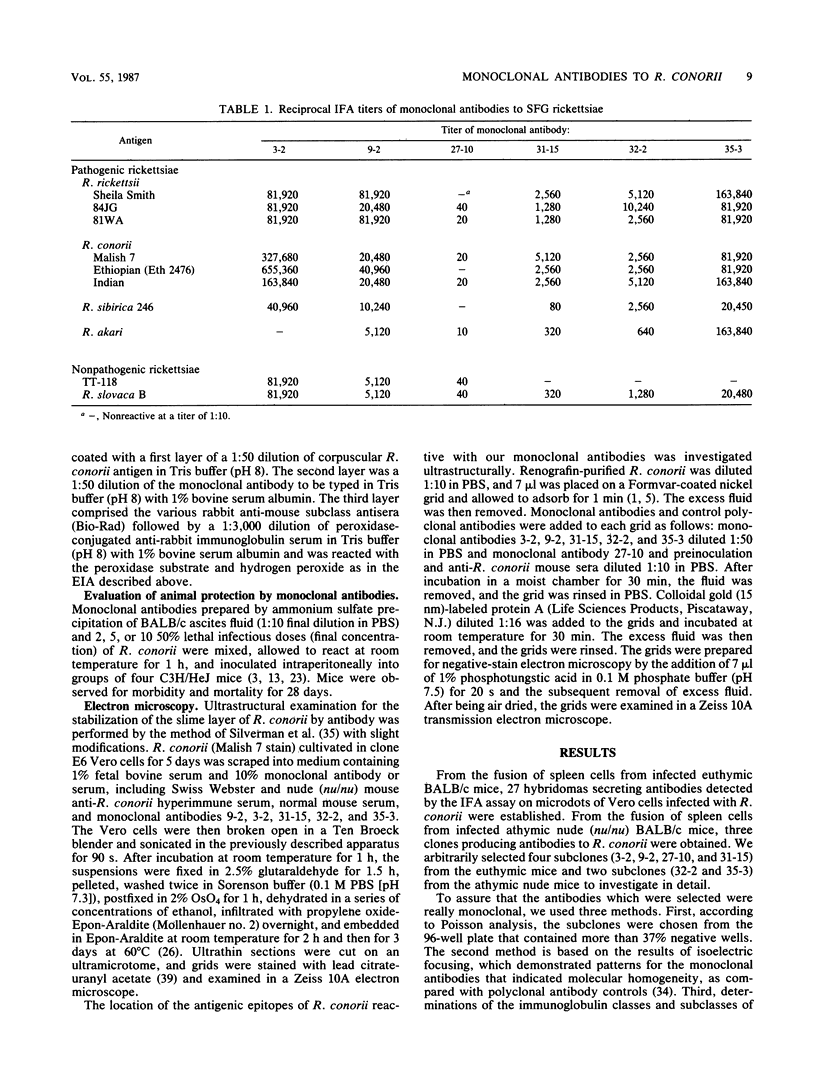
Abstract
Four monoclonal antibodies from euthymic mice and two monoclonal antibodies from athymic mice were directed against antigens of Rickettsia conorii, as shown by both indirect immunofluorescence and an enzyme immunoassay. There was extensive cross-reactivity with other spotted fever group rickettsiae. Euthymic monoclonal antibodies 3-2 and 9-2 (immunoglobulin G2a [IgG2a]) and 27-10 (IgG1) distinctly outlined the acetone-fixed rickettsial surface, as determined by indirect immunofluorescence; only monoclonal antibody 3-2 reacted with the intact rickettsial surface, as determined by colloidal gold-protein A negative-stain electron microscopy. Athymic monoclonal antibodies 32-2 and 35-3 (IgM) and euthymic monoclonal antibody 31-15 (IgG3) all demonstrated an irregular, extrarickettsial morphology, as determined by immunofluorescence, and ultrastructural cell wall blebs that were readily shed from the rickettsial surface. Monoclonal antibody 3-2, the only antibody to confer protection in lethally challenged mice, reacted with a high-molecular-weight protein in Western immunoblots. Monoclonal antibodies 31-15, 32-2, and 35-3 reacted with a "ladder" of proteinase K-resistant, lipopolysaccharidelike antigens. None of the monoclonal antibodies stabilized the ultrastructural rickettsial slime layer, but both athymic and euthymic polyclonal antibodies to R. conorii did. This is, to the best of our knowledge, the first report of the production of monoclonal antibodies to R. conorii and their use for antigenic analysis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., List R. H., Mann R. E., Hayes S. F., Thomas L. A. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985 Jun;151(6):1052–1060. doi: 10.1093/infdis/151.6.1052. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., List R. H., Mann R. E., Wiedbrauk D. L. Antigenic heterogeneity in high- and low-virulence strains of Rickettsia rickettsii revealed by monoclonal antibodies. Infect Immun. 1986 Feb;51(2):653–660. doi: 10.1128/iai.51.2.653-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Philip R. N., Casper E., Todd W. J., Mann R. E., Johnston M. R., Nauck C. J. Biological properties of rabbit antibodies to a surface antigen of Rickettsia rickettsii. Infect Immun. 1983 Apr;40(1):292–298. doi: 10.1128/iai.40.1.292-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL E. J., STOENNER H. G. Immunologic relationships among the spotted fever group of rickettsias determined by toxin neutralization tests in mice with convalescent animal serums. J Immunol. 1960 Feb;84:171–182. [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. Y., Limanek J. S., Chang C. C. A simple and efficient procedure for the rapid homogenization of cultured animal cells grown in monolayer. Anal Biochem. 1981 Sep 15;116(2):298–302. doi: 10.1016/0003-2697(81)90360-2. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Dumler J. S., Fiset P., Wisseman C. L., Jr, Snyder M. J., Levine M. M. Serodiagnosis of Rocky Mountain spotted fever: comparison of IgM and IgG enzyme-linked immunosorbent assays and indirect fluorescent antibody test. J Infect Dis. 1983 Nov;148(5):876–880. doi: 10.1093/infdis/148.5.876. [DOI] [PubMed] [Google Scholar]
- Eisemann C. S., Nypaver M. J., Osterman J. V. Susceptibility of inbred mice to rickettsiae of the spotted fever group. Infect Immun. 1984 Jan;43(1):143–148. doi: 10.1128/iai.43.1.143-148.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E. M., Goldwasser R. A., Bearman J. E., Sarov I., Sarov B., Torok V., Naggan L. Rickettsial antibody prevalence in southern Israel: IgG antibodies to Coxiella burnetii, Rickettsia typhi, and spotted fever group rickettsiae among urban- and rural-dwelling and Bedouin women. Am J Trop Med Hyg. 1983 Nov;32(6):1387–1391. doi: 10.4269/ajtmh.1983.32.1387. [DOI] [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Hanson B. A., Wisseman C. L., Jr, Waddell A., Silverman D. J. Some characteristics of heavy and light bands of Rickettsia prowazekii on Renografin gradients. Infect Immun. 1981 Nov;34(2):596–604. doi: 10.1128/iai.34.2.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Jarboe D. L., Eisemann C. S., Jerrells T. R. Production and characterization of cloned T-cell hybridomas that are responsive to Rickettsia conorii antigens. Infect Immun. 1986 Apr;52(1):326–330. doi: 10.1128/iai.52.1.326-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Eisemann C. S. Role of T-lymphocytes in production of antibody to antigens of Rickettsia tsutsugamushi and other Rickettsia species. Infect Immun. 1983 Aug;41(2):666–674. doi: 10.1128/iai.41.2.666-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange J. V., Walker D. H. Production and characterization of monoclonal antibodies to Rickettsia rickettsii. Infect Immun. 1984 Nov;46(2):289–294. doi: 10.1128/iai.46.2.289-294.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Mansueto S., Vitale G., Miceli M. D., Tringali G., Quartararo P., Picone D. M., Occhino C. A sero-epidemiological survey of asymptomatic cases of Boutonneuse fever in western Sicily. Trans R Soc Trop Med Hyg. 1984;78(1):16–18. doi: 10.1016/0035-9203(84)90163-9. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Montenegro N. R., Walker D. H., Hegarty B. C. Infection of genetically immunodeficient mice with Rickettsia conorii. Acta Virol. 1984 Nov;28(6):508–514. [PubMed] [Google Scholar]
- Pedersen C. E., Jr, Walters V. D. Comparative electrophoresis of spotted fever group rickettsial proteins. Life Sci. 1978 Feb;22(7):583–587. doi: 10.1016/0024-3205(78)90337-5. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- Public Health Weekly Reports for NOVEMBER 8, 1946. Public Health Rep. 1946 Nov 8;61(45):1605–1640. [PMC free article] [PubMed] [Google Scholar]
- Raoult D., Gallais H., Ottomani A., Resch J. P., Tichadou D., De Micco P., Casanova P. La forme maligne de la fièvre boutonneuse méditerranéenne. Six observations. Presse Med. 1983 Oct 29;12(38):2375–2378. [PubMed] [Google Scholar]
- STOENNER H. G., LACKMAN D. B., BELL E. J. Factors affecting the growth of rickettsias of the spotted fever group in fertile hens' eggs. J Infect Dis. 1962 Mar-Apr;110:121–128. doi: 10.1093/infdis/110.2.121. [DOI] [PubMed] [Google Scholar]
- Saravis C. A., Zamcheck N. Isoelectric focusing in agarose. J Immunol Methods. 1979;29(1):91–96. doi: 10.1016/0022-1759(79)90130-3. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. D., Jones M. External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun. 1978 Oct;22(1):233–246. doi: 10.1128/iai.22.1.233-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos T., Palmer E. L., Obijeski J. F., Martin M. L. Origin and structure of the group-specific, complement-fixing antigen of Rickettsia rickettsii. Appl Microbiol. 1974 Sep;28(3):481–488. doi: 10.1128/am.28.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Montenegro M. R., Hegarty B. C., Tringali G. R. Rocky Mountain spotted fever vaccine: a regional need. South Med J. 1984 Apr;77(4):447–449. doi: 10.1097/00007611-198404000-00009. [DOI] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Burgdorfer W. Plaque formation in tissue cultures by Rickettsia rickettsi isolated directly from whole blood and tick hemolymph. Infect Immun. 1972 Nov;6(5):736–738. doi: 10.1128/iai.6.5.736-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]










