Abstract
Objective
Minimally invasive repair of mitral valve prolapse (MVP) causing severe mitral regurgitation (MR) should reduce mitral regurgitation and have chronic durability. Our ex-vivo, acute in-vivo, and chronic in-vivo studies suggest that direct application of radiofrequency ablation (RFA) to mitral leaflets and chordae can effect these repair goals to decrease MR.
Methods
A total of seven canines were studied to assess the effects of RFA on mitral valve structure and function. RFA was applied ex-vivo (n=1), acutely in-vivo using a right lateral thoracotomy and cardiopulmonary bypass (n=3), and chronically in-vivo using percutaneous access to the heart (n=3). RFA was applied to the mitral valve and its associated chordae. Mitral valve structure and function (in-vivo preparations) were then assessed.
Results
Ex-vivo application of RFA resulted qualitative reduction in mitral leaflet surface area and chordal length. Acute in-vivo application of RFA to canines found to have MVP causing severe MR demonstrated a 43.7–60.7% statistically significant (p=0.039) reduction in post-ablation MR. Chronic, in-vivo, percutaneous application of RFA was found to be feasible and the engendered alterations durable.
Conclusion
These data suggest that myxomatous mitral valve repair using radiofrequency energy delivered via catheter is feasible.
Keywords: mitral valve prolapse, mitral regurgitation, repair, radiofrequency ablation
Introduction
Prevalence of Mitral Valve Prolapse
Mitral valve prolapse is the most common cause of mitral regurgitation in industrialized countries.1 The prevalence of mitral valve prolapse in the general population is 2.4%2. Recent United States census data places the population of the U.S. at 281,421,906. Thus, currently there are approximately 6,754,125 persons with mitral valve prolapse. Several trials have examined the prevalence of severe mitral regurgitation in the setting of mitral valve prolapse; severe mitral regurgitation ranges from 6.5–9.0% of all cases of mitral valve prolapse.3,2,4 The recent American College of Cardiology/American Heart Association Guidelines on Valvular Heart Disease (2006) state that in an asymptomatic patient with severe mitral regurgitation, mitral valve repair may be recommended to preserve the left ventricular size and function. Given the above prevalences, there are currently about 472,788 persons with severe mitral regurgitation from mitral valve prolapse who could benefit from a percutaneous mitral valve repair. In addition, given a current birth rate of approximately 14 per 1000 persons in the United States, an additional 6619 persons per year will have mitral valve prolapse with severe mitral regurgitation. These figures represent a staggering volume of the population that could benefit from a novel therapy that could percutaneously repair the mitral valve using existing technologies.
Minimally Invasive Mitral Valve Repair
Alain Carpentier pioneered mitral valve repair. He states there are two requirements for mitral valve repair: understanding of the anatomy and pathology of the mitral valve and excellent surgical exposure of the mitral valve by using large incisions in a patient’s chest wall to visualize the heart.5 This major surgical study was among the first to indicate that repair of the mitral valve offered a lower death rate and long term success comparable to valve replacement. Subsequent research has demonstrated that repair, as opposed to replacement, of the mitral valve has a lower operative death rate6, 7, 8, 9, lower rates of blood clot formation6, 8, decreased need for blood thinners which may cause excess bleeding6, 8, shorter post-surgical recovery times10, and longer survival6, 8, 9. In addition, repair allows the surgeon to leave the valve support structures (chordae tendinae) in place; traditional valve replacement removes these support structures. It has been found that leaving the chordae tendinae intact results in better mitral valve repairs7, 9.
As surgery has become more sophisticated, there has been a trend toward techniques that are less invasive. Less invasive surgical techniques have been shown to decrease the need for blood transfusions, decrease rates of infection, decrease wound complications, decrease patient pain, and shorten recovery times10. It is interesting to note that surgical repair is technically demanding11, the type of repair did not have an effect on long-term durability10, repair is possible in 90–95% of patients with valve regurgitation6, and the function of the repaired valve can be assessed by non-invasive echocardiographic imaging6. Currently, a minimally invasive mitral valve repair requires 89–186 minutes of cardiopulmonary bypass (CPB) time, 58–143 minutes of cross-clamp time, and 9–53 minutes of total repair time. 12 If mitral valve repair could be adapted to a percutaneous, closed chest, beating heart environment, the repair time could be reduced by the elimination of both CPB and cross-clamp times.
Percutaneous Mitral Valve Repair
We hypothesize that the application of radiofrequency energy to mitral valve leaflets and their associated structures is feasible to percutaneously repair myxomatous mitral valve prolapse. The application of energy may cause controlled damage which leads to fibrotic scarring and contracture of valve leaflets and surrounding structures. This direct application of energy to the leaflet and its associated structures may result in decreased mitral regurgitation.
Methods
Acute Ex-Vivo
A canine with no known structural heart disease was sacrificed as part of an unrelated protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The heart was then excised and dissected to expose the anterior mitral leaflet. The heart with leaflet and chordae exposed was placed in 0.9% saline solution. First, radiofrequency ablation (RFA) energy was applied to the anterior leaflet of the mitral valve using an RF ablation pen (Cardioblate™ Ablation System, Medtronic Inc., Minneapolis, MN). RFA was applied from the tip of the leaflet to the annulus. These ablations were performed every 2–3mm as allowed by the size of the leaflet itself. Next, RFA was applied in a similar manner to the chordae associated to the anterior leaflet of the mitral valve.
Acute In-Vivo
A colony of over 30000 beagles was screened for the presence of mitral valve prolapse causing severe mitral regurgitation. The initial screening consisted of identifying animals aged greater than 4 years (n=100). Next, these animals were auscultated to identify systolic murmurs of grade 2 or larger (n=13). Finally, these remaining animals underwent transthoracic echocardiograms (MicroMaxx, SonoSite, Inc., Bothell, WA) to evaluate mitral valve structure and function.
Canines (n=3) found to have anterior leaflet (n=1), posterior leaflet (n=1), and bileaflet MVP prolapse (n=1) causing severe MR with a mean ejection fraction of 66±3%(Mean±SD) underwent direct RFA in a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The animal was delivered to the operating theatre. On the day of surgery, dogs were sedated with acepromazine (0.1 mg/kg) and anesthetized with sodium thiopental (10 mg/kg) for induction and isoflurane (1.0–1.75%) for maintenance. Transthoracic echocardiograms were performed to reassess baseline mitral valve structure and function. Using standard techniques, both arterial and central venous pressure lines were placed in the leg for continuous blood pressure monitoring. In addition, venous catheters were placed in the front legs of the animals for medication and fluid administration.
The chest and infrascapular areas were shaved and scrubbed. Using sterile surgical technique a right lateral thoracotomy was performed in standard fashion. Following systemic heparinization, cardiopulmonary bypass was established via an aortic cannula and right atrial cannula. The cardiopulmonary bypass circuit consisted of a reservoir, a hollow fiber oxygenator/heat exchanger, and a roller pump. After stabilization, the aorta was cross-clamped and the heart arrested by infusion of cold (4 degrees Centigrade) hyperkalemic cardioplegia solution (30 mL/kg) into the aortic root.
The mitral valve apparatus was exposed by opening the left atrium. Next, radiofrequency energy, using an RF ablation pen (Cardioblate™ Ablation System, Medtronic Inc., Minneapolis, MN), was applied to the prolapsed leaflets of the mitral valve and any associated elongated chordae. Radial ablations were applied from the tip of the leaflet to the annulus. These ablations were performed every 2–3mm as allowed by the size of the leaflet itself. No attempts were made to reattach flail leaflets or torn chordae.
At the end of the procedure, the heart was closed, and echocardiograms were performed to reassess mitral valve structure and function. Specifically, mitral regurgitant volume was calculated using the proximal isovelocity surface area (PISA) on pre- and post-ablation echocardiograms. Regurgitant stroke volume determined by the PISA method has been shown an accurate and reproducible means to calculate mitral regurgitant volume in a canine model.13 Regurgitant volumes on pre- and post-ablation were compared using a two-sample Student t-test assuming equal variances.
Chronic Ex-Vivo
Canines with no known structural heart disease (n=3) underwent percutaneous mitral valve RFA in a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The animal was brought to the surgical suite. The dog was induced with acepromazine 0.1mg/kg IM once and sodium thiopental 10mg/kg IV once preoperatively. A 20 G venous catheter was placed for fluids (37 degree,5% dextrose) administration. The dog was intubated, ventilated, and maintained on Isoflurane (1.0–1.75%). First, an 11French sheath and an 8F sheath were placed in the right femoral vein and artery, respectively, using a femoral cutdown.
Next, a 10F intracardiac echocardiographic catheter (ACUSON, AcuNav Diagnostic Ultrasound Catheter, Siemens Medical Solutions USA, Inc., Malvern, PA, USA) was inserted into the right atrium (RA) via the right femoral vein. This ICE catheter was used to discern atrial, mitral valve, and contiguous anatomy, ensure firm/stable ablation electrode-valvular contact, and permit direct, continuous observation of the electrode-valvular interface before, during, and after radiofrequency ablation (RFA) energy applications.
Finally, the ablation catheter (Thermocool, 4mm, irrigated ablation electrode, Biosense Webster) was placed into the left ventricle retrograde across the aortic valve via the right femoral artery. The catheter was then placed in contact with the anterior mitral valve leaflet. RFA was selectively delivered to the anterior mitral valve leaflet until structural and/or functional alteration of the leaflet was demonstrated via intracardiac echo. Attempts were made to limit RFA to the anterior leaflet as the posterior leaflet served as the unablated control. For each ablation, power was titrated to achieve ablation electrode temperature of 40°C for 60seconds.
The animals were anesthetized for the entire procedure, which took approximately 3–4 hours to complete. The surgeries were completed under aseptic conditions. Post surgery, the animals were placed on a circulating water heating pad until recovery. They received antibiotics and analgesia. Dogs were allowed to recover from surgery for one week prior to further study. Weekly thereafter the dogs underwent transthoracic echocardiograms to assess mitral valve structure and function.
Results
Acute Ex-Vivo
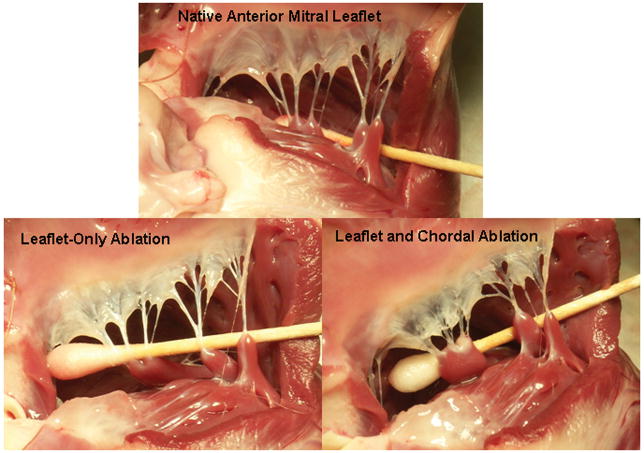
Figure 1 depicts the effects ex-vivo application of RFA had upon canine anterior mitral valve leaflets and chordae. These images demonstrate blanching and shortening that was characteristic of RFA applied to leaflet only. A similar blanching and shortening was noted when RFA was applied to the chordae.
Figure 1. Pre- and Post-Radiofrequency Ablation of Ex-Vivo Canine Anterior Mitral Leaflet.
The top image depicts a native canine anterior mitral leaflet and chordae. The bottom left image is status-post the application of 15sec irrigated RFA (20Watts) to the leaflet only. The bottom right image is status-post the application of an additional 5sec irrigated RFA (20Watts) to the chordae associated with the anterior leaflet.
Acute Ex-Vivo
Table 1 reports the screening echocardiograms of beagles aged greater than 4 years with systolic murmurs ultimately having mitral valve prolapse causing severe MR. Three canines found to have anterior leaflet (n=1), posterior leaflet (n=1), and bileaflet MVP prolapse (n=1) causing severe MR with a mean ejection fraction of 66±3%(±SD) underwent direct RFA. Sixty seven percent of the animals (n=2) had visually-confirmed torn chordae.
Table 1.
Echocardiograms of Beagles with Mitral Valve Prolapse Causing Severe MR
| EF (%) | Leaflets Prolapsed | MR | PV Flow Reversal? | Torn Chordae? | |
|---|---|---|---|---|---|
| Experiment #1 | 72 | Anterior | Severe | N/A | Yes |
| Experiment #2 | 65 | Posterior | Severe | N/A | No |
| Experiment #3 | 62 | Bileaflet | Severe | Yes | Yes |
Table 2 reports the operative outcomes for the animals undergoing mitral valve leaflet and chordae RFA. Each canine required an average of 4±1.7 RFA applications with an average power of 15.8±4.6W for an average duration of 39.4±22sec. Experiments 1, 2, and 3 demonstrated a 43.7%, 44.4%, and 60.7%, respectively, statistically significant (p=0.039) reduction in post-ablation MR.
Table 2.
Operative Findings of Animals Undergoing OPEN ACUTE Mitral Valve Leaflet and Chordal RFA
| Pre-Ablation Regurgitant Volume (cc) | Post-Ablation Regurgitant Volume (cc) | Percent Change in MR Post-Ablation | Number of Ablations | Average Power of Ablations (mean ±SD watt) | Average Duration of Ablations (mean ±SD sec) | |
|---|---|---|---|---|---|---|
| Experiment #1 | 59.3 | 33.4 | −43.7 | 3 | 12.2±1.4 W | 50±10 |
| Experiment #2 | 70.0 | 38.9 | −44.4 | 3 | 16.9±3.1 W | 50±10 |
| Experiment #3 | 102.5 | 40.3 | −60.7 | 6 | 16.9±1.4 W | 28.8±9.7 |
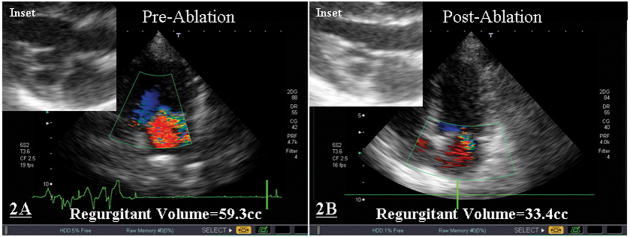
Figure 2 demonstrates the pre- and post-ablation structure and function of the myxomatous mitral valve in the first animal of the study. Transthoracic echocardiograms performed before (Figure 2A) and after RFA (Figure 2B) demonstrated a reduction in the degree of MVP, MR (regurgitant volume was decreased from 59.3cc to 33.4cc), and chordal length (see insets of Figure 2).
Figure 2. Pre- and Post-Ablation Morphology and Structure of Myxomatous Mitral Valve.
Transthoracic echocardiograms performed before (2A) and after RFA (2B) demonstrated a reduction in the degree of MVP, MR, and chordal length (see insets). This animal underwent 3 applications of RFA at an average power of 12.2±1.4W for an average of 50±10sec each.
Chronic In-Vivo
Experiment #4 underwent 19 applications (60sec in duration) with an average power of 69.5±17.9Watts (mean±SD). Experiment #5 underwent 23 applications (60sec in duration) with an average power of 52.3±14.0Watts. Experiment #6 underwent 23 applications (60sec in duration) with an average power of 51.3±26.9Watts (mean±SD). Of note, during the final application of RFA Experiment #5 had ventricular tachycardia when the electrode contacted the left ventricular endocardium. The VT terminated immediately on cessation of ablation. There were no other complications during ablations.
Figure 3 depicts the animal preparation used for ICE-guided ablation of mitral valve anterior leaflets. To guide mitral valve ablation, the ICE catheter was placed in the right atrium and positioned to give a modified three chamber view. The pre- and post-RFA images illustrate the typical change in anterior leaflet morphology after energy application was completed. The anterior leaflet thickened and its motion limited compared to pre-ablation morphology.
Figure 3. ICE-Guided Percutaneous Ablation of Mitral Valve Anterior Leaflet.
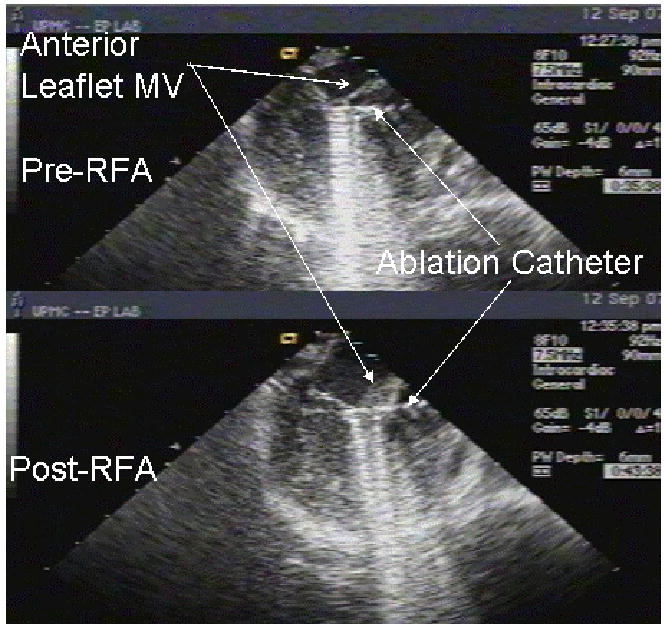
To guide mitral valve ablation, ICE catheter was placed in the right atrium and positioned to give a modified three chamber view. The left atrium is a 12 o’clock, the left ventricle is directly inferior to the left atrium, and the ablation catheter is advanced retrograde across the aortic valve and positioned adjacent to the anterior leaflet of the mitral valve. The top image depicts the mitral valve prior to RFA and the bottom image depicts the mitral valve following RFA. One notes the obvious thickening found post-RFA.
Table 3 reports the baseline and subsequent transthoracic echocardiograms of the animals undergoing percutaneous ablation. All animals had structurally normal appearing mitral valves and two of three animals had no mitral regurgitation prior to ablation. One animal had trivial mitral regurgitation prior to ablation. All animals experienced a minor increase in mitral regurgitation which peaked in weeks 1–3 and anterior mitral leaflet thickening which persisted the entire 5 week follow-up. There was no evidence of inadvertent chordal damage during the ablations or follow-up. Figure 4 shows the chronic echocardiographic changes after percutaneous anterior mitral leaflet ablation in Experiment #6. The baseline and subsequent echocardiograms depict the typical changes found before and after RFA. One can see thickening of the entire anterior leaflet, pronounced at the base and tip of the leaflet that persists throughout the entire follow-up.
Table 3.
Echocardiographic Findings of Animals Undergoing PERCUTANEOUS CHRONIC Mitral Valve Leaflet and Chordal RFA
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-RFA Mitral Regurgitation (MR) | MR | Leaflet Thickening | MR | Leaflet Thickening | MR | Leaflet Thickening | MR | Leaflet Thickening | MR | Leaflet Thickening | |
| Experiment #4 | None | 1+ | Yes | 1+ | Yes | 1+ | Yes | Triv | Yes | Triv | Yes |
| Experiment #5 | None | Triv | Yes | No | No | Triv | Yes | Triv | Yes | Triv | Yes |
| Experiment #6 | Triv | 1+ | No | Triv | Yes | 1+ | Yes | 1+ | Yes | Triv | Yes |
Figure 4. Chronic Echocardiographic Changes after Percutaneous Anterior Mitral Leaflet Ablation.
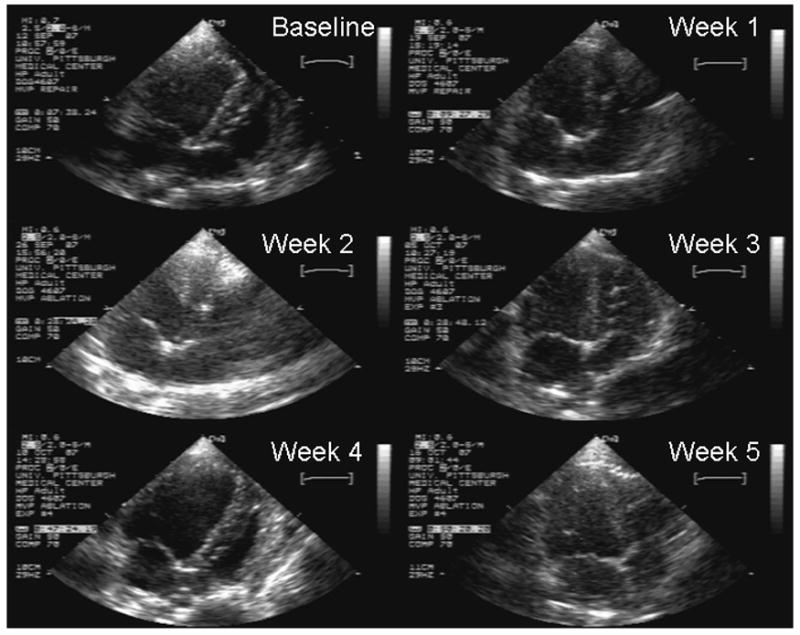
The transthoracic apical 4 chamber echocardiogram still frames were taken at baseline and weekly thereafter for 5 weeks. The baseline echo demonstrates a typical baseline mitral valve morphology. The subsequent echocardiograms depict the typical changes found after RFA. One can see thickening of the entire anterior leaflet, pronounced at the base and tip of the leaflet that persists throughout the entire follow-up.
The transthoracic apical 4 chamber echocardiogram still frames were taken at baseline and weekly thereafter for 5 weeks. The baseline echo demonstrates a typical baseline mitral valve morphology. The subsequent echocardiograms depict the typical changes found after RFA. One can see thickening of the entire anterior leaflet, pronounced at the base and tip of the leaflet that persists throughout the entire follow-up.
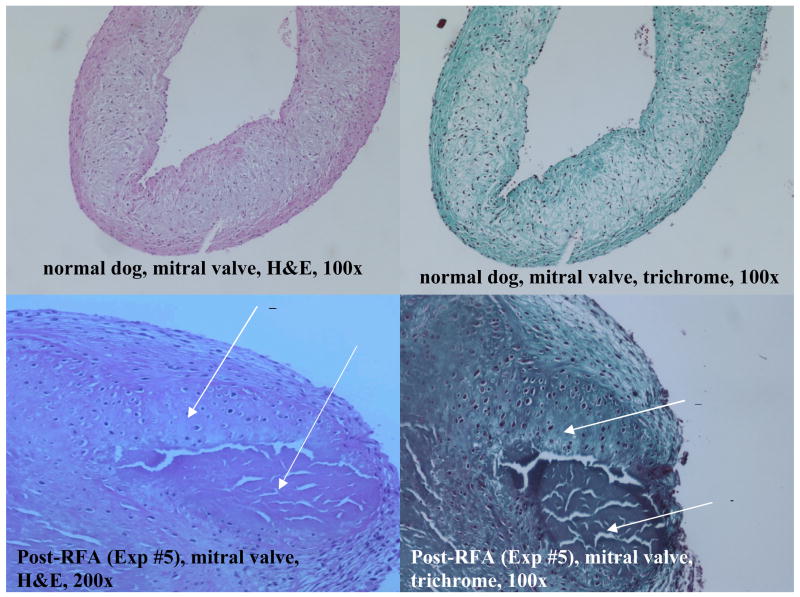
Histopathologic changes in mitral leaflets of these dogs were examined immediately post-mortem (Figure 5). Affected valves showed small areas of coagulation necrosis (consistent with RFA injury), labeled by arrows A, and surrounding fibroblastic proliferation, labeled by arrows B, as a feature of tissue repair.
Figure 5. Histopathologic Changes In Mitral Leaflets after Radiofrequency Ablation.
Affected valves show small areas of coagulation necrosis (consistent with RFA injury), labeled by arrows A, and surrounding fibroblastic proliferation, labeled by arrows B, as a feature of tissue repair.
Discussion
Acute ex-vivo study of mitral valve leaflet and chordae demonstrated a qualitative decrease in mitral leaflet surface area, increase in rigidity, and chordal shortening. In a naturally-occurring canine model of myxomatous mitral valve prolapse, three animals found to have anterior leaflet (n=1), posterior leaflet (n=1), and bileaflet MVP prolapse (n=1) causing severe MR with a preserved ejection fractions underwent direct RFA. The pre- and post-ablation regurgitant volumes ranged from 59.3–102.5cc and 33.4–40.3cc, respectively; corresponding to a 43.7–60.7% statistically significant (p=0.039) reduction in post-ablation MR. These data suggest that myxomatous mitral valve repair using radiofrequency energy delivered via catheter may be feasible. Finally, further investigation was performed to evaluate whether such a technique could be adapted to a percutaneous, closed chest, beating heart environment. ICE-guided anterior mitral leaflet ablation was performed percutaneously in normal canines. The chronic, in-vivo, percutaneous application of RFA was found to be feasible and the engendered alterations durable. This report adds to the body of knowledge about radiofrequency ablation because it is the first report to study chronic, albeit only 6 weeks, echocardiographic and histopathologic effects of intracardiac RFA applied to valve tissue.
Canine Model of Myxomatous MVP
There is a prevalence of MVP in canines that increases with age14,15,16. Most importantly, a majority of dogs develop myxomatous mitral valve disease that is very similar macroscopically and microscopically to primary mitral valve prolapse in humans.15 The similarity of the canine disease process thus makes translation of this therapy in the human model of myxomatous mitral valve prolapse relatively straightforward.
Pathophysiology of Mitral Valve Prolapse
Mitral valve prolapse (MVP) due to myxomatous disease causing severe mitral regurgitation (MR) is thought to be due to a variety of mechanisms: 1) Weakening of the central fibrous core of both the leaflets and chordae17, 2) Increased surface area and decreased density of the leaflets18, and 3) Decreased strength and increased extensibility of the chordae.19 Therefore, percutaneous repair of a regurgitant valve in the setting of MVP should consist of increasing the rigidity of the valve leaflet itself, decreasing the surface area of the valve leaflet, and decreasing the length of redundant chordae.
Radiofrequency energy produces cell damage by heating the tissue and causing coagulative necrosis (cell death). These areas of cell death will eventually be replaced with fibrosis (scar tissue).20,21,22 As one increases the energy applied to tissue, one will see an increase in the coagulation area and an increase in thermal damage.21 Though this technique has not been studied on heart valves one can infer that similar cellular changes may occur when this energy is applied to cardiac valves. Interestingly, this technique has been used in oral surgery to stiffen the palate to prevent snoring.23 It has been speculated that radiofrequency ablation (RFA) may cause direct valve tissue injury in patients undergoing ablation of accessory pathways.24 In fact, Wolfsohn et al20 found that when they accidentally applied energy to one of the valves of the heart they caused a fibrous scar. Another group25 found pathologic changes in the tricuspid valve when AV node or His bundle ablation was attempted in dogs. These changes included edema, swelling of the spongiosa, degenerative change of collagen fibers, endocardial thickening, and slight fibrosis and destruction of the valvular tissue. Additionally, collagen in heart tissue denatures (breaks down) and contracts when heated to >65° Celcius26. In fact, the chordae tendinae contract when heated to >65° Celcius.26 This contraction of heart tissue has also been noted by another group.22
The acute (1–7 days) effects of RFA (50-300J) include a lesion that is round-oval in shape, centrally-pale with varying amounts of a hemorrhagic rim.27 Occasionally, there is a mural thrombus over the lesion (~20% occurrence). There is central coagulation necrosis, peripheral contraction band necrosis, and interstitial edema and fibrinous material on the endocardium.25 During days 3–7, one observes a rim of granulation tissue composed of proliferating capillaries and fibroblasts admixed with acute and mononuclear inflammatory cells.27 Finally, the chronic effects are usually evident at 4–6 weeks after RFA. Chronic lesions are round-oval to irregular in shape, well-circumscribed, and fibrotic.27 The acute edema has almost disappeared and there are almost mature collagen fibers and a mild increase in elastic fibers.25 Much of these pathological observations were made in dog models however, Huang et al27 found that dog tissue damage was very similar to the tissue damage found in postmortem humans.
Understanding the effects of RFA on cardiac structures as demonstrated in both animal and human studies, this study adds to the body of knowledge about the effect of RFA on cardiac valvular tissue. The mitral valve tissue 5 weeks post-RFA in this study showed small areas of coagulation necrosis and surrounding fibroblastic proliferation as a feature of tissue repair. These findings are consistent with prior work on non-valvular intracardiac tissue.
Limitations
There are several limitations to this technique. First, the chronic effects (>6weeks) of radiofrequency ablation on cardiac valve tissue are unknown. It is possible that the engendered alterations effected by RFA lead to valve fragility/instability. Second, the percutaneous application of RFA did not demonstrate the utility of ablation in treating mitral valve prolapse. Though we show that RFA treatment can be successful when applied using an open surgical method, we did not demonstrate this for percutaneous method; we applied RFA to normal mitral valves. In order to limit the number of animals used in the percutaneous method, we (and our Institutional Animal Care and Use Committee) felt that RFA should be reliably applied to myxomatous mitral valves by direct visualization in an open surgical preparation prior to assessing the feasibility of percutaneous delivery of RFA to mitral valves. Finally, the great vessel and cardiac anatomy of canines is different than that of humans. It is not known if this technique could be reproduced in the human model.
Conclusion
These data suggest that myxomatous mitral valve repair using radiofrequency energy delivered via catheter is feasible. Furthermore, percutaneous repair of a cardiac valve using radiofrequency ablation may have additional advantages in that 1) It preserves the supporting structures of the valves (chordae tendinae) which have been shown to be beneficial, 2) It does not require the permanent placement of a foreign object into the human body and thus minimizes the chance of infection and blood clot formation, and 3) It may lead to improved patient tolerance, less complications, quicker recovery, and less cost. This catheter based approach may be expanded to percutaneous applications in the future.
Acknowledgments
The authors would like to acknowledge David Fischer and Mike Nakon for their dedicated work in the experimental animal laboratory.
Support: This work was supported in part by a National Institutes of Health Loan Repayment Program Clinical Research Award to Dr. Williams.
References
- 1.Devereux RB, Kramer-Fox R, et al. Mitral valve prolapse: causes, clinical manifestations, and management. Ann Intern Med, Vol. 1989;111:305–317. doi: 10.7326/0003-4819-111-4-305. [DOI] [PubMed] [Google Scholar]
- 2.Freed LA, Levy D, et al. Prevalence and Clinical Outcome of Mitral Valve Prolapse. New England Journal of Medicine. 1999 July 1;341(1):1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 3.Freed LA, Benjamin EJ, et al. Mitral Valve Prolapse in the General Population: The Benign Nature of Echocardiographic Features in the Framingham Heart Study. Journal of the American College of Cardiology. 2002;40(7):1298–1304. doi: 10.1016/s0735-1097(02)02161-7. [DOI] [PubMed] [Google Scholar]
- 4.Devereux RB, Jones EC, et al. Prevalence and Correlates of Mitral Valve Prolapse in a Population-based Sample of American Indians: the Strong Heart Study. American Journal of Medicine. 2001 December 15;111:679–685. doi: 10.1016/s0002-9343(01)00981-0. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier A, Relland J, et al. Conservative Management of the Prolapsed Mitral Valve. Annals Thoracic Surgery. 1978 October;26(4):294–302. doi: 10.1016/s0003-4975(10)62895-0. [DOI] [PubMed] [Google Scholar]
- 6.Galloway AC, Colvin SB, et al. Long-term Results of Mitral Valve Reconstruction With Carpentier Techniques in 148 Patients with Mitral Insufficiency. Circulation. 1988 September;78(3):I97–I105. [PubMed] [Google Scholar]
- 7.Cosgrove DM, Chavez AM, et al. Results of mitral valve reconstruction. Circulation. 1986 September;74(Supp I):I82–I87. [PubMed] [Google Scholar]
- 8.Matsumoto H, Sakata R, et al. Posterior Mitral Annuloplasty With a Flexible Linear Reducer. Jpn J Thorac Cardiovasc Surg. 2002 September;50(9):371–374. doi: 10.1007/BF02913186. [DOI] [PubMed] [Google Scholar]
- 9.Onnasch JF, Schneider F, et al. Mitral valve repair versus mitral valve replacement. Z Cardiol. 2001;90(Supp 6):75–80. [PubMed] [Google Scholar]
- 10.Galloway AC, Grossi EA, et al. Evolving Techniques for Mitral Valve Reconstruction. Annals of Surgery. 2002 September;236(3):288–294. doi: 10.1097/00000658-200209000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasol R, Joubert-Hubner E. Triangular Resection of the Anterior Leaflet for Repair of the Mitral Valve. Ann Thorac Surg. 2001;71:381–383. doi: 10.1016/s0003-4975(00)02065-8. [DOI] [PubMed] [Google Scholar]
- 12.Tatooles AJ, Pappas PS, Gordon PJ, Slaughter MS. Minimally Invasive Mitral Valve Repair Using the daVinci Robotic System. Ann Thoras Surg. 2004;77:1978–1984. doi: 10.1016/j.athoracsur.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Choi H, Lee K, Lee H, Lee Y, Chang D, Eom K, Youn H, Choi M, Yoon J. Quantification of mitral regurgitation using proximal isovelocity surface area method in dogs. J Vet Sci. 2004;5(2):163–171. [PubMed] [Google Scholar]
- 14.Zook BC, Blackbourne BD, Katz RJ, Bradley EW. Mitral Valve Prolapse. Comparative Pathology Bulletin. 1983 August;15:3–4. [Google Scholar]
- 15.Pederson HD, Haggstrom J. Mitral valve prolapse in the dog: a model of mitral valve prolapse in man. Cardiovascular Research. 2000;47:234–243. doi: 10.1016/s0008-6363(00)00113-9. [DOI] [PubMed] [Google Scholar]
- 16.Pederson HD, Kristensen BO, Lorentzen KA, Koch J, Jensen AL, Flagstad A. Mitral Valve Prolapse in 3-year-old Healthy Cavalier King Charles Spaniels. An Echocardiographic Study. Can J Vet Res. 1995;59:294–298. [PMC free article] [PubMed] [Google Scholar]
- 17.Davies MJ, Moore BP, Braimbridge MV. The floppy mitral valve: Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. British Heart Journal. 40(1978):468–481. doi: 10.1136/hrt.40.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King BD, Clark MA, Baba N, Kilman JW, Wooley CF. Myxomatous Mitral Valves: Collagen Dissolution as the Primary Defect. Circulation. 1982 August;66(2):288–296. doi: 10.1161/01.cir.66.2.288. [DOI] [PubMed] [Google Scholar]
- 19.Cosgrove DM, Stewart WJ. Mitral Valvuloplasty. Curr Probl Cardiol. 1989 July;14(7):353–416. doi: 10.1016/s0146-2806(89)80002-7. [DOI] [PubMed] [Google Scholar]
- 20.Wolfsohn AL, Green MS, Walley VM. Pathology of Radiofrequency Catheter Ablation of the Atrioventricular Node. Modern Pathology. 1994;7(4):494–496. [PubMed] [Google Scholar]
- 21.Remorgida V. Tissue thermal damage caused by bipolar forceps can be reduced with a combination of plastic and metal. Surgical Endoscopy. 1998;12:936–939. doi: 10.1007/s004649900751. [DOI] [PubMed] [Google Scholar]
- 22.Liem LB, Pomeranz M, et al. Electrophysiological Correlates of Transmural Linear Ablation. PACE. 2000 January;23:40–46. doi: 10.1111/j.1540-8159.2000.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 23.Mair EA, Day RH. Cautery-assisted palatal stiffening operation. Otolaryngol Head Neck Surg. 2000 April;122:547–555. doi: 10.1067/mhn.2000.106475. [DOI] [PubMed] [Google Scholar]
- 24.Minich LL, Snider AR, Dick M., II Doppler Detection of Valvular Regurgitation After Radiofrequency Ablation of Accessory Connections. Am J Cardiol. 1992 July 1;70:116–117. doi: 10.1016/0002-9149(92)91404-r. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Satake S, Kawahara Y, Sugiura M, Hirao K, Tanaka K, Kawara T, Masuda A, Nishikawa T, Kasajima T. Pathological Aspects of Radiofrequency Ablation of the Canine Atrioventricular Node and Bundle of His: With Special Reference to Chronic Incomplete Atrioventricular Block. Acta Pathol Jpn, V. 1991;41(7):487–498. doi: 10.1111/j.1440-1827.1991.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 26.Victal OA, Teerlink JR, et al. Left Ventricular Volume Reduction by Radiofrequency Heating of Chronic Myocardial Infarction in Patients with Congestive Heart Failure. Circulation. 2002 March 19;105:1317–1322. doi: 10.1161/hc1102.105566. [DOI] [PubMed] [Google Scholar]
- 27.Huang SKS, Graham AR, Bharati S, Lee MA, Gorman G, Lev M. Short- and Long-Term Effects of Transcatheter Ablation of the Coronary Sinus By Radiofrequency Energy. Circulation, V. 1988 August;78(2):416–427. doi: 10.1161/01.cir.78.2.416. [DOI] [PubMed] [Google Scholar]