Abstract
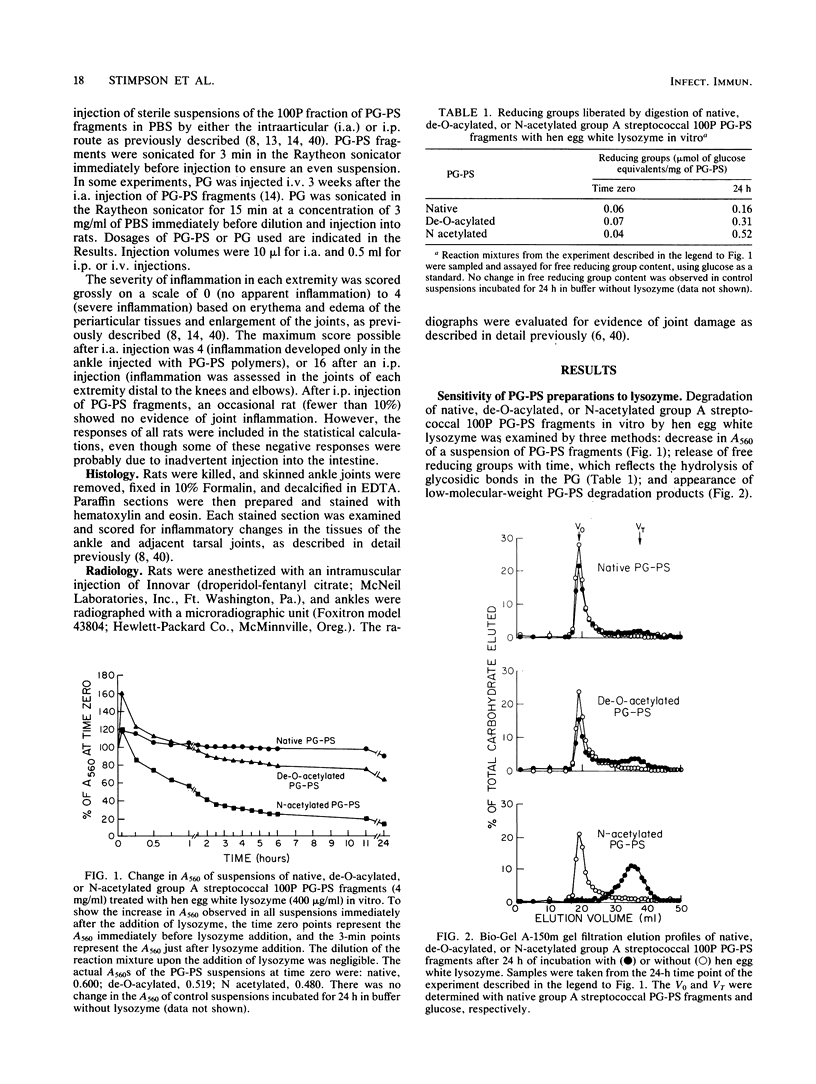
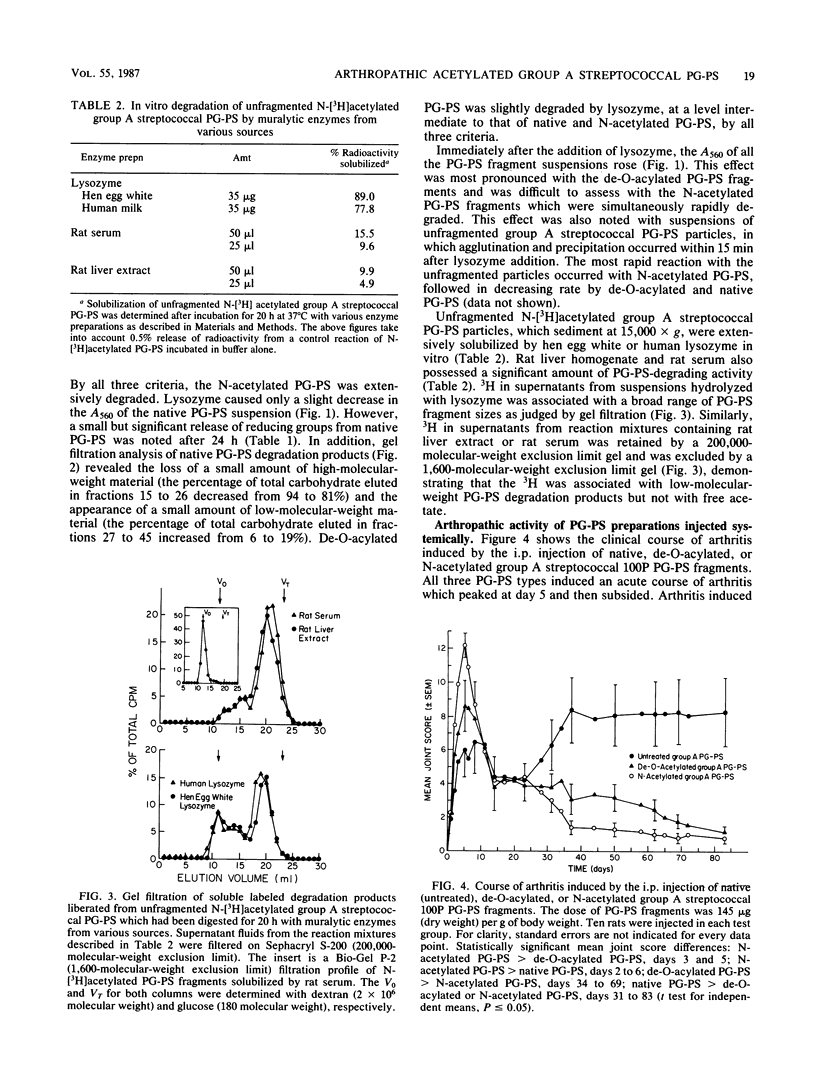
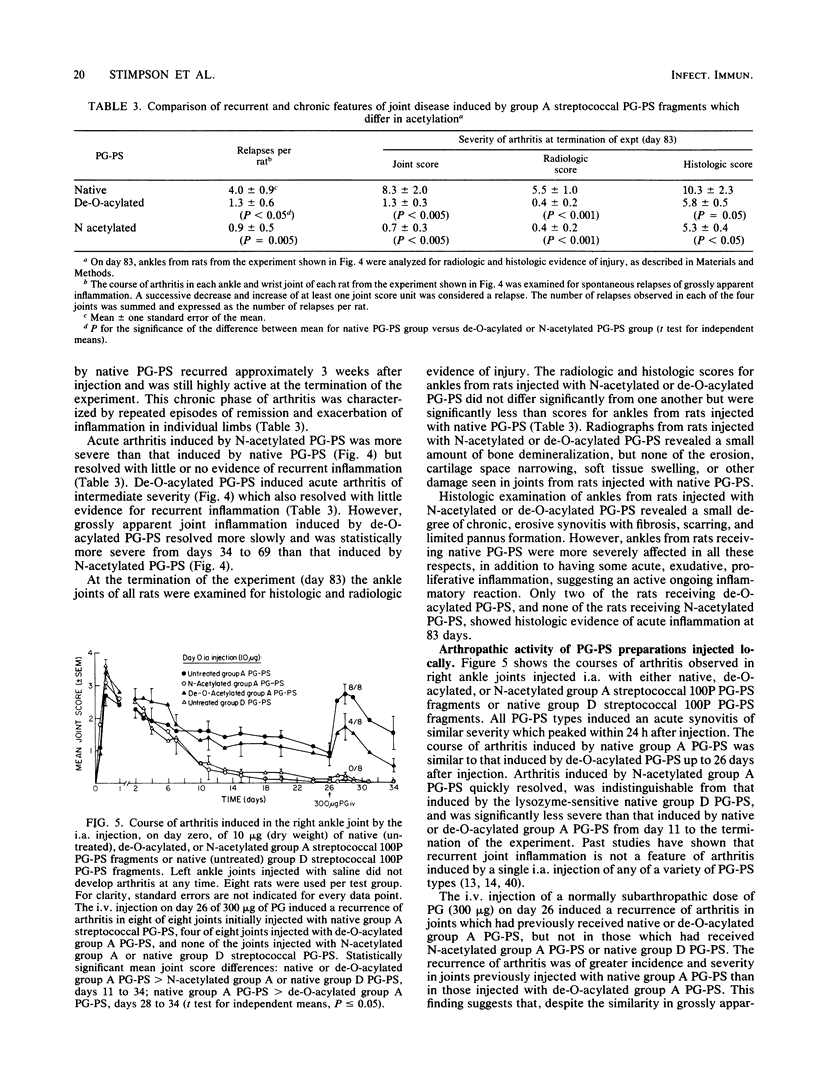
Purified group A streptococcal peptidoglycan-polysaccharide (PG-PS) fragments were either de-O-acylated, or acetylated and then de-O-acylated to yield N-acetylated PG-PS. Native PG-PS was poorly degraded, N-acetylated PG-PS was extensively degraded, and de-O-acylated PG-PS was only slightly degraded by hen egg white lysozyme. N-acetylated PG-PS was also extensively degraded by human lysozyme and partially degraded by rat serum or rat liver extract. After a single intraperitoneal injection of rats with a sterile, aqueous suspension, all PG-PS preparations induced acute arthritis. The acute arthritis induced by N-acetylated PG-PS was significantly more severe than that induced by native PG-PS; that induced by de-O-acylated PG-PS was of intermediate severity. After the acute reaction, rats injected with native PG-PS developed chronic relapsing erosive synovitis which remained severe for the duration of the experiment (83 days). In contrast, joint inflammation induced by N-acetylated PG-PS resolved within 6 weeks with little evidence of recurrent disease. Chronic arthritis induced by de-O-acylated PG-PS was of intermediate severity. In another assay of arthropathic activity, the arthritis in all rat ankle joints, which had been injected directly with native PG-PS, could be reactivated 3 weeks later by the intravenous injection of a small dose of PG. In contrast, only 50% of the joints initially injected with de-O-acylated PG-PS and none of the joints injected with N-acetylated PG-PS could be reactivated. These studies support the concepts that the resistance of PG-PS to muralytic digestion is crucial for chronic arthropathic activity and that the nature and degree of PG acetylation are important molecular determinants of the phlogistic activities of PG-PS polymers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. O-acetyl groups in the cell wall of Streptococcus faecalis. J Biol Chem. 1958 Feb;230(2):949–959. [PubMed] [Google Scholar]
- Abdulla E. M., Schwab J. H. Biological properties of streptococcal cell-wall particles. 3. Dermonecrotic reaction to cell-wall mucopeptides. J Bacteriol. 1966 Jan;91(1):374–383. doi: 10.1128/jb.91.1.374-383.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- Araki Y., Oba S., Araki S., Ito E. Enzymatic deacetylation of N-acetylglucosamine residues in cell wall peptidoglycan. J Biochem. 1980 Aug;88(2):469–479. doi: 10.1093/oxfordjournals.jbchem.a132994. [DOI] [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Cuttino J. T., Jr, Anderle S. K., Cromartie W. J., Schwab J. H. Radiologic analysis of arthritis in rats after systemic injection of streptococcal cell walls. Arthritis Rheum. 1979 Jan;22(1):25–35. doi: 10.1002/art.1780220105. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R. E., Anderle S. K., Chetty C., Stimpson S. A., Cromartie W. J., Schwab J. H. Comparison of inflammatory reactions induced by intraarticular injection of bacterial cell wall polymers. Am J Pathol. 1986 Feb;122(2):323–334. [PMC free article] [PubMed] [Google Scholar]
- Esser R. E., Stimpson S. A., Cromartie W. J., Schwab J. H. Reactivation of streptococcal cell wall-induced arthritis by homologous and heterologous cell wall polymers. Arthritis Rheum. 1985 Dec;28(12):1402–1411. doi: 10.1002/art.1780281213. [DOI] [PubMed] [Google Scholar]
- Fleming T. J., Wallsmith D. E., Rosenthal R. S. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect Immun. 1986 May;52(2):600–608. doi: 10.1128/iai.52.2.600-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis H. A., Miller S. E., Wheat R. W. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun. 1976 May;13(5):1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I., Gallis H. A., Cole R. M. Group A streptococci: localization in rabbits and guinea pigs following tissue injury. Science. 1969 Nov 28;166(3909):1161–1163. doi: 10.1126/science.166.3909.1161. [DOI] [PubMed] [Google Scholar]
- Glick A. D., Ranhand J. M., Cole R. M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect Immun. 1972 Sep;6(3):403–413. doi: 10.1128/iai.6.3.403-413.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYMANN H., MANNIELLO J. M., BARKULIS S. S. STRUCTURE OF STREPTOCOCCAL CELL WALLS. 3. CHARACTERIZATION OF AN ALANINE-CONTAINING GLUCOSAMINYLMURAMIC ACID DERIVATIVE LIBERATED BY LYSOZYME FROM STREPTOCOCCAL GLYCOPEPTIDE. J Biol Chem. 1964 Sep;239:2981–2985. [PubMed] [Google Scholar]
- Janusz M. J., Chetty C., Eisenberg R. A., Cromartie W. J., Schwab J. H. Treatment of experimental erosive arthritis in rats by injection of the muralytic enzyme mutanolysin. J Exp Med. 1984 Nov 1;160(5):1360–1374. doi: 10.1084/jem.160.5.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz M. J., Esser R. E., Schwab J. H. In vivo degradation of bacterial cell wall by the muralytic enzyme mutanolysin. Infect Immun. 1986 May;52(2):459–467. doi: 10.1128/iai.52.2.459-467.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisango K., Saiki I., Tanio Y., Okumura H., Araki Y., Sekikawa I., Azuma I., Yamamura Y. Structures and biological activities of peptidoglycans of Listeria monocytogenes and Propionibacterium acnes. J Biochem. 1982 Jul;92(1):23–33. doi: 10.1093/oxfordjournals.jbchem.a133918. [DOI] [PubMed] [Google Scholar]
- Lahav M., Ne'eman N., Adler E., Ginsburg I. Effect of leukocyte hydrolases on bacteria. I. Degradation of 14C-labeled Streptococcus and Staphylococcus by leukocyte lysates in vitro. J Infect Dis. 1974 May;129(5):528–537. doi: 10.1093/infdis/129.5.528. [DOI] [PubMed] [Google Scholar]
- Lehman T. J., Allen J. B., Plotz P. H., Wilder R. L. Bacterial cell wall composition, lysozyme resistance, and the induction of chronic arthritis in rats. Rheumatol Int. 1985;5(4):163–167. doi: 10.1007/BF00541517. [DOI] [PubMed] [Google Scholar]
- Lehman T. J., Allen J. B., Plotz P. H., Wilder R. L. Polyarthritis in rats following the systemic injection of Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 1983 Oct;26(10):1259–1265. doi: 10.1002/art.1780261013. [DOI] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H., Cromartie W. J. Relation of rheumatic-like cardiac lesions of the mouse to localization of group A streptococcal cell walls. J Exp Med. 1969 Jan 1;129(1):37–49. doi: 10.1084/jem.129.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H. Persistence of group a streptococcal cell walls related to chronic inflammation of rabbit dermal connective tissue. J Exp Med. 1967 Jun 1;125(6):1137–1148. doi: 10.1084/jem.125.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Blundell J. K., Perkins H. R. Strain-related differences in lysozyme sensitivity and extent of O-acetylation of gonococcal peptidoglycan. Infect Immun. 1982 Aug;37(2):826–829. doi: 10.1128/iai.37.2.826-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Folkening W. J., Miller D. R., Swim S. C. Resistance of O-acetylated gonococcal peptidoglycan to human peptidoglycan-degrading enzymes. Infect Immun. 1983 Jun;40(3):903–911. doi: 10.1128/iai.40.3.903-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Cromartie W. J., Ohanian S. H., Craddock J. G. Association of experimental chronic arthritis with the persistence of group A streptococcal cell walls in the articular tissue. J Bacteriol. 1967 Nov;94(5):1728–1735. doi: 10.1128/jb.94.5.1728-1735.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Processing of streptococcal cell walls by rat macrophages and human monocytes in vitro. Infect Immun. 1977 Sep;17(3):591–598. doi: 10.1128/iai.17.3.591-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Goodrum K. J., Warejcka D. J. Rat arthritis due to whole group B streptococci. Clinical and histopathologic features compared with groups A and D. Am J Pathol. 1983 Jul;112(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Goodrum K. J., Warejcka D. J., Weaver J. L., Miller H. L., Babcock L. Modulation of complement fixation and the phlogistic capacity of group A, B, and D streptococci by human lysozyme acting on their cell walls. Infect Immun. 1986 Jun;52(3):803–811. doi: 10.1128/iai.52.3.803-811.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Brown R. R., Anderle S. K., Klapper D. G., Clark R. L., Cromartie W. J., Schwab J. H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986 Jan;51(1):240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Esser R. E., Cromartie W. J., Schwab J. H. Comparison of in vivo degradation of 125I-labeled peptidoglycan-polysaccharide fragments from group A and group D streptococci. Infect Immun. 1986 May;52(2):390–396. doi: 10.1128/iai.52.2.390-396.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Wells A., Pararajasegaram G., Baldwin M., Yang C. H., Hammer M., Fox A. Uveitis and arthritis induced by systemic injection of streptococcal cell walls. Invest Ophthalmol Vis Sci. 1986 Jun;27(6):921–925. [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]



