Abstract
Simply observing other individuals interacting has been shown to affect subsequent behaviour and also hormones in ‘bystander’ individuals. However, immediate physiological responses of an observer have been hardly investigated. Here we present results on individuals' heart rate (HR) responses during various situations, which occur regularly in a flock of greylag geese (Anser anser, e.g. agonistic encounters, vehicles passing by). We recorded simultaneously HR and behaviour of 21 semi-tame free-roaming geese, equipped with fully implanted transmitters. We considered 304 social and 81 non-social events during which the focal individuals did not respond behaviourally. Independent of the spatial distance to the event, these HR responses were significantly greater in social contexts (e.g. departing or landing geese, agonistic interactions) than in non-social situations (e.g. vehicles passing by, thunder). Focal individuals showed a significantly higher maximum HR as well as a greater HR increase in response to agonistic interactions, in which the pair partner or a family member was involved, as compared with a non-affiliated goose. Also, HR was significantly higher when the bystander watched non-affiliated geese interacting, which were higher ranking than the focal. We conclude that these differences are due to different relevance of the recorded events for the focal individual, depending on the individuals involved in the observed interaction.
Keywords: bystander events, physiological response, heart rate, relevance of stimuli, greylag geese
1. Introduction
In group-living animals, social interactions may have pronounced effects on individuals' subsequent behaviour (de Waal & Yoshihara 1983; Black & Owen 1989; Briffa & Elwood 2001) and physiology (Hack 1997; Otten et al. 1997; Kotrschal et al. 2000; Briffa & Elwood 2005). Social interactions affect not only individuals actively involved but also individuals that observe an interaction (bystanders). Individuals are known to change their behaviour in subsequent interactions according to the information gained by observing others (eavesdropping, e.g. Naguib et al. 1999; Earley & Dugatkin 2001; Johnstone 2001; Peake 2005; Zulandt et al. 2008), and observing aggressive encounters modulates individuals' physiology (e.g. neurotransmitters, Edwards & Kravitz 1997; steroid hormones, Oliveira et al. 2001, 2002).
No information is available on heart rate (HR) changes of bystanders in response to observing social interactions. HR is known to be modulated by physical activity (Tatoyan & Cherkovich 1972; MacArthur et al. 1979; Dressen et al. 1990; Kreeger et al. 1990; Arnold et al. 2004) as well as by active involvement in social interactions (Kanwisher et al. 1978; Marchant et al. 1995; Ely et al. 1999; Sgoifo et al. 1999; Wascher et al. 2008). If a focal individual observes an event without showing any physical activity or change in body posture, the potential relevance of a stimulus situation may be evaluated via HR (Bastian 1984). In bystanders, HR changes probably are not due to physical activity but mainly due to ‘psychological’ factors. These include emotions and affects, defined as mediators between the perception of the social and physical environments, and individuals' behavioural and physiological responses (Panksepp 1989; Lott 1991; Aureli 1997) and motivation, defined as processes and structures of an organism directing action towards the satisfaction of needs (Hebb 1949; Deci & Ryan 1985). HR increases in response to relevant stimuli in the absence of physical activity have been anecdotically reported from bighorn sheep (Ovis canadensis: MacArthur et al. 1982), herring gulls (Larus argentatus: Kanwisher et al. 1978) and guide dogs (Fallani et al. 2007). Also, HR has been used in several studies to assess emotions in animals (Aureli et al. 1999; Cabanac & Aizawa 2000; Bradley & Lang 2001; Preston & de Waal 2002; Désiré et al. 2004). Affective responses, including physiological, behavioural and subjective components, are a result of the cognitive evaluation (not necessarily ‘conscious’) of an event in terms of its significance for the survival and well-being of the individual (Scherer 1999; Désiré et al. 2006). Therefore, HR has been used as a parameter to judge the relevance of certain stimuli, for example, if individuals can differentiate between familiar and non-familiar animals (Nakagawa et al. 2001) or between conspecifics and heterospecifics (Berntson & Boysen 1989; Boysen & Berntson 1989; Hauber et al. 2002).
Greylag geese (Anser anser) are long-term monogamous birds (Lorenz 1991). Young stay with their parents for at least one year after fledging. Pair partner and family members support each other actively in agonistic interactions as well as passively, via the mere presence of social allies (Frigerio et al. 2003; Weiß & Kotrschal 2004; Scheiber et al. 2005b), and virtually no aggression occurs within such a bond (Lorenz 1991; Kotrschal et al. 2006; Weiß et al. in press) whereas agonistic encounters with non-affiliated flock members are quite common. Greylag geese form a dominance hierarchy, in which families dominate pairs, which tend to win against singletons in social interactions (Lamprecht 1986).
In this study, we investigated HR responses of individual greylag geese observing naturally occurring social and non-social events. Upregulation of HR via the sympathetico-adrenergic system prepares an organism to respond adequately to a certain stressor (Smith et al. 2000). Owing to the overwhelming importance of the social environment for reproductive success (Silk 2007), social contexts are among the most potent stressors (de Vries et al. 2003). We therefore predict that HR responses will be more pronounced when experiencing social interactions (e.g. agonistic encounters) as compared with non-social events (e.g. vehicles passing by). Furthermore, because greylag geese form complex dyadic relationships (Scheiber et al. 2008), we predict that HR of bystanders would be affected by the identity of the observed individuals (e.g. affiliated versus non-affiliated individuals, lower versus higher ‘ranking’).
2. Material and methods
(a) The flock
A non-migratory flock of greylag geese was introduced in the Almtal (Upper Austria) by the late Konrad Lorenz in 1973. The geese are unrestrained and roam the valley between the Konrad Lorenz Forschungsstelle (KLF) and a lake approximately 10 km to the south, where they roost at night. At the time of the study, the flock consisted of approximately 150 individuals, marked with coloured leg bands for identification.
The flock is supplemented with pellets and grain twice per day. Geese are habituated to the close presence of humans and do not show avoidance if approached at up to 1 m distance. Also, they do not excrete elevated levels of corticosterone metabolites following such situations (Scheiber et al. 2005a) or change HR significantly when familiar humans approach (C. A. F. Wascher 2007, unpublished data).
(b) Transmitter technology and implantation technique
Twenty-one focal individuals (8 females and 13 males) were fitted with a fully implanted sensor–transmitter package without external antennas or repeater, weighing approximately 60 g, with a battery lifetime of 18 months (technical solution by Franz Schober et al., Research Institute for Wildlife Ecology, Vienna). HR was instantaneously transmitted on a beat-to-beat basis (Prinzinger et al. 2002). The transmitter was calibrated to record and transmit in the range of 30 to over 500 beats per minute (b.p.m.). Transmitters were implanted by an experienced team of veterinarians (Walzer et al. 2000). This was approved by animal experimental licence (GZ68.210/41-BrGT/2003) of the Austrian Federal Ministry of Science and Research (for further technical details, see also Wascher et al. 2008).
(c) Data collection
HR and behaviour (using Observer v. 4.1.126; Noldus 2002) of 21 focal individuals were recorded simultaneously by one of us (C.A.F.W.) at a maximal distance of 10 m to the focal individual. Recordings were done after feeding (08.00–08.30), when most of the flock rested and all events that occurred in the surrounding of the focal individual could be clearly distinguished. We only analysed bystander events during which no other event occurred in parallel. We characterized the different events as ‘social’ (departing or landing geese and agonistic interactions) or ‘non-social’ (vehicles passing by and loud noise). We also noted the distance of the event to the focal individual. Departing and landing events occurred at a distance of 0.5 to 300 m (mean±s.d. 37.1±61.2 m) to the focal individual and agonistic interactions at a distance of 0.5 to 100 m (mean±s.d. 9.8±38.7 m). Non-social events occurred from 10 to 500 m (mean±s.d. 161.3±172.9 m) away. In cases of agonistic interactions, their intensity was noted and, whenever possible, the identity of the individuals involved. We differentiated four intensities of agonistic interactions defined as follows. Intensity 1: threat postures without any locomotory activity. Intensity 2: goose walking towards another goose in a threat posture. Intensity 3: goose running towards another goose without body contact. Intensity 4: fight with body contact (e.g. biting, wing beating). For the analysis, we included events where the focal individual showed no or only slight changes in body posture (raising the head slightly). Data were collected daily over a period of 18 months (June 2005–November 2006). Independent of this study, the ‘percentage won interactions’ for each individual of the flock as an approximation of rank was evaluated (B. M. Weiß 2005 and 2006, unpublished data). Based on these data, we defined the focal individual either higher or lower ranking than the interacting individuals, which means that the focal individual is either winning or losing a higher percentage of interactions than the individuals involved in an interaction.
(d) Statistical analysis
Data were analysed using SigmaStat v. 3.5. (Systat Software, Inc. 2006) statistical package. Results of all tests are given two-tailed and significance was set to p=0.05. As data partly deviated from normal distribution and equal variance, we resorted to non-parametric tests. We analysed maximum HR (b.p.m.) reached within the first 10 s after an event started, HR increase (b.p.m.) and duration of HR increase (s) in response to different events. All mean and range values are summarized in table 1. In cases where more than one event was recorded per individual, we calculated the mean HR response (mean maximum HR, mean HR increase and mean duration of the HR response) over all of the observed events. HR increase was based on the difference between a momentary ‘baseline’ value 3 s before the start of the event and the maximum HR reached. In fact, when a focal individual was involved in an agonistic encounter, HR changed significantly only in the last 3 s preceding an event, but not before (C. A. F. Wascher 2007, unpublished data). In the case of bystander events, HR does not significantly change in the last 9 s before an event. For the duration of the HR response, we calculated the time in seconds until HR returned from the maximum to the baseline level. We could not calculate a mean HR over an event because we recorded only the starting point but not the duration of an event.
Table 1.
Mean HR and HR ranges in response to different events. (Maximum HR and HR increase is given in b.p.m. and the duration of the HR response in seconds (s).)
| maximum HR (b.p.m.) | HR increase (b.p.m.) | duration of HR response (s) | ||||
|---|---|---|---|---|---|---|
| mean | range | mean | range | mean | range | |
| social versus non-social events | 169.9 versus 146.3 | 331.5–116 versus 284–89 | 57.3 versus 44 | 159.5–26 versus 174–4 | 11.6 versus 13.7 | 8.25–20 versus 2.5–34 |
| departing/landing geese versus agonistic interactions | 157 versus 162.8 | 255.5–100 versus 197.6–144.4 | 52.6 versus 54.7 | 106–8 versus 84.8–29.2 | 10.6 versus 11 | 20–2 versus 18–8.2 |
| males versus females | 155.3 versus 167 | 219.5–127.8 versus 220.4–138.1 | 43 versus 59 | 65–21.8 versus 129.8–29.8 | 10.8 versus 15.6 | 16.6–5.5 versus 23.1–11.4 |
| single versus paired males | 142 versus 183 | 163.6–134.5 versus 313–111.5 | 31.6 versus 56.6 | 50.8–17 versus 165–16 | 12.6 versus 10 | 18.7–6.5 versus 14–6 |
| Affiliated versus non-affiliated | 199.2 versus 140.9 | 252.7–102 versus 179–102 | 81.3 versus 37 | 137.3–10 versus 78–8 | 11.6 versus 9.6 | 17.4–2 versus 14–5 |
| pair partner won versus lost | 232.5 versus 151.5 | 354–94 versus 184–126 | 108 versus 57 | 222–4 versus 118–24 | 14.2 versus 13.7 | 31–2 versus 17–10 |
| lower versus higher ‘ranking’ | 143.5 versus 175.6 | 184–124 versus 227–132 | 50.1 versus 72.6 | 118–14 versus 148–31 | 16.9 versus 11.5 | 15.5–6 versus 31–7 |
To compare HR increases in response to social versus non-social events, as well as between affiliated (pair partner and family member) and non-affiliated individuals involved in an interaction, we used Wilcoxon signed-rank tests. The p-values are based on T+, if the sample size was smaller than 15, and on z when larger than 15 (Siegel & Castellan 1998). We only analysed interactions within comparable intensities. To compare HR responses in paired versus single males, we applied Mann–Whitney U tests; for the comparison of HR response with different intensities of attacks, we used a Friedman test (Siegel & Castellan 1998). A Spearman rho correlation was applied to investigate the effect of distance to the event in respect to HR. Therefore, we divided the distance of an event to the focal individual into seven categories (0–0.5, 0.5–1, 1–3, 3–5, 5–10, 10–50 and more than 50 m). As it was not possible to observe a sufficient number of focal individuals watching agonistic interactions between both individuals who are lower and higher in ‘rank’ than the focal individual, we had to apply Mann–Whitney U tests for this comparison. In all recorded events, both opponents were either lower or higher ranking than the focal individual. Every focal individual was used only in one condition (either observing lower or higher ranking geese) and each condition contained paired males, one single male and one female. Sample size changes between tests because not all implanted individuals could be observed in all conditions and interactions of high intensity are generally rare. Also, during the course of the study, some of the implanted individuals disappeared and a few transmitter devices stopped working.
3. Results
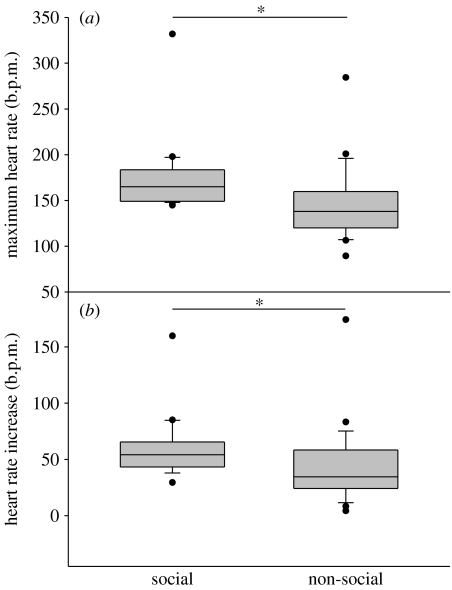
We recorded 385 ‘bystander events’ of 21 focal individuals, 304 and 81 in social and non-social contexts, respectively. HR responses were significantly greater when focal individuals watched social events (agonistic interactions and departing or landing geese) than when they watched non-social events (vehicles passing by, loud noise; Wilcoxon test, maximum HR: n=21, z=−2.381, p=0.018; figures 1 and 2a; and HR increase: n=21, z=−2.068, p=0.04; figure 2b). The social and non-social events did not differ significantly in their duration of HR response (Wilcoxon test: n=21, z=−0.939, p=0.357). Also, we found no significant differences in the HR response of the bystander, depending on the nature of the social context (departing/landing geese or agonistic interactions; Wilcoxon test, maximum HR: n=21, z=−0.317, p=0.756; HR increase: n=21, z=0.278, p=0.278; and duration of the HR response: n=21, z=1.218, p=0.230). Sexes did not differ in maximum HR (Mann–Whitney U test: p=0.294, n1=8, n2=13, U=37) or HR increase (Mann–Whitney U test: p=0.292, n1=8, n2=13, U=36), but the responses lasted for significantly longer periods of time in the females (Mann–Whitney U test: p=0.007, n1=8, n2=13, U=14). Male singletons (n=4) did not differ in HR responses from paired males (n=9; Mann–Whitney U test, maximum HR: p=0.487, n1=4, n2=9, U=13; HR increase: p=0.871, n1=4, n2=9, U=16; and duration of the HR response: p=0.7, n1=4, n2=9, U=21). HR responses to agonistic encounters were independent of intensity of the agonistic encounter (Friedman test, maximum HR: p=0.368, Χ22=2, n=4; HR increase: p=0.472, Χ22=1.5, n=4; and duration of the HR response: p=0.174, Χ22=3.5, n=4). There was no correlation between HR response and spatial distance to the observed event (Spearman's rho test, maximum HR: r=0.000, p=1, n=7; HR increase: r=0.107, p=0.819, n=7; and duration of the HR response: r=0.393, p=0.383, n=7).
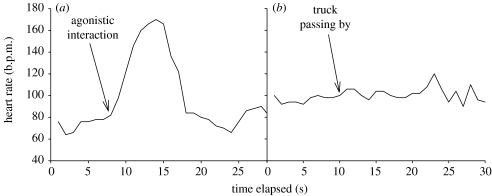
Figure 1.
Examples of a social and a non-social bystander event of one focal individual. (a) The focal individual watched an agonistic interaction at a distance of approximately 5 m. The two interacting individuals were non-affiliated with the focal individual that was resting all the time. (b) A truck passed by, at a distance of approximately 10 m. In this case, the focal individual was vigilant and did not change its behaviour in response to the event.
Figure 2.
(a) Maximum HR and (b) HR increase when watching social events as compared with non-social events in 21 greylag geese. Box plots show the median and the interquartile range from the 25th to the 75th percentiles. Whiskers above and below the box indicate the 10th and 90th percentiles. *p<0.05.
(a) HR responses during observation of agonistic interactions
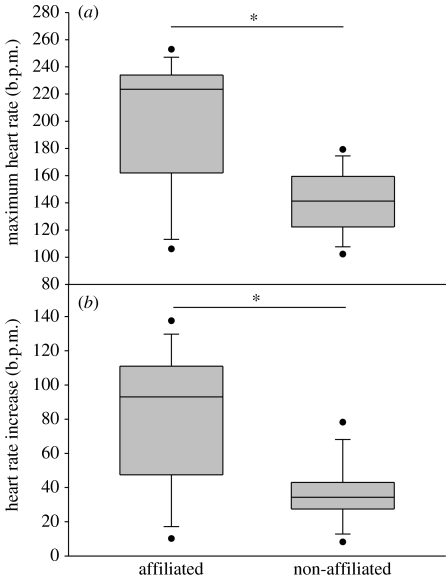
We found a significantly greater maximum HR (Wilcoxon test: n=8, T+=3, p=0.039; figure 3a) and HR increase (Wilcoxon test: n=8, T+=2, p=0.023; figure 3b) in response to interactions in which affiliated individuals were involved when compared with similar situations with non-affiliated individuals. The duration of the response did not differ between the two groups of events (Wilcoxon test: n=8, T+=11, p=0.383). However, whether the interacting partner won or lost had no effect on the bystanders' HR (Wilcoxon test, maximum HR: n=4, T+=3, p=0.625; HR increase: n=4, T+=10, p=0.125; and duration of the HR response: n=4, T+=0, p=0.125).
Figure 3.
(a) Maximum HR and (b) HR increase in b.p.m. of eight bystanders showing no behavioural response when watching agonistic interactions with affiliated or non-affiliated geese involved. Other parameters are the same as given in figure legend 2.
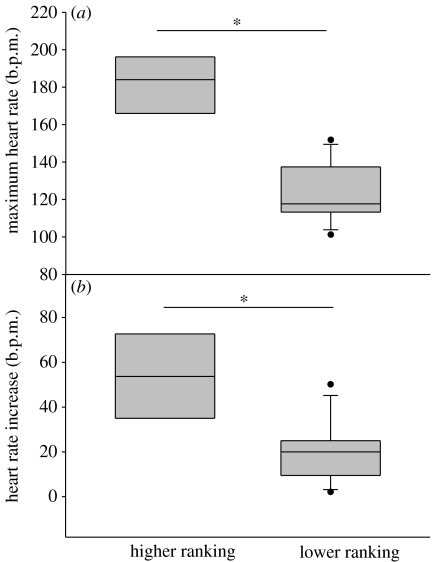
The bystanders' HR were influenced by the rank of the interacting non-affiliated individuals. Seven focal individuals observed encounters, involving geese, which lost more interactions and four different focal individuals watched individuals interacting which both won more interactions. Maximum HR (Mann–Whitney U test: p=0.012, n1=7, n2=4, U=1; figure 4a) and HR increase (Mann–Whitney U test: p=0.024, n1=7, n2=4, U=2; figure 4b) were significantly higher when both interacting individuals were higher in rank than the focal individual. The duration of a HR response, however, did not differ between individuals watching lower or higher ranking geese interacting (Mann–Whitney U test: p=0.230, n1=7, n2=4, U=7). HR responses of the focal individuals were unaffected by the rank of the opponent when an affiliated individual was involved (Mann–Whitney U test, maximum HR: p=0.4, n1=4, n2=3, U=3; HR increase: p=0.4, n1=4, n2=3, U=3; and duration of the HR response: p=0.629, n1=4, n2=3, U=4).
Figure 4.
(a) Maximum HR and (b) HR increase of focal individuals depending on the ‘rank’ relationship of the two interacting individuals with the bystander. In all events, the opponents were both either lower (n=7) or higher (n=4) ‘ranking’ than the observing focal individual. All opponents were non-affiliated to the bystander. Other parameters are the same as given in figure legend 2.
4. Discussion
In this study, we show that in immobile bystanders, social stimuli have a stronger modulatory effect on HR than non-social events. Generally, suddenness, unfamiliarity and unpredictability of a stimulus have been found to be most effective HR modulators (Weiss 1970; Désiré et al. 2006). In our case, however, the social situations occurred much more frequently, regularly and predictably than non-social events. Also, in most cases, social stimuli were less intense (e.g. loud) than non-social stimuli. The individuals involved in an interaction had a stronger influence on HR than the event per se (e.g. its intensity or distance to the focal individual), although in some cases (e.g. high-intensity interactions) our low sample size requires cautious interpretation. Still, these results indicate that social context is not only quantitatively but may also be qualitatively different from non-social stressors.
This raises the question, whether these specific physiological responses reflect the emotional states of bystanders. Recently, emotions and empathy in animals have been discussed (Panksepp 1989; Preston & de Waal 2002; Paul et al. 2005; de Waal 2008). Affective states and emotional arousal can be assessed via physiological and behavioural parameters in humans and non-human animals (Blix et al. 1974; Aureli et al. 1999; Cabanac & Guillemette 2001; Désiré et al. 2004; Fallani et al. 2007). It was suggested that the behavioural expression of emotions may be contagious and thereby affect the emotional states of bystanders (Darwin 1872; Hatfield et al. 1994; Preston & de Waal 2002; de Waal 2008). This is the more plausible, as mirror neurons have recently been found in birds (Prather et al. 2008). The fact that HR responses depended on the identity of the performing individuals suggests that ‘bystanding’ does not just result in a nonspecific arousal.
Our results indicate that greylag geese may be able to estimate other flock members' success in agonistic encounters and probably also the identity of other flock members, as HR increased more strongly when focal individuals watched higher ranking than lower ranking geese interacting, but only when these geese were non-affiliated with the focal goose. It is unlikely that bystanders identify individuals' success in agonistic interactions only by behavioural cueing, because within any interaction, one individual was dominant, the other subordinate, but in relation to our focal subject both opponents were either higher or lower in rank. Still, our results remain somewhat anecdotal, because we did not manage to get complete recordings from all focal individuals, that is, observing both lower ranking as well as higher ranking individuals interact. Therefore, we cannot exclude the possibility that individual differences influence HR responses most as we have previously documented behavioural phenotype-related differences in HR (Wascher et al. 2006).
If the pair partner or a family member was involved in an interaction, HR was increased, irrespective of the rank of the opponents or the outcome of the interaction. In general, upregulation of HR may be interpreted as becoming ready for action. The bystander should modulate HR in proportion to the relevance and potential consequences of an event. For example, a bystander could prepare for the increased risk of being attacked, because not only in primates but also in geese, redirected aggression is common (Watanabe 1979; Aureli et al. 1992; B. M. Weiß 2007, unpublished data). HR upregulation in the bystander watching non-associated individuals involved depended on the rank of the interacting individuals, possibly because the bystander could subsequently be targeted by one of the opponents.
In sum, we have shown that HR is modulated to a greater extent by social than non-social contexts. Also, HR is modulated by just watching an interaction, without active involvement of the focal individual or any observable behavioural response. Such HR responses indicate differences in the relevance of various events, depending on the identity of the individuals involved. At present, we remain cautious of interpreting our HR results as an indication of ‘emotional’ or even ‘empathic’ involvement (Preston & de Waal 2002; de Waal 2008), although the specificity of the arousal caused by watching social interactions points in this direction.
Acknowledgments
This research was approved by animal experimental licence (GZ68.210/41-BrGT/2003) of the Austrian Federal Ministry of Science and Research.
We thank Anna Braun, Iulia Nedelcu and Brigitte Weiß for their helpful discussions on the topic and Christan Schloegl for statistical advice. Walter Arnold, Franz Schober, Gerhard Fluch and Thomas Paumann provided technical advice. We also thank two anonymous reviewers for their valuable comments. This project was funded by FWF projects 18601-B17 to I.B.R.S. and 15766-B03 to K.K., permanent support was provided by the ‘Verein der Förderer’ and the Herzog von Cumberland Stiftung.
References
- Arnold W, Ruf T, Reimoser S, Tataruch F, Onderscheka K, Schober F. Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus) Am. J. Physiol. 2004;286:174–181. doi: 10.1152/ajpregu.00593.2002. doi:10.1152/ajpregu.00593.2002 [DOI] [PubMed] [Google Scholar]
- Aureli F. Post-conflict anxiety in nonhuman primates: the mediating role of emotion in conflict resolution. Aggress. Behav. 1997;23:315–328. doi:10.1002/(SICI)1098-2337(1997)23:5<315::AID-AB2>3.0.CO;2-H [Google Scholar]
- Aureli F, Cozzolino R, Cordischi C, Scucchi S. Kin-oriented redirection among Japanese macaques: an expression of a revenge system? Anim. Behav. 1992;44:283–291. doi:10.1016/0003-3472(92)90034-7 [Google Scholar]
- Aureli F, Preston S.D, de Waal F.B.M. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J. Comp. Psychol. 1999;113:59–65. doi: 10.1037/0735-7036.113.1.59. doi:10.1037/0735-7036.113.1.59 [DOI] [PubMed] [Google Scholar]
- Bastian H.-V. Die Änderung der Herzfrequenz als Maß der Erregung- eine Literaturübersicht. Die Vogelwarte. 1984;32:226–233. [Google Scholar]
- Berntson G.G, Boysen S.T. Specificity of the cardiac response to conspecific vocalization in chimpanzees. Behav. Neurosci. 1989;103:235–245. doi: 10.1037//0735-7044.103.2.235. doi:10.1037/0735-7044.103.2.235 [DOI] [PubMed] [Google Scholar]
- Black J.M, Owen M. Agonistic behaviour in barnacle goose flocks: assessment, investment and reproductive success. Anim. Behav. 1989;37:199–209. doi:10.1016/0003-3472(89)90110-3 [Google Scholar]
- Blix A.S, Stromme S.B, Ursin H. Additional heart rate—an indicator of psychological activation. Aerosp. Med. 1974;45:1219–1222. [PubMed] [Google Scholar]
- Boysen S.T, Berntson G.G. Conspecific recognition in the chimpanzee (Pan troglodytes): cardiac responses to significant others. J. Comp. Psychol. 1989;103:215–220. doi: 10.1037/0735-7036.103.3.215. doi:10.1037/0735-7036.103.3.215 [DOI] [PubMed] [Google Scholar]
- Bradley M.M, Lang P.J. Measuring emotion: behavior, feeling, and physiology. In: Lane R.D, Nadel L, editors. Cognitve neuroscience of emotion. Oxford University Press; Oxford, UK: 2001. pp. 242–276. [Google Scholar]
- Briffa M, Elwood R.W. Decision rules, energy metabolism and vigour of hermit-crab fights. Proc. R. Soc. B. 2001;268:1841–1848. doi: 10.1098/rspb.2001.1752. doi:10.1098/rspb.2001.1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa M, Elwood R.W. Rapid change in energy status in fighting animals: causes and effects of strategic decicions. Anim. Behav. 2005;70:119–124. doi:10.1016/j.anbehav.2004.10.013 [Google Scholar]
- Cabanac M, Aizawa S. Fever and tachycardia in a bird (Gallus domesticus) after simple handling. Physiol. Behav. 2000;69:541–545. doi: 10.1016/s0031-9384(00)00227-4. doi:10.1016/S0031-9384(00)00227-4 [DOI] [PubMed] [Google Scholar]
- Cabanac A.J, Guillemette M. Temperature and heart rate as stress indicators of handled common eider. Physiol. Behav. 2001;74:475–479. doi: 10.1016/s0031-9384(01)00586-8. doi:10.1016/S0031-9384(01)00586-8 [DOI] [PubMed] [Google Scholar]
- Darwin C. Murray; London, UK: 1872. The expression of emotions in man and animals. [Google Scholar]
- Deci E.L, Ryan R.M. Plenum Press; New York, NY: 1985. Intrinsic motivation and self-determination in human behavior. [Google Scholar]
- Désiré L, Veissier I, Després G, Boissy A. On the way to assess emotions in animals: do lambs (Ovis aries) evaluate an event through its suddenness, novelty, or unpredictability? J. Comp. Psychol. 2004;118:363–374. doi: 10.1037/0735-7036.118.4.363. doi:10.1037/0735-7036.118.4.363 [DOI] [PubMed] [Google Scholar]
- Désiré L, Veissier I, Després G, Delval E, Toporenko G, Boissy A. Appraisal process in sheep (Ovis aries): interactive effect of suddenness and unfamiliarity on cardiac and behavioral responses. J. Comp. Psychol. 2006;120:280–287. doi: 10.1037/0735-7036.120.3.280. doi:10.1037/0735-7036.120.3.280 [DOI] [PubMed] [Google Scholar]
- de Vries A.C, Glasper E.R, Detillion C.E. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. doi:10.1016/S0031-9384(03)00152-5 [DOI] [PubMed] [Google Scholar]
- de Waal F.B.M. Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. doi:10.1146/annurev.psych.59.103006.093625 [DOI] [PubMed] [Google Scholar]
- de Waal F.B.M, Yoshihara D. Reconciliation and redirected affection in rhesus monkeys. Behaviour. 1983;85:224–241. [Google Scholar]
- Dressen W, Grün H, Hendrichs H. Radio telemetry of heart rate in male tammar wallabies (Marsupialia: Macropodidae)—temporal variations and behavioural correlates. Aust. J. Zool. 1990;38:89–103. doi:10.1071/ZO9900089 [Google Scholar]
- Earley R.L, Dugatkin L.A. Eavesdropping on visual cues in green swordtail (Xiphophorus helleri) fights: a case for networking. Proce. R. Soc. B. 2001;269:943–952. doi: 10.1098/rspb.2002.1973. doi:10.1098/rspb.2002.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D.H, Kravitz E.A. Serotonin, social status and aggression. Curr. Opin. Neurobiol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. doi:10.1016/S0959-4388(97)80140-7 [DOI] [PubMed] [Google Scholar]
- Ely C.R, Ward D.H, Bollinger K.S. Behavioral correlates of heart rates of free-living greater white-fronted geese. Condor. 1999;101:390–395. doi:10.2307/1370002 [Google Scholar]
- Fallani G, Prato Previde E, Valsecchi P. Behavioral and physiological responses of guide dogs to a situation of emotional distress. Physiol. Behav. 2007;90:648–655. doi: 10.1016/j.physbeh.2006.12.001. doi:10.1016/j.physbeh.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Frigerio D, Weiss B, Dittami J, Kotrschal K. Social allies modulate corticosterone excretion and increase success in agonistic interactions in juvenile hand-raised greylag geese (Anser anser) Can. J. Zool. 2003;81:1746–1754. doi:10.1139/z03-149 [Google Scholar]
- Hack M.A. The energetic costs of fighting in the house cricket, Acheta domesticus L. Behav. Ecol. 1997;8:28–36. doi:10.1093/beheco/8.1.28 [Google Scholar]
- Hatfield E, Cacioppo J.T, Rapson R.L. Cambridge University Press; Cambridge, UK: 1994. Emotional contagion. [Google Scholar]
- Hauber M.E, Pearson H.E, Reh A, Merges A. Discrimination between host songs by brood parasitic brown-headed cowbirds (Molothrus ater) Anim. Cogn. 2002;5:129–137. doi: 10.1007/s10071-002-0143-x. doi:10.1007/s10071-002-0143-x [DOI] [PubMed] [Google Scholar]
- Hebb D.O. Wiley; New York, NY: 1949. The organization of behavior. [Google Scholar]
- Johnstone R.A. Eavesdropping and animal conflict. Proc. Natl Acad. Sci. USA. 2001;98:9177–9180. doi: 10.1073/pnas.161058798. doi:10.1073/pnas.161058798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher J.W, Williams T.C, Teal J.M, Lawson K.O.J. Radiotelemetry of heart rates from free-ranging gulls. Auk. 1978;95:288–293. [Google Scholar]
- Kotrschal K, Dittami J, Hirschenhauser K, Möstl E, Peczely P. Effects of physiological and social challenges in different seasons on fecal testosterone and corticosterone in male domestic geese (Anser domesticus) Acta Ethol. 2000;2:115–122. doi:10.1007/s102110000016 [Google Scholar]
- Kotrschal K, Hemetsberger J, Weiß B.M. Homosociality in greylag geese (Anser anser): making the best of a bad situation. In: Vasey P, Sommer V, editors. Homosexual behaviour in animals: an evolutionary perspective. Cambridge University Press; Cambridge, UK: 2006. pp. 45–76. [Google Scholar]
- Kreeger T.J, Kuechle V.B, Mech L.D, Tester J.R, Seal U.S. Physiological monitoring of gray wolves (Canis lupus) by radiotelemetry. J. Mammal. 1990;71:258–261. doi:10.2307/1382180 [Google Scholar]
- Lamprecht J. Structure and causation of the dominance hierarchy in a flock of bar-headed geese (Anser indicus) Behaviour. 1986;96:28–48. [Google Scholar]
- Lorenz K. Harper Collins; London, UK: 1991. Here I am—where are you? [Google Scholar]
- Lott D.F. Cambridge University Press; Cambridge, UK: 1991. Intraspecific variation in the social system of wild vertebrates. [Google Scholar]
- MacArthur R.A, Johnston R.H, Geist V. Factors influencing heart rate in free-ranging bighorn sheep: a physiological approach to the study of wildlife harassment. Can. J. Zool. 1979;57:2010–2021. [Google Scholar]
- MacArthur R.A, Geist V, Johnston R.H. Physiological correlates of social behaviour in bighorn sheep: a field study using electrocardiogram telemetry. J. Zool. Lond. 1982;196:401–415. [Google Scholar]
- Marchant J.N, Mendl M.T, Rudd A.R, Broom D.M. The effect of agonistic interactions on the heart rate of group-housed sows. Appl. Anim. Behav. Sci. 1995;46:49–56. doi:10.1016/0168-1591(95)00636-2 [Google Scholar]
- Naguib M, Fichtel C, Todt D. Nightingales respond more strongly to vocal leaders of simulated dyadic interactions. Proc. R. Soc. B. 1999;266:537–542. doi:10.1098/rspb.1999.0669 [Google Scholar]
- Nakagawa S, Waas J.R, Miyazaki M. Heart rate changes reveal that little blue penguin chicks (Eudyptula minor) can use vocal signatures to discriminate familiar from unfamiliar chicks. Behav. Ecol. Sociobiol. 2001;50:180–188. doi:10.1007/s002650100355 [Google Scholar]
- Noldus. Noldus Information Technology; Wageningen, The Netherlands: 2002. The Observer®. [Google Scholar]
- Oliveira R.F, Lopes M, Carneiro L.A, Canario A.V.M. Watching fights raises fish hormone levels. Nature. 2001;409:475. doi: 10.1038/35054128. doi:10.1038/35054128 [DOI] [PubMed] [Google Scholar]
- Oliveira R.F, Hirschenhauser K, Carneiro L.A, Canario A.V.M. Social modulation of androgen levels in male teleost fish. Comp. Biochem. Physiol. B. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. doi:10.1016/S1096-4959(01)00523-1 [DOI] [PubMed] [Google Scholar]
- Otten W, Puppe B, Stabenow B, Kanitz E, Schön P.C, Brüssow K.P, Nürnberg G. Agonistic interactions and physiological reactions of top- and bottom-ranking pigs confronted with a familiar and an unfamiliar group: preliminary results. Appl. Anim. Behav. Sci. 1997;55:79–90. doi:10.1016/S0168-1591(97)00036-1 [Google Scholar]
- Panksepp J. The psychobiology of emotions: the animal side of human feelings. In: Gainotti G, Caltagirone C, editors. Emotions and the dual brain. Springer; Berlin, Germany: 1989. pp. 31–55. [Google Scholar]
- Paul E.S, Harding E.J, Mendl M. Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005;29:469–491. doi: 10.1016/j.neubiorev.2005.01.002. doi:10.1016/j.neubiorev.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Peake T.M. Eavesdropping in communication networks. In: McGregor P.K, editor. Animal communication networks. Cambridge University Press; Cambridge, UK: 2005. pp. 13–37. [Google Scholar]
- Prather J.F, Peters S, Nowicki S, Mooney R. Precise auditory–vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. doi:10.1038/nature06492 [DOI] [PubMed] [Google Scholar]
- Preston S.D, de Waal F.B.M. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. doi:10.1017/S0140525X02000018 [DOI] [PubMed] [Google Scholar]
- Prinzinger R, Nagel B, Bahat O, Bögel R, Karl E, Weihs D, Walzer C. Energy metabolism and body temperature in the griffon vulture (Gyps fulvus) with comparative data on the hooded vulture (Necrosyrtes monachus) and the white-backed vulture (Gyps africanus) J. Ornithol. 2002;143:456–467. doi:10.1046/j.1439-0361.2002.02039.x [Google Scholar]
- Scheiber I.B.R, Kralj S, Kotrschal K. Sampling effort/frequency necessary to infer individual acute stress responses from fecal analysis in greylag geese (Anser anser) Ann. N. Y. Acad. Sci. 2005a;1946:154–167. doi: 10.1196/annals.1343.012. doi:10.1196/annals.1343.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiber I.B.R, Weiß B.M, Frigerio D, Kotrschal K. Active and passive social support in families of greylag geese (Anser anser) Behaviour. 2005b;142:1535–1557. doi: 10.1163/156853905774831873. doi:10.1163/156853905774831873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiber I.B.R, Weiß B.M, Hirschenhauser K, Nedelcu I.T, Wascher C.A.F, Kotrschal K. Does ‘relationship intelligence’ make big brains in birds? Open Biol. J. 2008;1:6–8. doi: 10.2174/1874196700801010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer K.R. Appraisal theory. In: Dalgleish T, Power M, editors. Handbook of cognition and emotion. Wiley; Chichester, UK: 1999. pp. 637–663. [Google Scholar]
- Sgoifo A, Koolhaas J.M, de Boer S, Musso E, Stilli D, Buwalda B, Meerlo P. Social stress, autonomic neural activation, and cardiac activity in rats. Neurosci. Biobehav. Rev. 1999;23:915–923. doi: 10.1016/s0149-7634(99)00025-1. doi:10.1016/S0149-7634(99)00025-1 [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan N.J. McGraw-Hill; Boston, MA: 1998. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- Silk J.B. Social components of fitness in primate groups. Science. 2007;317:1347–1351. doi: 10.1126/science.1140734. doi:10.1126/science.1140734 [DOI] [PubMed] [Google Scholar]
- Smith F.M, West N.H, Jones D.R. The cardiovascular system. In: Whittow G.C, editor. Sturkie's avian physiology. Academic Press; San Diego, CA: 2000. pp. 141–232. [Google Scholar]
- Systat Software, Inc. 2006 SigmaStatfor Windows Round Rock, TX: Sub Systems, Inc.
- Tatoyan S.K, Cherkovich G.M. The heart rate in monkeys (baboons and macaques) in different physiological states recorded by radiotelemetry. Folia Primatol. 1972;17:255–266. doi: 10.1159/000155436. [DOI] [PubMed] [Google Scholar]
- Walzer C, Boegel R, Fluch G, Karl E, Schober F, Prinzinger R. Intra-abdominal implantation of a multi-sensor telemetry system in a free-ranging Eurasian griffon (Gyps fulvus) In: Lumeij J.T, Remple J.D, Redig P.T, Lierz M, Cooper J.E, editors. Raptor biomedicine III. Zoological Education Network; Lake Worth, FL: 2000. pp. 313–319. [Google Scholar]
- Wascher A.F.C, Krajl S, Scheiber I.B.R, Kotrschal K. Heart rate and coping styles in greylag geese. J. Ornithol. 2006;147(Suppl.):269–270. [Google Scholar]
- Wascher A.F.C, Arnold W, Kotrschal K. Heart rate modulation by social contexts in greylag geese (Anser anser) J. Comp. Psychol. 2008;122:100–107. doi: 10.1037/0735-7036.122.1.100. doi:10.1037/0735-7036.122.1.100 [DOI] [PubMed] [Google Scholar]
- Watanabe K. Alliance formation in a free-ranging troop of Japanese macaques. Primates. 1979;20:459–474. doi:10.1007/BF02373429 [Google Scholar]
- Weiß B.M, Kotrschal K. Effects of passive social support in juvenile greylag geese (Anser anser): a study from fledging to adulthood. Ethology. 2004;110:429–444. doi:10.1111/j.1439-0310.2004.00979.x [Google Scholar]
- Weiß, B. M., Kotrschal, K., Frigerio, D., Hemetsberger, J. & Scheiber, I. B. R. In press. Birds of a feather stay together: extended family bonds, clan structures and social support in greylag geese (Anser anser). In Family relations. Issues and challenges (ed. R. N. Ramirez). New York, NY: Nova Science Publishers.
- Weiss J.M. Somatic effects of predictable and unpredictable shock. Psychosom. Med. 1970;32:397–408. doi: 10.1097/00006842-197007000-00008. [DOI] [PubMed] [Google Scholar]
- Zulandt T, Zulandt-Schneider R.A, Moore P.A. Observing agonistic interactions alters subsequent fighting dynamics in the crayfish, Orconectes rusticus. Anim. Behav. 2008;75:13–20. doi:10.1016/j.anbehav.2007.04.017 [Google Scholar]