Abstract
High vitamin D intake is associated with reduced insulin resistance. Expression of extra-renal 1α,25-dihydroxyvitamin D hydroxylase (1α-hydroxylase) has been reported in several tissues and contributes to local synthesis of 1α,25-dihydroxyvitamin D3 (1,25(OH)2D) from the substrate 25-hydroxyvitamin D (25OHD). Expression and dietary regulation of 1α-hydroxylase in tissues associated with energy metabolism, including adipose tissue, has not been assessed. Male Wistar rats were fed a high calcium (1.5%) and high vitamin D (10,000 IU/kg) or a low calcium (0.25%), low vitamin D (400 IU/kg) with either a high fat (40% energy) or high sucrose (66% energy) dietary background for 14 weeks. Expression of 1α-hydroxylase, assessed by real time PCR, was detected in adipose tissue and did not differ with dietary level of calcium and vitamin D. 1α-hydroxylase mRNA was also detected in 3T3-L1 preadipocytes and 25OHD treatment at 10 nM levels induced 1,25(OH)2D responsive gene, CYP24, and this response was reduced in the presence of the p450 inhibitor, ketoconazole. In addition, 3H 25OHD was converted to 3H 1,25(OH)2D in intact 3T3-L1 preadipocytes. Cumulatively, these results demonstrate that 1α-hydroxylase is expressed in adipose tissue and is functional in cultured adipocytes. Thus, the capacity for local production may play a role in regulating adipocyte growth and metabolism.
Keywords: Vitamin D; 1,25-dihydroxyvitamin D; 1α-hydroxylase; adipocyte; diet; rats
1. Introduction
In addition to the well known function of regulating calcium homeostasis, it is proposed that vitamin D modulates a broader range of physiological functions. For example, low serum 25-hydroxyvitamin D3 (25OHD) level is associated with higher body mass index (BMI) [1,2] and insulin resistance [3]. It is therefore important to investigate the metabolism of vitamin D in the adipocyte to understand how vitamin D status may impact both fat mass and local insulin resistance in adipose tissue.
Vitamin D is converted in the liver to 25OHD and circulates bound to serum vitamin D binding protein. The vitamin D metabolite, 25OHD, a status marker of vitamin D sufficiency, is converted to 1,25-dihydroxyvitamin D (1,25(OH)2D), the most active form of vitamin D. This active vitamin D metabolite, 1,25(OH)2D, mediates genomic regulation through the nuclear vitamin D receptor (VDR), a member of the steroid hormone receptor family [4]. Although 25OHD binds to the VDR, it's affinity is 1000 fold less than 1,25(OH)2D. Finally, 1,25(OH)2D is targeted for degradation by a further hydroxylation at the 24 position by the enzyme, 24-hydroxylase, which is product of the CYP24 gene. The activity of 24-hydroxylase is highly induced by 1,25(OH)2D, therefore, providing a negative feedback mechanism.
The enzyme 1α-hydroxylase (CYP27B1) is primarily expressed in the kidney [5] and converts 25OHD to the active form of vitamin D, 1α,25 dihydroxyvitamin D3 (1,25(OH)2D). Previous literature demonstrates that there is also extra-renal expression of 1α-hydroxylase, which may contribute to local production of 1,25(OH)2D. This locally produced 1,25(OH)2D may regulate cellular responses, including modulating cell proliferation, and differentiation through paracrine, autocrine action, or both [6, 7, 8]. Extra-renal tissues in which expression of 1α-hydroxylase are described include bone [9], ovarian [10], pancreatic islets [11], brain [12], and parathyroid gland [13]. In addition, activity of 1α-hydroxylase in extra-renal tissues has also been shown in placenta [14], immune cells [15,16,17], keratinocyte [18], lung [19], prostate [20], cervical tissue [21], intestine [22], vascular endothelial cells [23] and smooth muscle [24]. In addition, the substrate of 1α-hydroxylase, 25OHD, has growth inhibitory action similar to the active metabolite (1,25(OH)2D), in cultured mammary cells [25]. Liver, a tissue that plays an active role in energy metabolism and glucose homeostasis, expresses 1α-hydroxylase and is able to convert 25OHD into 1,25(OH)2D [26,27]. However, the expression of the 1α-hydroxylase in extrahepatic tissues including skeletal muscle and adipose tissue, which are critical in regulating energy metabolism, has not been investigated.
The expression and activity of the renal 1α-hydroxylase is tightly regulated by serum calcium [28], phosphorus [29], parathyroid hormone (PTH) [30], calcitonin [31], and 1,25(OH)2D [32]. On the other hand, regulation of 1α-hydroxylase has been shown to be independent of PTH in untransformed, primary culture of prostate epithelial cells [33] and dietary phytoestrogens upregulate activity of the 1α-hydroxylase in colon [34] and breast cells [34]. Although high vitamin D and calcium in the diet down regulates renal 1α-hydroxylase expression, regulation of extra-renal 1α-hydroxylases by dietary components has not been determined. The purpose of the current study was to determine the expression of 1α-hydroxylase in adipose tissue; to determine the responsiveness of 1α-hydroxylase in adipose tissue to dietary vitamin D, calcium, and macronutrient background; and to determine the activity of the 1α-hydroxylase in adipocytes.
2. Material and Methods
2.1 Animals and diets
Male Wistar rats (n=32; Harlan, Indianapolis, IN) ) weighing 175-190 gms were individually housed and maintained on a 12 hour light/dark cycle at constant room temperature (22 ± 2 °C). Animals were fed a pelleted chow diet (#2014, Harlan Teklad, Indianapolis, IN) for one week and then randomly assigned to the experimental diets (Research Diets Inc., New Brunswick, New Jersey) shown in Table 1. The treatment diets were based on the AIN 93 G diet with modifications as described. Rats were fed either a control diet (n=8), a high fat diet with 40% of energy primarily from soybean oil, or high sucrose with 66% of energy primarily from sucrose. Diets contained either low dairy product component diet (LD) or high dairy product components (HD) as shown in Table 1 (n=6/group). Rats were fed the experimental diets for 14 weeks. At the end of the study, the rats were fasted overnight, euthanized with CO2 and exsanguinated by decapitation. Epididymal fat tissues were rapidly minced, and frozen in Trizol (Invitrogen, Carlsbad, CA) in liquid nitrogen before storage in -80 °C.
Table 1. Content of Intervention Diets*.
| High Fat | High Sucrose | ||||
|---|---|---|---|---|---|
| Control | LD | HD | LD | HD | |
| Protein (% of energy) | 18 | 18 | 18 | 18 | 18 |
| Carbohydrate (% of energy) | 66 | 42 | 42 | 66 | 65 |
| Fat (% of energy) | 16 | 40 | 40 | 16 | 16 |
| Casein (gm)* | 200 | 200 | 149 | 200 | 149 |
| Non-Fat Dry Milk (gm) | 0 | 0 | 125 | 0 | 125 |
| Corn Starch (gm) | 397 | 166 | 99 | 67 | 0 |
| Maltodextrin (gm) | 132 | 132 | 132 | 0 | 0 |
| Sucrose (gm) | 100 | 100 | 100 | 562 | 562 |
| Cellulose (gm) | 50 | 50 | 50 | 50 | 50 |
| Soybean Oil (gm) | 70 | 173 | 173 | 70 | 70 |
| Calcium (gm%) | 0.5 | 0.25 | 1.5 | 0.25 | 1.5 |
| Vitamin D3 (IU/kg) | 1000 | 400 | 10000 | 400 | 10000 |
All diets contain adequate vitamins and minerals unless indicated otherwise. Equivalent t-Butylhydroquinone (0.014 gm/kg) and 3 gm added L-cystine to each diet.
2.2 Culture of 3T3-L1 cells
Preadipocytes (3T3-L1) were seeded in 100 mm dishes and used to determine the effects of 25OHD to induce CYP24 expression and activity. Cells were maintained in high glucose DMEM (Invitrogen, Carlsbad, CA) containing 10% FBS (Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37 °C with 5% CO2. Media was changed every other day and cells were treated when 100% confluent with serum free medium for 2 hours with vehicle (ethanol), 1,25(OH)2D or 25OHD at the concentrations indicated. In some experiments, cells were co-treated with the P450 inhibitor, ketoconazole (100 nM), which inhibits both the 24-hydroxylase and the 1α-hydroxylase [35,36].
2.3 RNA Expression
RNA from tissue and cells was isolated and treated with TURBO DNA free reagents (Ambion, Foster City, CA) according to manufacturer's protocol. A 1:1 ratio of oligo-dT (Promega, Madison, WI) and random primer (Promega, Madison, WI) were used in reverse transcription (RT) reaction (Affinity Script, Stratagene). The 1α-hydroxylase mRNA was amplified in real-time polymerase chain reaction (PCR) using the following primers: 5′ CACCCATTTGCATCTCTTCC3′ and 5′GATGGATGCTCCTCTCAGGT3′ (Brilliant SYBR Green Mastermix, Stratagene). To confirm primer specificity PCR products from control group in these tissues were separated by agrose gel electrophoresis. mRNA from kidney served as a positive control. The expected PCR product is 187 base pair (bp) in length. To determine relative gene expression, the expression of 1α-hydroxylase was normalized to 18S expression (5′TTAGAGTGTTCAAAGCAGGCCCGA3′ and 5′TCTTGGCAAATGCTTTCGCTCTGG3′), and determined using delta CT methods. Gene expression is always reported relative to the control group. To determine 24-hydroxylase gene expression, 3T3 L1 preadipocytes were treated at confluence either 1,25(OH)2D or 25OHD at 1, 10, 100 or 1000 nM for 2 hours as indicated. Vehicle control was ethanol. Gene expression was determined using the method described using the following primers: 5′CAAACCCTGGAAAGCCTATCG3′ and 5′CGCTGCCACTCCTGTCCTT3′. The expression was of the 24 hydroxylase was normalized to GAPDH: 5′TCACCATCTTCCAGGAGCG3′ and 5′CTGCTTCACCACCTTCTTGA3′. The 24-hydroxylase gene expression is reported relative to the vehicle treated control group.
2.4 1α-Hydroxylase Assay
In separate experiments, 3T3 L1 adipocytes were treated at confluence with 2 μCi of 25-hydroxy[26,27-methyl-3H] (3H 25OHD) (specific acitivity: 9.99 Ci/mmol) and with unlabeled 25OHD at a final concentration of 100 nM in 1.5 ml serum-free medium. After 2 hours, cells were scraped into 200 ul cold calcium magnesium free-phosphate buffered saline (CMF-PBS, 137 M sodium chloride, 1.5 mM potassium phosphate, 7.2 mM sodium phosphate, 2.7 mM potassium chloride, pH 7.4), the dish was rinsed twice with CMF-PBS and combined with cell lysate. Acetic acid was added to the cell lysate to achieve a pH of 3.5. To each sample unlabeled 1,25(OH)2D was added. The sample was extracted with ethyl acetate (1.5 ml). The aqueous phase was re-extracted and the combined organic phases sampled to quantify total radioacitivity, dried with nitrogen and resuspended in ethyl acetate. The vitamin D metabolites were separated by thin layer chromatography using a LK5D Silica Gel 150A TLC plate (Whatman, Mobile, AL) which included unlabeled 25OHD, 1,25(OH)2D, and 24,25(OH)2D, eluted with benzene:ethyl acetate (1:1) and unlabeled metabolites visualized with iodine. In addition, a similar amount of 25-hydroxy[26,27-methyl-3H] as loaded in the sample lanes was included for the background. Areas eluting with 25OHD, 1,25(OH)2D, and 24,25(OH)2D were scraped and quantified by liquid scintillation counting (Beckman LS 6500 Liquid Scintillation Counter, Beckman, Fullerton, CA). Total protein amount was determined concurrently from separate plates of 3T3-L1 cells by bicinchoninic acid (BCA) protein assay. The synthesis rate of 1,25(OH)2D was expressed as pmol/hour/mg total protein. The extraction recovery of radioactivity is 94.6%.
2.5 Statistical Analysis
Results were analyzed by two-way analysis of variance using SAS general linear model program (SAS/GLM Version 9.0, SAS Institute Inc., Cary, NC). Main effects of dairy products or macronutrient background were examined by contrast analysis. Means were considered different when P < 0.05.
3. Results
After 14 weeks on their respective diets (Table 1), there were no significant differences in the body weights of the rats (521±21, 570±16, 535±30, 535±30 and 552±12 gm for control, high fat LD, high fat HD, high sucrose LD and high sucrose HD, respectively). On the other hand, there were significant differences in the intake of vitamin D (25.8±1.13, 9.92±0.87, 265.7±23.3, 10.4±.84 and 267.9±13.5 IU/day for control, high fat LD, high fat HD, high sucrose LD and high sucrose HD, respectively) among dietary groups. 1α-hydroxylase gene expression was determined in liver, skeletal muscle, adipose and kidney following the intervention. In addition to kidney, 1α-hydroxylase is expressed in adipose tissues of rats in the control group (Figure 1A). The relative expression of the 1α-hydroxylase was assessed following 14 weeks of dietary intervention of control, LD or HD diets with either a high fat or high sucrose dietary background. There were similar levels of 1α-hydroxylase gene expression in liver (Figure 1B), skeletal muscle (Figure 1C) and adipose tissue (Figure 1D) in rats fed the HD, LD, and control diets. In addition, there were similar levels of 1α-hydroxylase gene expression in rats fed high fat and high sucrose diets after collapsing the LD and HD groups in adipose tissue (Figures 1C and 1D, respectively).
Figure 1. Dietary regulation of 1α-hydroxylase in liver, skeletal muscle and adipose tissue.
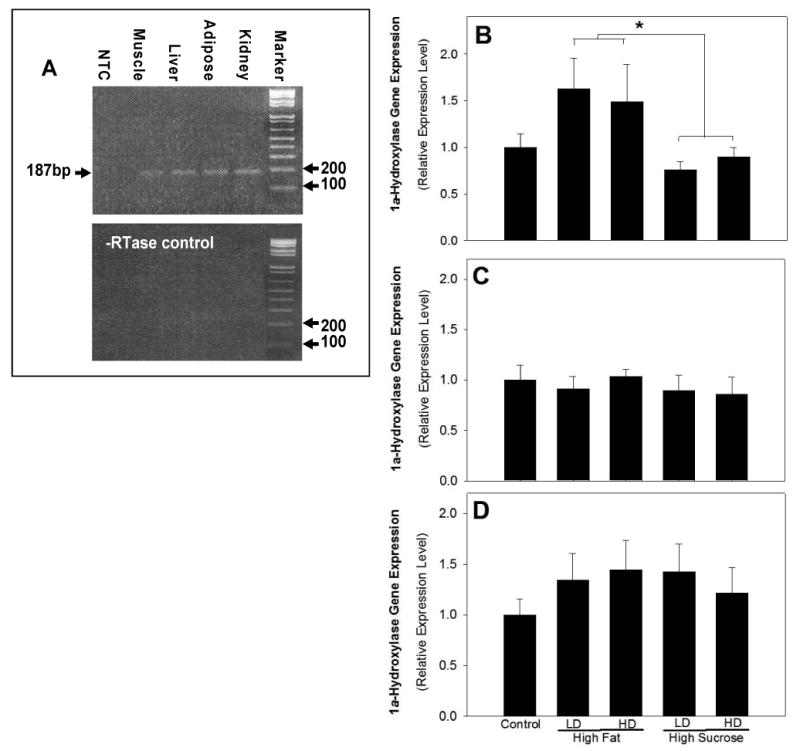
mRNA was isolated from Wistar rats following dietary intervention and relative 1α-hydroxylase expression assessed by real time RT-PCR with specific mouse 1α-hydroxylase primer pair, which yields 187 bp of PCR product. Panel A is a representative visualization of products, PCR product size was verified after loading together with a molecular weight marker on a 1.5% agrose gel. The lower panel displays the results of the no RT control samples. Male Wistar rats were fed the LD or HD diet with either a high fat (40% energy) or high sucrose (66% energy) dietary background for 14 weeks. Relative 1α-hydroxylase gene expression level was shown for liver (B), skeletal muscle (C), and adipose (D) tissue (mean±SE). 1α-hydroxylase gene expression is reported relative to vehicle treatment group. The HD and LD dietary groups were collapsed to test the impact of dietary background and asterisk (*) indicates p≤ 0.05 between high fat compared to high sucrose dietary groups.
To determine whether or not 1α-hydroxylase is active in 3T3 L1 adipocytes, confluent 3T3L1 adipocytes were treated with 25OHD and the CYP24 gene expression measured. This serves as an indirect measure of 1α-hydroxylase activity because at lower levels, 25OHD needs to be converted to 1,25(OH)2D to regulate gene transcription of CYP24. CYP24 gene expression was increased by 25OHD at doses as low as 10 nM at a similar level as 1 nM 1,25(OH)2D (Figure 2), suggesting that the 1α-hydroxylase is functional in the 3T3 L1. In addition, treatment with the P450 inhibitor, ketoconazole (100 nM), significantly reduced the induction of CYP24 following treatment 25OHD at 10 and 100 nM. Treatment of cells with 100 nM 1,25(OH)2D and 1000 nM 25OHD reduced induction of CYP24 gene expression compared to 10 nM 1,25(OH)2D and 100 nM 25OHD, respectively. Co-treatment with ketoconazole and 100 nM 1,25(OH)2D significantly increased CYP24 induction compared to cells without ketoconazole, suggesting a p450 enzyme was involved with the reduction in CYP24 expression with 100 nM 1,25(OH)2D. Cumulatively, these results support that the 1α-hydroxylase is active in the cultured adipocytes and at least in part plays a role in the 25OHD mediated induction of CYP24.
Figure 2. Activation of 1α-hydroxylase gene expression in 3T3 L1 preadipocytes.
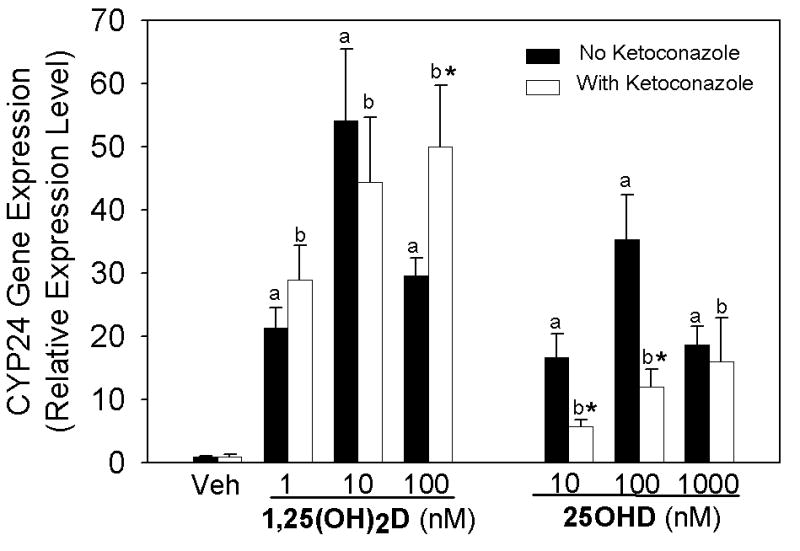
Preadipocytes were treated with vehicle (Veh), 25OHD or 1,25(OH)2D without (dark bars) or with (open bars) ketoconazole (100 nM) in serum-free medium for 2 hours. The level of CYP24 expression was assessed using real time RT-PCR and results are expressed relative to vehicle treated control group (mean±SE) or ketoconazole only control group for all ketoconazole treated cells. Significance was determined for vehicle compared to treated cells without ketoconazole (a) or ketoconazole with vehicle compared to ketoconazole with treatment (b) and asterisk (*) indicates p<0.05 comparison of the treatment group with or without Ketoconazole.
The ability of 3T3-L1 adipocytes to convert 25OHD to 1,25(OH)2D was assessed by employing radiolabeled 25OHD (100 nM, 2 hours), and determining appearance of the radiolabel in 1,25(OH)2D. The rate of 1,25(OH)2D synthesis by 3T3-L1 cells was 1.16±0.07 pmol/mg protein/hour from 25OHD over background.
4. Discussion
The results of the current study demonstrate 1α-hydroxylase gene expression in extra-renal tissues that contribute to energy balance in rats. We found that the 1α-hydroxylase gene is expressed in liver, skeletal muscle and adipose tissue. In addition, 1α-hydroxylase gene expression was increased in the liver of rats fed a high fat diet, but not by dietary vitamin D or calcium. It is also not regulated by dietary vitamin D or calcium in adipose tissue. Finally, our results demonstrate that the 1α-hydroxylase gene is expressed and active in 3T3 L1 preadipocyte cells. To our knowledge, this is the first report showing 1α-hydroxylase expression in adipose tissue and that the enzyme is functional in adipocytes.
Adipose tissue harvested from animals is comprised of a heterogeneous population including adipocytes as well as other cell types such as macrophages. This is particularly relevant as activated macrophages express functional 1α-hydroxylase [17]. Our studies in 3T3 L1 preadipocytes suggest that 1α-hydroxylase gene expression in adipose tissue of rats is likely due to adipocytes as well as activated macrophages. In addition, our studies support that the 1α-hydroxylase in adipocytes is functional, shown by induction of CYP24 gene expression following 25OHD treatment, as well as the production of radiolabeled 1,25(OH)2D from 3H 25OHD. The activity measured in the adipocytes in our study (1.16±0.07 pmol/mg protein/hour) is comparable to that in other cell lines, including prostate (0.07-3.08 pmol/mg protein/hour, 20), vascular endothelial cells (318 fmol/mg protein/hour, 23) and in human renal tissue (0.20 pmoles/mg protein/20 minutes, 37). The lower induction of CYP24 with 100 nM compared to 10 nM 1,25(OH)2D is likely due to the increased degradation of the metabolite by 24-hydroxylase. This conclusion is supported by the results shown in Figure 2 in which co-treatment with the p450 inhibitor, ketoconazole, increases the induction of CYP24 by 1,25(OH)2D. Although 25OHD binds the VDR approximately 1000 fold less well than 1,25(OH)2D [34], only a 10 fold higher dose of 25OHD (10 nM), within a physiological range for this metabolite, induced CYP24 expression to a similar level compared to 1 nM 1,25(OH)2D. In addition, co-treatment with the p450 inhibitor, ketoconazole, significantly reduced the induction of the CYP24 expression by 25OHD, supporting that the activity of the 1α-hydroxyalse enzyme is required, at least in part, for the induction. Therefore, the 1α-hydroxylase is expressed in adipose tissue and is functional in cultured adipocytes.
Gene regulation of the extra-renal 1α-hydroxylase is currently under investigation. It has been shown to be independent of PTH in a primary culture of normal prostate epithelial cells and HPV18 DNA transformed normal prostate epithelial cell line, PZ-HPV-7 [33], although PTH suppressed 1α-hydroxylase reporter construct in ROS 17/2.8 osteoblast [38]. Several cytokines, such as interferon (IFN)γ [39], cytokine interleukin (IL)-1β [40], and epidermal growth factor (EGF) [41] have been shown to induce extra-renal 1α-hydroxylase expression; while transforming growth factor-beta (TGF-beta) [38] or insulin-like growth factor-1 (IGF-1) [38], growth factor independent-1 (GFI1) [42] decreased its expression of the 1α-hydroxylase. One of the few studies which have investigated the role of diet in regulation of 1-hydroxylae shows that phytoestrogens upregulate 1α-hydroxylase in the colon [43]. The current study investigated the effect of high calcium and vitamin D diets consumption on 1α-hydroxylase regulation. There were no differences in 1α-hydroxylase expression in liver, skeletal muscle and adipose tissue between intake of high calcium and vitamin D compared to low intakes in the rats demonstrating a lack of dietary regulation by vitamin D and calcium.
On the other hand, in the current study, 1α-hydroxylase expression was higher in liver tissue when rats were fed with high fat diet compared to high sucrose diet. It is important to consider that the mRNA is isolated from liver tissue, which contains a variety of cell types. A high fat diet may induce fat accumulation in the liver, leading to a fatty liver. This high fat diet-induced change may lead to macrophage infiltration. Macrophages express 1α-hydroxylase, which is induced when the macrophages are activated [17]. Therefore, it is critical to determine if the increase in 1-hydroxylase in liver tissue is due to macrophage infiltration or a specific effect on hepatocytes.
The function of 1,25(OH)2D in regulating cellular decisions in the adipocyte is not clear. Evidence suggests that 1,25(OH)2D may increase fat synthesis in human adipocytes [44] however, other studies demonstrate that 1,25(OH)2D increases the expression of Insig-2, whose protein product is involved in down regulating fat synthesis [45]. Our studies demonstrate that the 1α-hydroxylase is functional in adipocytes and capable of localized 1,25(OH)2D production. The autocrine and paracrine activity of extrarenal 1,25(OH)2D production and localized concentrations are not yet known but it is possible that localized 1,25(OH)2D may stimulate different cellular signals compared with exogenously produced hormone. Addressing the role of the locally produced hormone in adipocyte function is critical to a more complete understanding of the role of vitamin D in controlling adiposity and body composition.
Footnotes
Supported by NIH DK069965
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bischof MG, Heinze G, Vierhapper H. Vitamin D status and its relation to age and body mass index. Horm Res. 2006;66:211–215. doi: 10.1159/000094932. [DOI] [PubMed] [Google Scholar]
- 3.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 4.Zehnder D, Bland R, Walker EA, Bradwell AR, Howie AJ, Hewison M, Stewart PM. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in the human kidney. J Am Soc Nephrol. 1999;10:2465–2473. doi: 10.1681/ASN.V10122465. [DOI] [PubMed] [Google Scholar]
- 5.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–134. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 7.Townsend K, Evnas KN, Campbell MJ, Colston KW, Adams JS, Hewison M. J Steroid Biochem and Mol Biology. 2005;97:103–109. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D2-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 9.Weber L, Hugel U, Reichrath J, Sieverts H, Mehls O, Klaus G. Cultured rat growth plate chondrocytes express low levels of 1alpha-hydroxylase. Recent Results Cancer Res. 2003;164:147–149. doi: 10.1007/978-3-642-55580-0_10. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–246. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- 11.Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 12.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Segersten U, Correa P, Hewison M, Hellman P, Dralle H, Carling T, Akerstrom G, Westin G. 25-hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab. 2002;87:2967–2972. doi: 10.1210/jcem.87.6.8604. [DOI] [PubMed] [Google Scholar]
- 14.Delvin EE, Arabian A, Glorieux FH, Mamer OA. In vitro metabolism of 25-hydroxycholecalciferol by isolated cells from human decidua. J Clin Endocrinol Metab. 1985;60:880–885. doi: 10.1210/jcem-60-5-880. [DOI] [PubMed] [Google Scholar]
- 15.Koeffler HP, Reichel H, Bishop JE, Norman AW. Gamma-interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun. 1985;127:596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- 16.Reichel H, Koeffler HP, Norman AW. Synthesis in vitro of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by interferon-gamma-stimulated normal human bone marrow and alveolar macrophages. J Biol Chem. 1987;262:10931–10937. [PubMed] [Google Scholar]
- 17.Dusso AS, Finch J, Brown A, Ritter C, Delmez J, Schreiner G, Slatopolsky E. Extrarenal production of calcitriol in normal and uremic humans. J Clin Endocrinol Metab. 1991;72:157–164. doi: 10.1210/jcem-72-1-157. [DOI] [PubMed] [Google Scholar]
- 18.Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25:1545–1548. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- 19.Jones G, Ramshaw H, Zhang A, Cook R, Byford V, White J, Petkovich M. Expression and activity of vitamin D-metabolizing cytochrome P450s (CYP1alpha and CYP24) in human nonsmall cell lung carcinomas. Endocrinology. 1999;140:3303–3310. doi: 10.1210/endo.140.7.6799. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7:391–395. [PubMed] [Google Scholar]
- 21.Friedrich M, Villena-Heinsen C, Axt-Fliedner R, Meyberg R, Tilgen W, Schmidt W, Reichrath J. Analysis of 25-hydroxyvitamin D3-1alpha-hydroxylase in cervical tissue. Anticancer Res. 2002;22:183–186. [PubMed] [Google Scholar]
- 22.Cross HS, Peterlik M, Reddy GS, Schuster I. Vitamin D metabolism in human colon adenocarcinoma-derived Caco-2 cells: expression of 25-hydroxyvitamin D3-1alpha-hydroxylase activity and regulation of side-chain metabolism. J Steroid Biochem Mol Biol. 1997;62:21–28. doi: 10.1016/s0960-0760(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 23.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 24.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 25.Kemmis CM, Salvador SM, Smith KM, Welsh J. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J Nutr. 2006;136:887–892. doi: 10.1093/jn/136.4.887. [DOI] [PubMed] [Google Scholar]
- 26.Negrea LA, Slatopolsky E, Dusso AS. 1,25-Dihydroxyvitamin D synthesis in rat liver microsomes. Horm Metab Res. 1995;27:461–464. doi: 10.1055/s-2007-980002. [DOI] [PubMed] [Google Scholar]
- 27.Axén E, Harmeyer J, Wikvall K. Renal and hepatic 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in piglets suffering from pseudo vitamin D-deficiency rickets, type I. Biochim Biophys Acta. 1998;1407(3):234–42. doi: 10.1016/s0925-4439(98)00047-7. [DOI] [PubMed] [Google Scholar]
- 28.Omdahl JL, Gray RW, Boyle IT, Knutson J, DeLuca HF. Regulation of metabolism of 25-hydroxycholecalciferol by kidney tissue in vitro by dietary calcium. Nat New Biol. 1972;237:63–64. doi: 10.1038/newbio237063a0. [DOI] [PubMed] [Google Scholar]
- 29.Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T, DeLuca HF, Suda T. Cloning and expression of rat 25-hydroxyvitamin D3-1alpha-hydroxylase cDNA. Proc Natl Acad Sci U S A. 1997;94:12920–12925. doi: 10.1073/pnas.94.24.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zierold C, Nehring JA, DeLuca HF. Nuclear receptor 4A2 and C/EBPbeta regulate the parathyroid hormone-mediated transcriptional regulation of the 25-hydroxyvitamin D3-1alpha-hydroxylase. Arch Biochem Biophys. 2007;460:233–239. doi: 10.1016/j.abb.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1alpha-hydroxylase gene in normocalcemic rats. Proc Natl Acad Sci U S A. 1999;96:8253–8258. doi: 10.1073/pnas.96.14.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 33.Young MV, Schwartz GG, Wang L, Jamieson DP, Whitlatch LW, Flanagan JN, Lokeshwar BL, Holick MF, Chen TC. The prostate 25-hydroxyvitamin D-1 alpha-hydroxylase is not influenced by parathyroid hormone and calcium: implications for prostate cancer chemoprevention by vitamin D. Carcinogenesis. 2004;25:967–971. doi: 10.1093/carcin/bgh082. [DOI] [PubMed] [Google Scholar]
- 34.Lechner D, Bajna E, Adlercreutz H, Cross HS. Genistein and 17beta-estradiol, but not equol, regulate vitamin D synthesis in human colon and breast cancer cells. Anticancer Res. 2006;26:2597–2603. [PubMed] [Google Scholar]
- 35.Adams JS, Beeker TG, Hongo T, Clemens TL. Constitutive expression of a vitamin D 1-hydroxylase in a myelomonocytic cell line: a model for studying 1,25-dihydroxyvitamin D production in vitro. J Bone Miner Res. 1990;12:1265. doi: 10.1002/jbmr.5650051212. [DOI] [PubMed] [Google Scholar]
- 36.Yee SW, Campbell MJ, Simons C. Inhibition of Vitamin D3 metabolism enhances VDR signaling in androgen-independent prostate cancer cells. J Steroid Biochem. 2006;98:228–235. doi: 10.1016/j.jsbmb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Satomura K, Seino Y, Yamaoka K, Tanaka Y, Ishida M, Yabuuchi H, Tanaka Y, DeLuca HF. Renal 25-hydroxyvitamin D3-1-hydroxylase in patients with renal disease. Kidney International. 1988;34:712–716. doi: 10.1038/ki.1988.237. [DOI] [PubMed] [Google Scholar]
- 38.Turner AG, Dwivedi PP, May BK, Morris HA. Regulation of the CYP27B1 5′-flanking region by transforming growth factor-beta in ROS 17/2.8 osteoblast-like cells. J Steroid Biochem Mol Biol. 2007;103:322–325. doi: 10.1016/j.jsbmb.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 39.Overbergh L, Stoffels K, Valckx D, Giulietti A, Bouillon R, Mathieu C. Regulation of 25-hydroxyvitamin d-1alpha-hydroxylase by IFNgamma in human monocytic THP1 cells. J Steroid Biochem Mol Biol. 2004;89-90:453–455. doi: 10.1016/j.jsbmb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Vigano P, Lattuada D, Mangioni S, Ermellino L, Vignali M, Caporizzo E, Panina-Bordignon P, Besozzi M, Di Blasio AM. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J Mol Endocrinol. 2006;36:415–424. doi: 10.1677/jme.1.01946. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Flanagan JN, Whitlatch LW, Jamieson DP, Holick MF, Chen TC. Regulation of 25-hydroxyvitamin D-1alpha-hydroxylase by epidermal growth factor in prostate cells. J Steroid Biochem Mol Biol. 2004;89-90:127–130. doi: 10.1016/j.jsbmb.2004.03.091. [DOI] [PubMed] [Google Scholar]
- 42.Dwivedi PP, Anderson PH, Omdahl JL, Grimes HL, Morris HA, May BK. Identification of growth factor independent-1 (GFI1) as a repressor of 25-hydroxyvitamin D 1-alpha hydroxylase (CYP27B1) gene expression in human prostate cancer cells. Endocr Relat Cancer. 2005;12:351–365. doi: 10.1677/erc.1.00920. [DOI] [PubMed] [Google Scholar]
- 43.Kallay E, Adlercreutz H, Farhan H, Lechner D, Bajna E, Gerdenitsch W, Campbell M, Cross HS. Phytoestrogens regulate vitamin D metabolism in the mouse colon: relevance for colon tumor prevention and therapy. J Nutr. 2002;132:3490S–3493S. doi: 10.1093/jn/132.11.3490S. [DOI] [PubMed] [Google Scholar]
- 44.Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. Faseb J. 2001;15:2751–2753. doi: 10.1096/fj.01-0584fje. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Lee DK, Choi E, Lee JW. Identification of a functional vitamin D response element in the murine Insig-2 promoter and its potential role in the differentiation of 3T3-L1 preadipocytes. Mol Endocrinol. 2005;19:399–408. doi: 10.1210/me.2004-0324. [DOI] [PubMed] [Google Scholar]


