Abstract
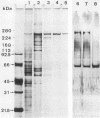
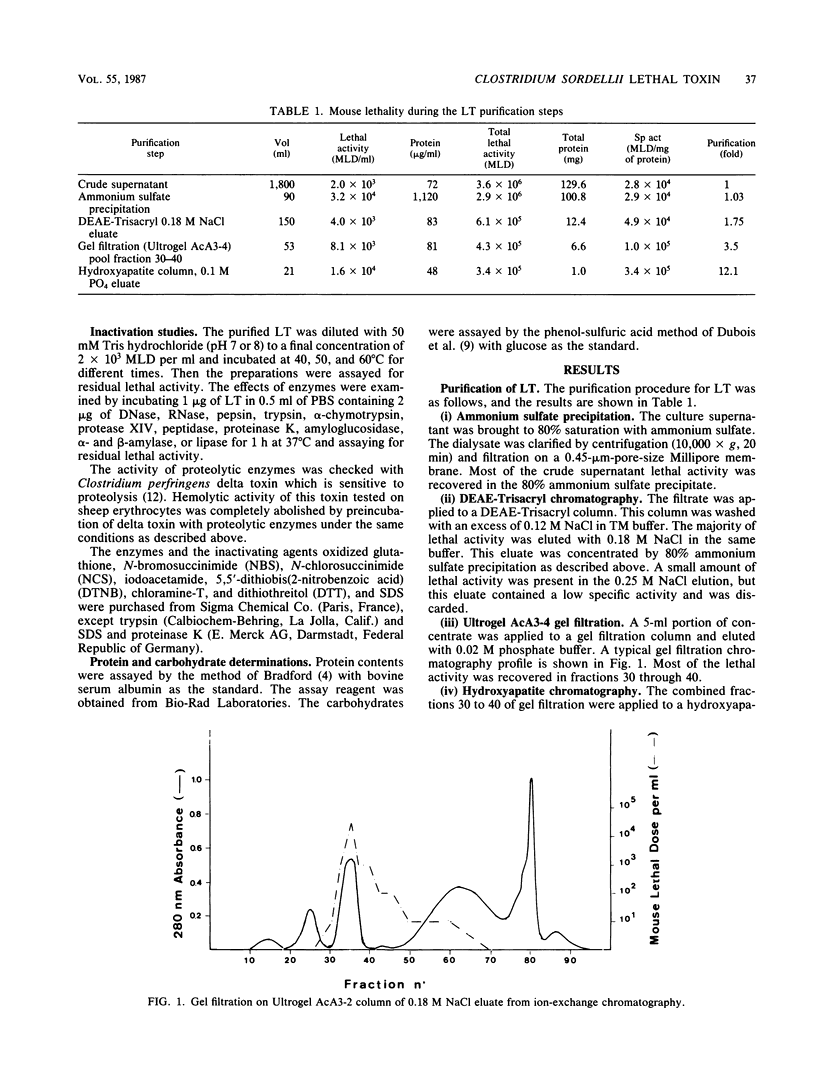
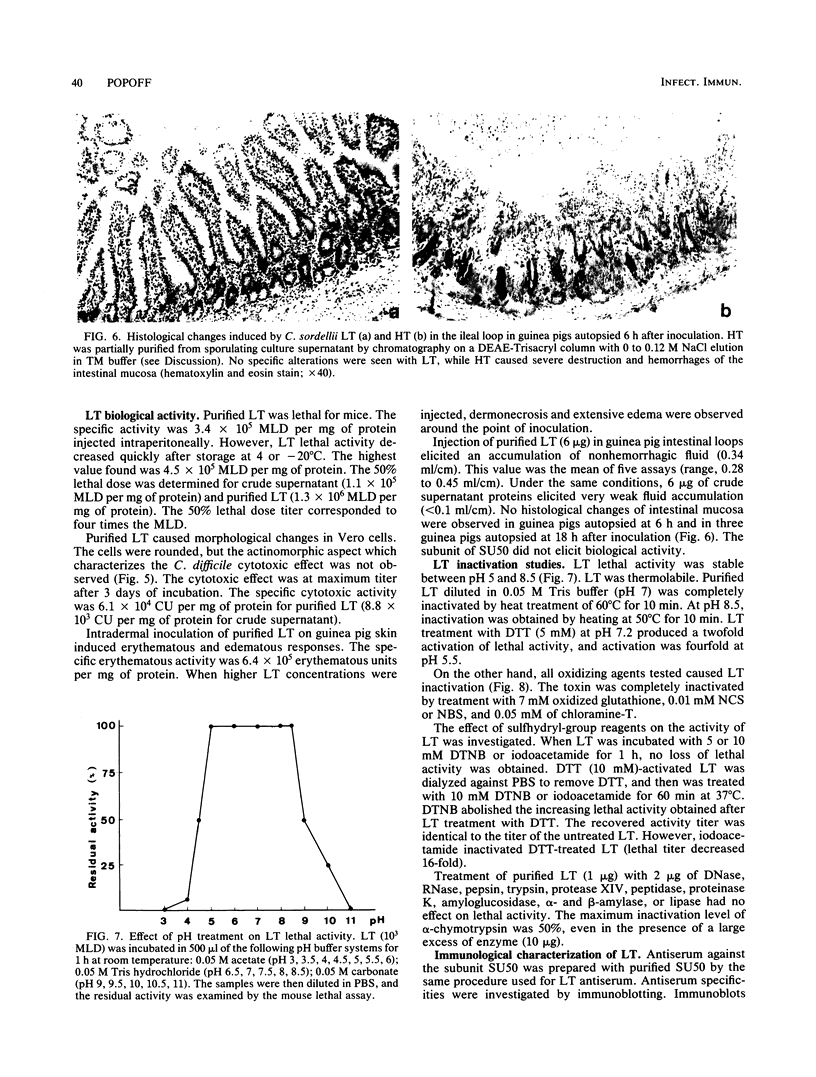
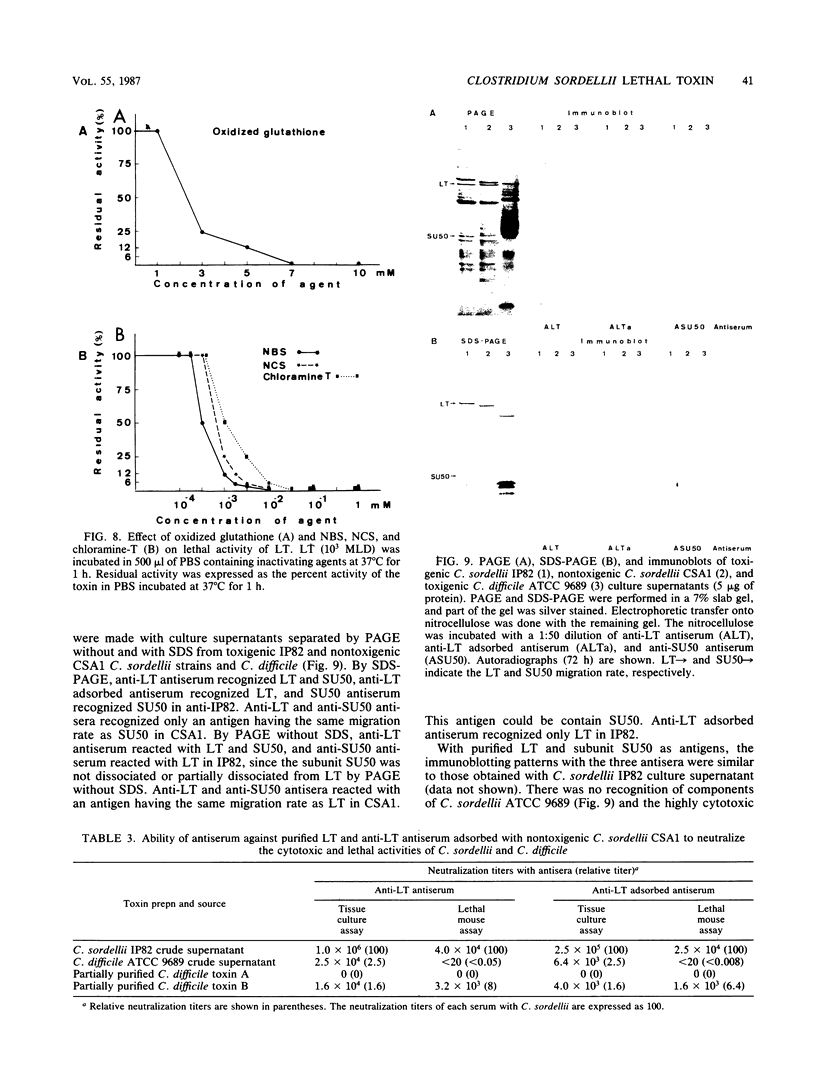
Lethal toxin (LT) was purified from Clostridium sordellii IP82 by DEAE-Trisacryl, Ultrogel AcA3-4 gel filtration, and hydroxyapatite column chromatography. The molecular weight of purified LT was estimated to be 240,000 to 250,000, and the pI was at pH 4.55. LT was lethal for mice by intraperitoneal injection (3.4 X 10(5) mouse lethal doses per mg of protein), cytotoxic for Vero cells (6.1 X 10(4) cytotoxic units per mg of protein), erythematous and edematous by intradermal injection in guinea pigs, and induced a moderate fluid accumulation in the guinea pig intestinal loop test. The lethal activity was inactivated by N-bromosuccinimide, N-chlorosuccinimide, chloramine-T, and sodium dodecyl sulfate. The data suggest that tryptophan and methionine residues present in the toxin are important for lethal activity. Furthermore, LT was inactivated by oxidized glutathione and activated by dithiothreitol. Inactivation by sulfhydryl-group reagents 5,5'-dithiobis(2-nitrobenzoic acid) and iodoacetamide was only obtained with dithiothreitol-treated LT. Thiol groups which are protected as a disulfide bond(s) seem to be essential for the LT activity. A specific antiserum against LT neutralized the biological activities of LT and also cytotoxic activity and lethal activity of Clostridium difficile toxin B but not of C. difficile toxin A. However, this serum did not recognize antigen from C. difficile culture supernatant by immunoblotting. It was concluded that antibodies prepared from C. sordellii LT that neutralized C. difficile cytotoxic activity recognized a low number of epitopes or tertiary structures of C. difficile cytotoxin.
Full text
PDF

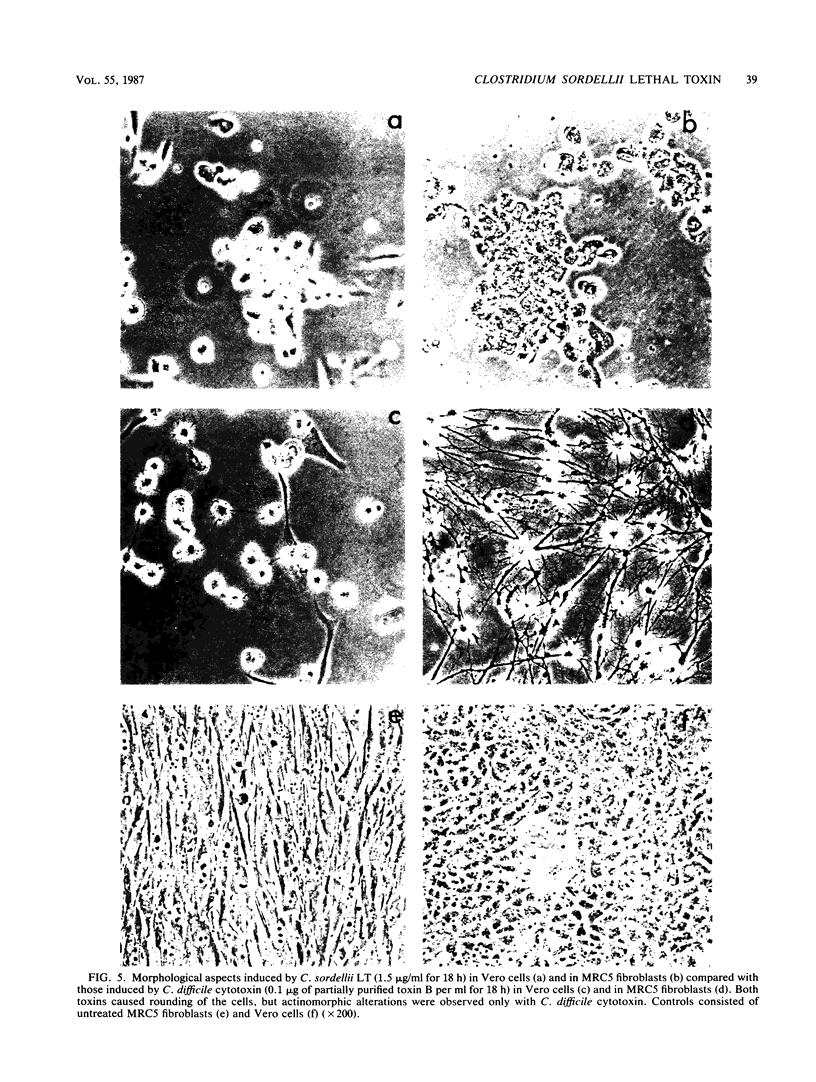


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Mashat R. R., Taylor D. J. Bacteria in enteric lesions of cattle. Vet Rec. 1983 Jan 1;112(1):5–10. doi: 10.1136/vr.112.1.5. [DOI] [PubMed] [Google Scholar]
- Arseculeratne S. N., Panabokké R. G., Wijesundera S. The toxins responsible for the lesions of Clostridium sordelli gas gangrene. J Med Microbiol. 1969 Feb;2(1):37–53. doi: 10.1099/00222615-2-1-37. [DOI] [PubMed] [Google Scholar]
- BROOKS M. E., EPPS H. B. Taxonomic studies of the genus Clostridium: Clostrididum bifermentans and C. sordellii. J Gen Microbiol. 1959 Aug;21:144–155. doi: 10.1099/00221287-21-1-144. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Gorbach S. L., Bartlett J. B. Neutralization of Clostridium difficile toxin by Clostridium sordellii antitoxins. Infect Immun. 1978 Nov;22(2):418–422. doi: 10.1128/iai.22.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dévényi T. Modified single-column procedure for the automatic analysis of amino acids. Acta Biochim Biophys Acad Sci Hung. 1969;4(3):297–299. [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Jolivet-Reynaud C., Alouf J. E. Binding of Clostridium perfringens 125I-labeled delta-toxin to erythrocytes. J Biol Chem. 1983 Feb 10;258(3):1871–1877. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Libby J. M., Wilkins T. D. Production of antitoxins to two toxins of Clostridium difficile and immunological comparison of the toxins by cross-neutralization studies. Infect Immun. 1982 Jan;35(1):374–376. doi: 10.1128/iai.35.1.374-376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Ogura H., Tanaka J., Tanabe N., Yamakawa K., Hatano M., Nishida S. Difference in susceptibility of various cell cultures to cytotoxic culture filtrates of Clostridium sordellii. Microbiol Immunol. 1984;28(4):493–497. doi: 10.1111/j.1348-0421.1984.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Tanabe N., Yamakawa K., Nishida S. Cytotoxin production by Clostridium sordellii strains. Microbiol Immunol. 1983;27(6):495–502. doi: 10.1111/j.1348-0421.1983.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Popoff M. R. Bacteriological examination in enterotoxaemia of sheep and lamb. Vet Rec. 1984 Mar 31;114(13):324–324. doi: 10.1136/vr.114.13.324. [DOI] [PubMed] [Google Scholar]
- Popoff M. R., Guillou J. P., Carlier J. P. Taxonomic position of lecithinase-negative strains of Clostridium sordellii. J Gen Microbiol. 1985 Jul;131(7):1697–1703. doi: 10.1099/00221287-131-7-1697. [DOI] [PubMed] [Google Scholar]
- Popoff M. R., Ravisse P. Lesions produced by Clostridium butyricum strain CB 1002 in ligated intestinal loops in guinea pigs. J Med Microbiol. 1985 Jun;19(3):351–357. doi: 10.1099/00222615-19-3-351. [DOI] [PubMed] [Google Scholar]
- Richards S. M., Hunt B. W. Clostridium sordellii in lambs. Vet Rec. 1982 Jul 3;111(1):22–22. doi: 10.1136/vr.111.1.22-a. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Fujii Y., Matsuura M. Effect of oxidizing agents and sulfhydryl group reagents on beta toxin from Clostridium perfringens type C. Microbiol Immunol. 1980;24(7):595–601. doi: 10.1111/j.1348-0421.1980.tb02862.x. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Role of one tryptophan residue in the lethal activity of Clostridium perfringens epsilon toxin. Biochem Biophys Res Commun. 1985 Apr 30;128(2):760–766. doi: 10.1016/0006-291x(85)90112-3. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Burstein Y., Patchornik A. Selective oxidation of methionine residues in proteins. Biochemistry. 1975 Oct 7;14(20):4497–4503. doi: 10.1021/bi00691a025. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Patchornik A., Burstein Y. Selective chemical cleavage of tryptophanyl peptide bonds by oxidative chlorination with N-chlorosuccinimide. Biochemistry. 1976 Nov 16;15(23):5071–5075. doi: 10.1021/bi00668a019. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K., Nakamura S., Nishida S. Separation of two cytotoxins of Clostridium sordellii strains. Microbiol Immunol. 1985;29(6):553–557. doi: 10.1111/j.1348-0421.1985.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Yamakawa K., Tanabe N., Okada Y., Nishida S., Nakamura S. Rabbit ileal loop responses to Clostridium sordellii strains. Microbiol Immunol. 1983;27(9):807–809. doi: 10.1111/j.1348-0421.1983.tb00646.x. [DOI] [PubMed] [Google Scholar]