Abstract
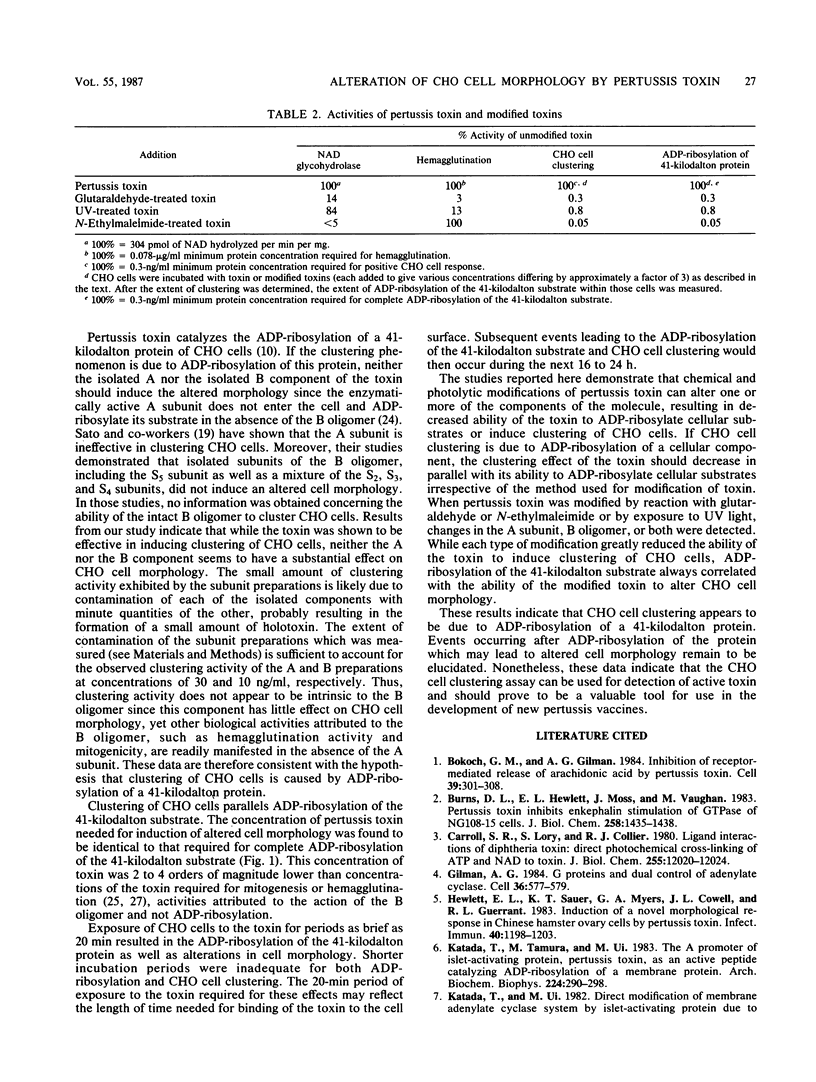
The mechanism by which pertussis toxin induces morphological changes in Chinese hamster ovary cells was studied to determine whether the resulting clustered growth pattern is due to toxin-catalyzed ADP-ribosylation of a cellular substrate. While pertussis toxin was extremely potent in inducing morphological changes in Chinese hamster ovary cells, preparations of isolated A subunit or B oligomer exhibited greatly reduced activity. The clustered growth response of these cells correlated with ADP-ribosylation of a 41-kilodalton cellular substrate for the toxin in that the toxin concentration and time of exposure to the toxin required for ADP-ribosylation were the same as those needed for alterations in cellular morphology. Moreover, pertussis toxin modified by either chemical or photolytic methods exhibited similar decreases in the ability to ADP-ribosylate the cellular substrate and alter cell morphology. These results suggest that clustering of Chinese hamster ovary cells is due to toxin-catalyzed ADP-ribosylation of a 41-kilodalton substrate. Therefore, alteration in Chinese hamster ovary cell morphology can be used as a measure of toxin activity. This assay should prove to be a useful tool in the development and evaluation of new pertussis vaccines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Burns D. L., Hewlett E. L., Moss J., Vaughan M. Pertussis toxin inhibits enkephalin stimulation of GTPase of NG108-15 cells. J Biol Chem. 1983 Feb 10;258(3):1435–1438. [PubMed] [Google Scholar]
- Carroll S. F., Lory S., Collier R. J. Ligand interactions of diphtheria toxin. III. Direct photochemical cross-linking of ATP and NAD to toxin. J Biol Chem. 1980 Dec 25;255(24):12020–12024. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Tamura M., Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983 Jul 1;224(1):290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Bruni P., Hsia J. A., Tsai S. C., Watkins P. A., Halpern J. L., Burns D. L., Kanaho Y., Chang P. P., Hewlett E. L. Pertussis toxin-catalyzed ADP-ribosylation: effects on the coupling of inhibitory receptors to the adenylate cyclase system. J Recept Res. 1984;4(1-6):459–474. doi: 10.3109/10799898409042567. [DOI] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Burns D. L., Hsia J. A., Yost D. A., Myers G. A., Hewlett E. L. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein). J Biol Chem. 1983 Oct 10;258(19):11879–11882. [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Bergman R. K., Sadowski P. L. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981 Sep;33(3):820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Ito K., Nakamura T., Kajikawa N., Maruyama Y., Ui M. Dual mechanisms involved in development of diverse biological activities of islet-activating protein, pertussis toxin, as revealed by chemical modification of lysine residues in the toxin molecule. Biochim Biophys Acta. 1984 Sep 28;801(2):232–243. doi: 10.1016/0304-4165(84)90072-2. [DOI] [PubMed] [Google Scholar]
- Oda M., Cowell J. L., Burstyn D. G., Manclark C. R. Protective activities of the filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella pertussis in mice. J Infect Dis. 1984 Dec;150(6):823–833. doi: 10.1093/infdis/150.6.823. [DOI] [PubMed] [Google Scholar]
- Okajima F., Katada T., Ui M. Coupling of the guanine nucleotide regulatory protein to chemotactic peptide receptors in neutrophil membranes and its uncoupling by islet-activating protein, pertussis toxin. A possible role of the toxin substrate in Ca2+-mobilizing receptor-mediated signal transduction. J Biol Chem. 1985 Jun 10;260(11):6761–6768. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Sato H., Ito A., Chiba J., Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984 Nov;46(2):422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Role of antibody to leukocytosis-promoting factor hemagglutinin and to filamentous hemagglutinin in immunity to pertussis. Infect Immun. 1981 Mar;31(3):1223–1231. doi: 10.1128/iai.31.3.1223-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kimura M., Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984 Jan 21;1(8369):122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Watkins P. A., Burns D. L., Kanaho Y., Liu T. Y., Hewlett E. L., Moss J. ADP-ribosylation of transducin by pertussis toxin. J Biol Chem. 1985 Nov 5;260(25):13478–13482. [PubMed] [Google Scholar]
- Zhang J. M., Cowell J. L., Steven A. C., Carter P. H., McGrath P. P., Manclark C. R. Purification and characterization of fimbriae isolated from Bordetella pertussis. Infect Immun. 1985 May;48(2):422–427. doi: 10.1128/iai.48.2.422-427.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]




