Random mutagenesis of the zebrafish genome using chemicals, retroviruses or transposons has uncovered mutations in hundreds of genes1. The ability to engineer specific mutations, however, has remained elusive. Two papers in this issue, by Meng et al. 2 and Doyon et al. 3, introduce a method for targeted mutagenesis in zebrafish. Both studies employ zinc-finger nucleases (ZFNs)—chimeric molecules consisting of a DNA-binding zinc-finger domain and the FokI restriction endonuclease—to induce mutations in specific zebrafish genes (Fig. 1). This technique makes it possible to disrupt any gene of interest and may facilitate more sophisticated manipulations of the zebrafish genome.
Figure 1. Targeted mutagenesis of zebrafish genes with zinc-finger nucleases (ZFNs).
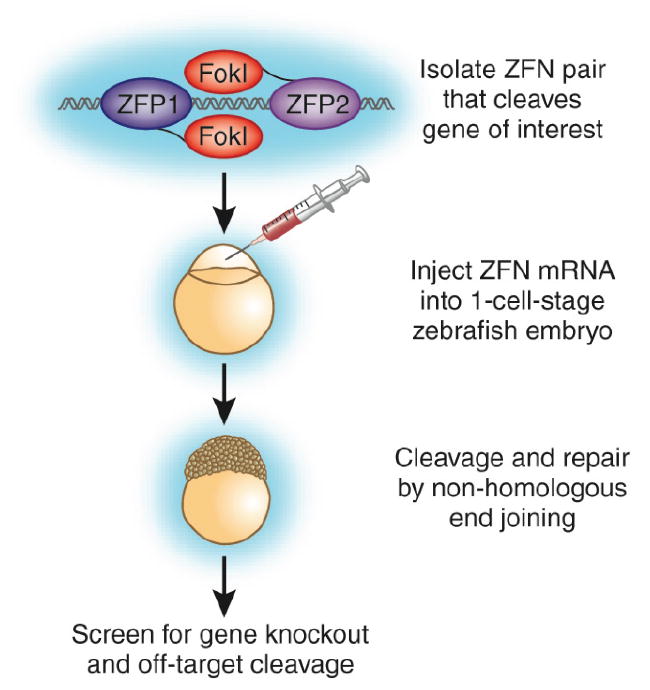
Two ZFNs that target the locus of interest are generated through rational design and screening. mRNAs encoding the ZFNs are injected into zebrafish embryos at the one-cell stage. After cleavage of the target sequence, the endogenous repair machinery can religate the DNA through nonhomologous end joining, which often adds or deletes nucleotides at the cleavage site.
ZFNs induce targeted double-strand breaks in the genome4,5,6. The specificity of DNA cleavage is conferred by varying a ZFN’s repertoire of zinc fingers, each of which interacts with a particular triplet of DNA base pairs. Combining three or four zinc fingers allows specific binding to 9- or 12-bp motifs, respectively. Double-strand breaks occur when two ZFNs bind to target DNA, bringing their nuclease domains together. Active only as a dimer, the nuclease domains cleave the DNA between the bound ZFNs.
The endogenous double-strand-break repair machinery can then edit the genome through two pathways. If a matching template sequence is available, repair can occur by homologous recombination. In the absence of a template, the DNA can be religated by nonhomologous end joining, often with the addition or deletion of bases. The ability of ZFNs to induce targeted double-strand breaks has been exploited in numerous applications, including the creation of knockouts in cell lines7 and invertebrates8 and gene editing in mammalian cells9.
Both Meng et al. 2 and Doyon et al. 3 use ZFNs to generate mutations through nonhomologous end joining: mRNAs encoding two ZFNs are injected into fertilized eggs, and ZFN activity is assayed by PCR2 and phenotypic screening3 in the injected fish and their progeny (Fig. 1). Importantly, 30–50% of injected fish transmit ZFN-induced mutations to their progeny, and many (18% in ref. 2; 7% in ref. 3) of these progeny are mutant. These results indicate that screening for mutagenic events is very efficient.
Both groups also show that ZFN-induced DNA cleavage is highly specific. Meng et al. 2, identify 41 regions of the zebrafish genome with sequences similar (differing by 1–4 nucleotides) to their intended ZFN target. Solexa sequencing of these regions in ZFN-injected embryos reveals that the rate of off-target cleavage is 1% in morphologically normal embryos and 5% in embryos with nonspecific “monster” phenotypes. Doyon et al. 3 analyze the five genomic regions with sequences most similar to their intended target in progeny of ZFN-injected fish and detect no off-target cleavage.
Interestingly, Doyon et al. 3 observe that both copies of the targeted gene are disrupted in some cells of injected embryos, leading to mosaic mutant phenotypes. Meng et al. 2, however, do not report mosaic phenotypes. Although the reason for this difference is unclear, one possibility is that Doyon et al. 3 use ZFNs with four zinc fingers whereas Meng et al. 2 use ZFNs containing three zinc fingers. Increasing the number of zinc fingers enhances the target specificity of ZFNs and can reduce off-target cleavage of DNA4. Accordingly, embryos injected by Doyon et al. 3 tolerate nanogram amounts of injected ZFN mRNA, whereas 50-pg doses of ZFN mRNA are toxic to most embryos in the Meng et al. 2 study. Hence, the higher levels of ZFNs used by Doyon et al. 3 may be sufficient to disrupt both copies of the targeted gene. Another reason for the difference may be that some mutant cells are more readily observable in a wild-type background than others—Doyon et al. 3 score obvious pigment and body pattern phenotypes, whereas Meng et al.2 analyze more subtle vascular defects. Further work will clarify these issues.
How easily can this technology be implemented in a standard zebrafish lab? Injection, genotyping and mutant analysis are well-established procedures. Therefore, the remaining obstacles involve the design, selection and validation of ZFNs. Indeed, both studies stem from collaborations between zebrafish researchers and ZFN experts. Web-based tools10 and published protocols11 are available to assist researchers in designing and synthesizing ZFNs. It should be noted that the construction of modular ZFNs based on individual zinc finger–DNA interactions has been generally unsuccessful unless the repertoire of zinc fingers is restricted to those with particularly well-validated target sequences12. A commercial source of ZFN expertise, design and optimization is under development3.
Intriguingly, zebrafish embryos themselves might provide an excellent in vivo test and optimization system for ZFNs. The Meng et al. study2 exemplifies this potential: ZFNs were designed such that a restriction enzyme recognition sequence was situated between their binding targets, enabling embryos to be tested for ZFN activity by PCR and restriction digestion shortly after injection. A skilled zebrafish researcher can inject and assay hundreds of embryos in a single day, thus allowing multiple candidate ZFNs to be tested in parallel.
It is likely that ZFNs will find widespread use in the zebrafish community and complement other approaches currently used to disrupt the function of specific genes1. For example, antisense morpholino oligonucleotides can block translation or splicing of specific RNAs but often induce off-target effects and are unsuited for phenotypic analyses at later stages of development. True genetic mutants can be generated through TILLING, in which large libraries of mutagenized fish are screened by PCR and sequenced for lesions in target genes. Although specific regions within genes can be analyzed for disruptions, the mutations obtained by TILLING are random; moreover, the required resources are beyond the scope of most laboratories. Retroviral insertions have also been successfully used to disrupt zebrafish genes. Because each insert can be mapped within the genome, large collections of insertions could in principle be created with inserts in nearly every gene. However, the potential to specifically edit the zebrafish genome is unique to ZFNs.
In summary, Doyon et al.3 and Meng et al.2 convincingly demonstrate that ZFNs can induce mutations in zebrafish via nonhomologous end joining. The next step will be to coerce the DNA repair machinery to use homologous recombination, rather than nonhomologous end joining, to repair ZFN-induced double-strand breaks. Homologous recombination techniques would allow for exquisite control over mutagenesis and could also facilitate the introduction of transgenes that reflect endogenous gene expression and protein localization. Finally, the two studies suggest that ZFN technology can be applied to other organisms that have a sequenced genome and that are amenable to RNA injection. ZFNs might therefore become the major technology for genome manipulation.
Contributor Information
Ian G. Woods, Email: ianwoods@mcb.harvard.edu.
Alexander F. Schier, Email: schier@fas.harvard.edu.
References
- 1.Amsterdam A, Hopkins N. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Meng X, et al. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyon Y, et al. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porteus MH, Baltimore D. Science. 2003;300:763. doi: 10.1126/science.1078395. [DOI] [PubMed] [Google Scholar]
- 5.Bibikova M, Beumer K, Trautman JK, Carroll D. Science. 2003;300:764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Kandavelou K, Chandrasegaran S. Cell Mol Life Sci. 2007;64:2933–2944. doi: 10.1007/s00018-007-7206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santiago Y, et al. Proc Natl Acad Sci USA. 2008;105:5809–5814. doi: 10.1073/pnas.0800940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibikova M, Golic M, Golic KG, Carroll D. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urnov FD, et al. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 10.Mandell JG, Barbas CF. Nucleic Acids Res. 2006;34:W516–523. doi: 10.1093/nar/gkl209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll D, Morton JJ, Beumer KJ, Segal DJ. Nat Protocols. 2006;1:1329–1341. doi: 10.1038/nprot.2006.231. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez CL, et al. Nat Methods. 2008;5:374–375. doi: 10.1038/nmeth0508-374. [DOI] [PMC free article] [PubMed] [Google Scholar]


