Abstract
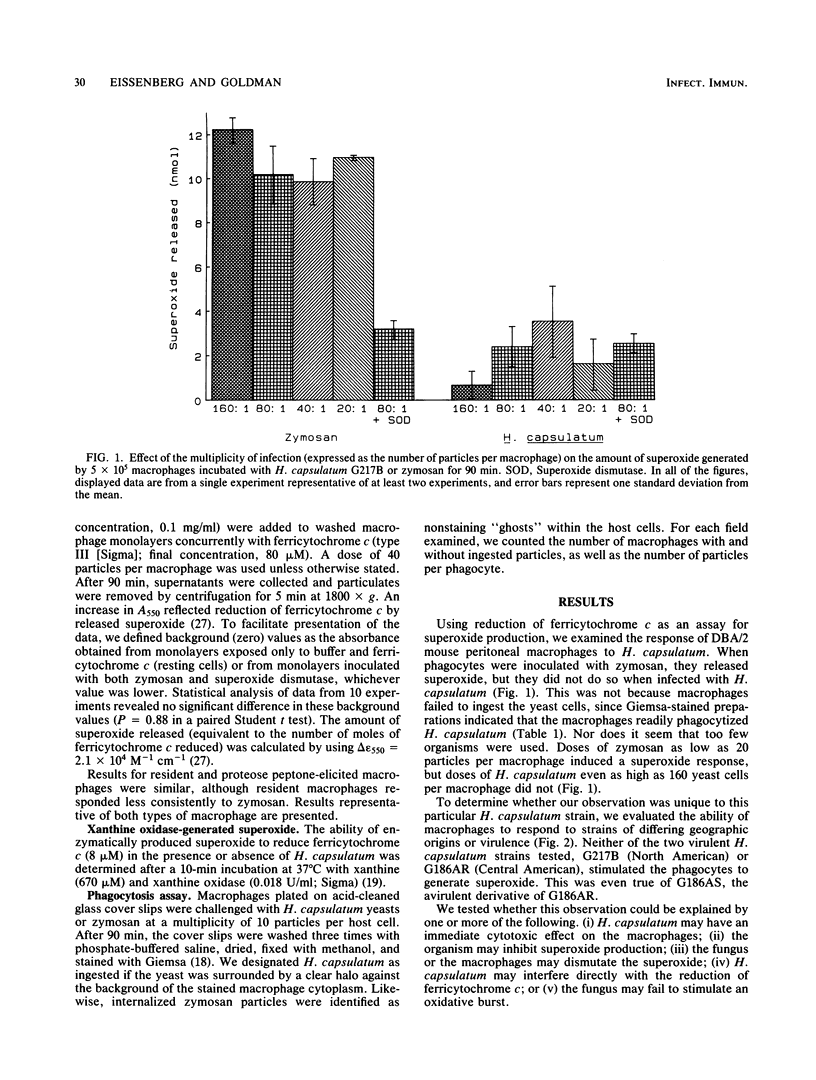
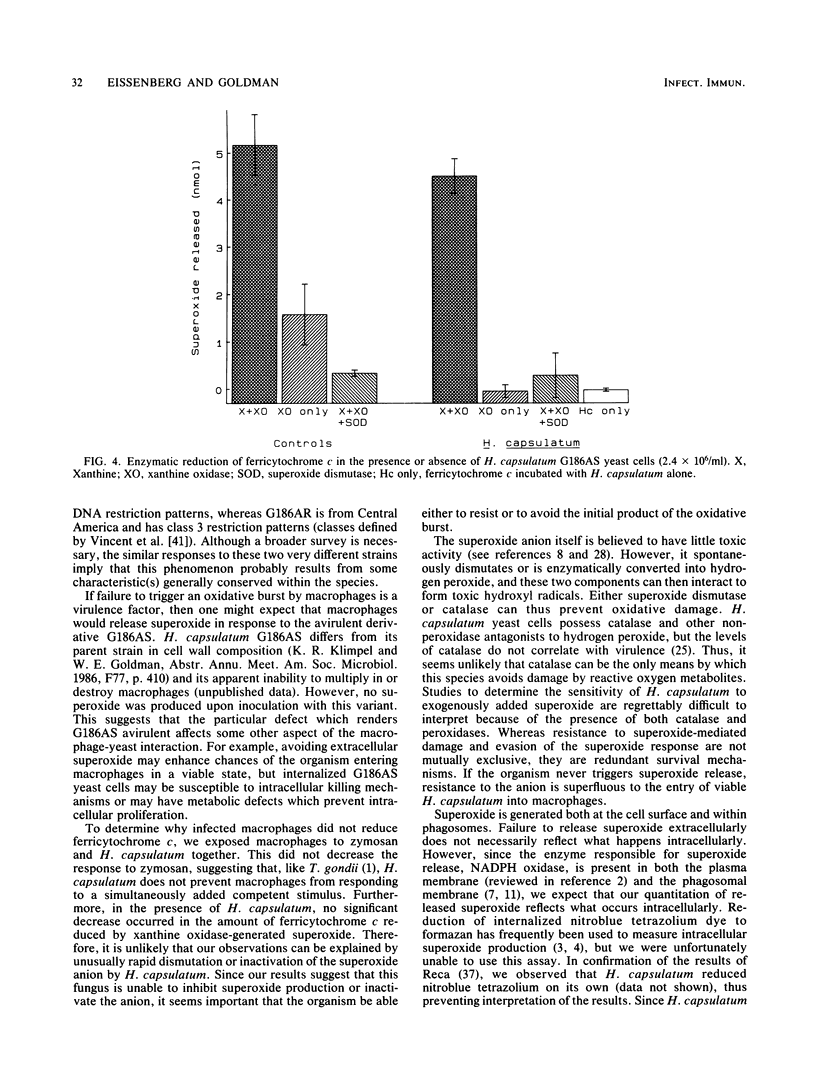
The yeast form of the dimorphic fungus Histoplasma capsulatum survives within macrophages after phagocytosis. To do so, it must avoid, inhibit, or resist a variety of toxic oxygen metabolites. Using ferricytochrome c reduction to assay superoxide release, we examined the response of mouse macrophages to the yeast form of various H. capsulatum strains. Doses of zymosan as low as 20 particles per macrophage elicited superoxide, whereas H. capsulatum failed to induce superoxide even at 160 yeast cells per macrophage. This phenomenon was observed with two virulent strains of H. capsulatum (G217B and G186A) and with an avirulent variant of G186A. Over a 15- to 150-min observation period, zymosan stimulated increasing reduction of ferricytochrome c, but H. capsulatum did not. When added concurrently with zymosan, H. capsulatum had no effect on superoxide production. Therefore, H. capsulatum was unable either to inactivate the oxygen radical or inhibit host cell superoxide response to other competent stimuli. Enzymatically generated superoxide reduced ferricytochrome c even in the presence of H. capsulatum, again implying that the organism does not readily inactivate superoxide. This experiment also demonstrated that the yeast did not interfere with the assay used. Thus, rather than inhibiting superoxide generation or inactivating the anion, H. capsulatum yeast cells appear to avoid the toxic effects of superoxide by failing to trigger its release.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976 Aug;48(2):309–313. [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Scates S. M., Moring S. E., Deem R., Misra H. P. Purification and properties of a unique superoxide dismutase from Nocardia asteroides. J Biol Chem. 1983 Jan 10;258(1):91–96. [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Bellavite P., Serra M. C., Davoli A., Rossi F. Selective enrichment of NADPH oxidase activity in phagosomes from guinea pig polymorphonuclear leukocytes. Inflammation. 1982 Mar;6(1):21–29. doi: 10.1007/BF00910716. [DOI] [PubMed] [Google Scholar]
- Bus J. S., Gibson J. E. Mechanisms of superoxide radical-mediated toxicity. J Toxicol Clin Toxicol. 1982 Aug;19(6-7):689–697. doi: 10.3109/15563658208990398. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon J. Y., Roberts M. B., Blackwell J. M. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology. 1984 Oct;53(2):345–355. [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Shuford W. W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect Immun. 1985 Jan;47(1):234–241. doi: 10.1128/iai.47.1.234-241.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Newburger P. E., Chovaniec M. E. NAD(P)H-dependent superoxide production by phagocytic vesicles from guinea pig and human granulocytes. J Biol Chem. 1980 Jul 25;255(14):6584–6588. [PubMed] [Google Scholar]
- Domer J. E., Hamilton J. G., Harkin J. C. Comparative study of the cell walls of the yeastlike and mycelial phases of Histoplasma capsulatum. J Bacteriol. 1967 Aug;94(2):466–474. doi: 10.1128/jb.94.2.466-474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., MacPherson G. G., Gordon S. Interaction of human monocytes, macrophages, and polymorphonuclear leukocytes with zymosan in vitro. Role of type 3 complement receptors and macrophage-derived complement. J Clin Invest. 1985 Dec;76(6):2368–2376. doi: 10.1172/JCI112249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Krick J. A., Remington J. S. Effects of human neutrophils and monocytes on Nocardia asteroides: failure of killing despite occurrence of the oxidative metabolic burst. J Infect Dis. 1980 Sep;142(3):432–438. doi: 10.1093/infdis/142.3.432. [DOI] [PubMed] [Google Scholar]
- HOWARD D. H. INTRACELLULAR BEHAVIOR OF HISTOPLASMA CAPSULATUM. J Bacteriol. 1964 Jan;87:33–38. doi: 10.1128/jb.87.1.33-38.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag M. R., Suntharalingam K., Fikrig S. The effect of Chlamydia trachomatis on luminol-dependent chemiluminescence of human polymorphonuclear leukocytes: requirements for opsonization. J Infect Dis. 1985 Jun;151(6):1045–1051. doi: 10.1093/infdis/151.6.1045. [DOI] [PubMed] [Google Scholar]
- Hassan H. M. Determination of microbial damage caused by oxygen free radicals, and the protective role of superoxide dismutase. Methods Enzymol. 1984;105:404–412. doi: 10.1016/s0076-6879(84)05056-4. [DOI] [PubMed] [Google Scholar]
- Hed J., Stendahl O. Differences in the ingestion mechanisms of IgG and C3b particles in phagocytosis by neutrophils. Immunology. 1982 Apr;45(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Holzer T. J., Nelson K. E., Crispen R. G., Andersen B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect Immun. 1986 Feb;51(2):514–520. doi: 10.1128/iai.51.2.514-520.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H. Fate of Histoplasma capsulatum in guinea pig polymorphonuclear leukocytes. Infect Immun. 1973 Sep;8(3):412–419. doi: 10.1128/iai.8.3.412-419.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H. Studies on the catalase of Histoplasma capsulatum. Infect Immun. 1983 Mar;39(3):1161–1166. doi: 10.1128/iai.39.3.1161-1166.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978 Jan;104(1):37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Measurement of O2- secreted by monocytes and macrophages. Methods Enzymol. 1984;105:365–369. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi G. S., Guiliacci P. L. Cell wall studies of Histoplasma capsulatum. Sabouraudia. 1967 Feb;5(3):180–188. [PubMed] [Google Scholar]
- Kossack R. E., Guerrant R. L., Densen P., Schadelin J., Mandell G. L. Diminished neutrophil oxidative metabolism after phagocytosis of virulent Salmonella typhi. Infect Immun. 1981 Feb;31(2):674–678. doi: 10.1128/iai.31.2.674-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Dreyfus L. A., Robertson D. C. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):737–742. doi: 10.1128/iai.23.3.737-742.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Snyder I. S. Mannose-sensitive stimulation of human leukocyte chemiluminescence by Escherichia coli. Infect Immun. 1979 Dec;26(3):1014–1019. doi: 10.1128/iai.26.3.1014-1019.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Pearson R. D., Harcus J. L., Symes P. H., Romito R., Donowitz G. R. Failure of the phagocytic oxidative response to protect human monocyte-derived macrophages from infection by Leishmania donovani. J Immunol. 1982 Sep;129(3):1282–1286. [PubMed] [Google Scholar]
- Reca M. E. Reduction of a tetrazolium salt in determining growth activity of yeast-phase Histoplasma capsulatum. Appl Microbiol. 1968 Feb;16(2):236–238. doi: 10.1128/am.16.2.236-238.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Rossi F. Reversible metabolic stimulation of polymorphonuclear leukocytes and macrophages by concanavalin A. Nat New Biol. 1973 May 23;243(125):111–112. [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent R. D., Goewert R., Goldman W. E., Kobayashi G. S., Lambowitz A. M., Medoff G. Classification of Histoplasma capsulatum isolates by restriction fragment polymorphisms. J Bacteriol. 1986 Mar;165(3):813–818. doi: 10.1128/jb.165.3.813-818.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Johnston R. B., Jr Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. J Exp Med. 1984 Feb 1;159(2):405–416. doi: 10.1084/jem.159.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]



