Abstract
E. coli ribonucleotide reductase (RNR) catalyzes the conversion of nucleoside 5′-diphosphates to deoxynucleoside 5′-diphosphates and is a 1:1 complex of two homodimeric subunits: α2 and β2. As a first step towards mapping the subunit interface, β2 (V365C) was labeled with [14C]-benzophenone (BP) iodoacetamide. The resulting [14C]-BP-β2 (V365C) was complexed with α2 and irradiated at 365 nm for 30 min at 4°C. The cross-linked mixture was purified by anion exchange chromatography and digested with trypsin. The peptides were purified by reverse phase chromatography, identified by scintillation counting and analyzed by Edman sequencing. Three [14C]-labeled peptides were identified: two contained a peptide in β to which the BP was attached. The third contained the same β peptide and a peptide in α found in its αD helix. These results provide direct support for the proposed docking model of α2β2.
E. coli ribonucleotide reductase (RNR) catalyzes the conversion of nucleoside 5′-diphosphates (NDPs) to deoxynucleoside 5′-diphosphates. 1, 2 It is a 1:1 complex of two homodimeric subunits: 〈2 and ®2. 3–5 α2 binds both NDP substrates (CDP, UDP, GDP, ADP) and the dNTP (TTP, dGTP, dATP)/ATP allosteric effectors that govern the specificity and enzymatic turnover rate. β2 contains the diferric tyrosyl radical (Y•) cofactor 6 that is required to initiate the nucleotide reduction in α2 by radical propagation over 35 Å. 7 No structure of an active αnβn of any class Ia RNR is currently available.8 Understanding this interaction is essential to the elucidation of the radical propagation mechanism between the two subunits, 7 the mechanism by which the allosteric effector binding to α2 triggers electron transfer between the subunits, 9, 10 and how one might take advantage of this knowledge to design inhibitors of RNR that disrupt subunit interactions. 11 The present communication provides methodology to map the subunit interface at the molecular level.
The interaction between α2 and β2 is weak (Kd = 0.4 μM)12 and is largely governed by the C-terminal 20 amino acids of β2.13, 14 The current model for the interactions between α2 and β2 is based on the docking model that Eklund and coworkers constructed from the structure of β2 and the structure of α2 complexed with a peptide to the last 20 amino acids (356–375) of β2.7 The binding mode of this 20-mer peptide (only 15 residues of which are observed) was proposed to be representative of how the C-terminal tail of β2 binds to α2. Recent pulsed electron-electron double resonance experiments have provided support for the long distance radical transfer consistent with the docking model.15, 16 Molecular insight into the subunit interactions, however, is not provided by this method. In the absence of a structure of the class Ia RNR complex17, we have recently developed a methodology with the potential to provide us with the desired molecular insight.12 The methodology involves the site-specific labeling of a Cys at fifteen different positions within the C-terminus of β2, with the photo cross-linker benzophenone (BP). The photo cross-linking reaction of each BP-β2 variant with α2 demonstrated that BP-β2 (V365C) had the highest photo cross-linking efficiency (~18 %) and was selected to identify the residues at the interface of α2β2 in the present study.12
To facilitate the isolation of cross-linked peptides from α2β2, we synthesized a [14C]-iodoacetamide analog of BP. Our previous studies used BP maleimide to attach BP to a single Cys within the C-terminus of β2.12 Due to the difficulties in making this material [14C]-labeled from available [14C]-starting material, the BP iodoacetamide (BPI) was selected as an alternative. The synthesis of [14C]-BPI was accomplished by coupling of [14C]-iodoacetic acid with 4-aminobenzophenone using dicyclohexylcarbodiimide (DCC). 18 The reaction time, temperature, and the stoichiometry of reactants were optimized to yield [14C]-BPI in ~95% yield with a specific activity of 6600 cpm/nmol. Labeling of β2 (V365C) with [14C]-BPI occurred quantitatively and the photo cross-linking reaction of the resultant BP variant with α2 gave ~18% cross-linked product, estimated by SDS PAGE.12
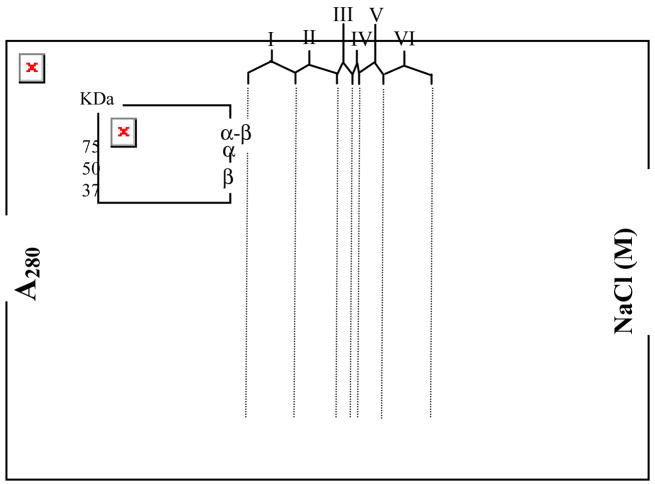
The α2β2 complex has a molecular weight of ~260 kDa, presenting a challenge for the identification of cross-linked peptide(s). In an effort to enrich the desired α-β (cross-linked) product, the reaction mixture was purified by anion exchange chromatography on POROS 10HQ by FPLC. A control experiment (supporting information, Fig S1) showed that anion exchange chromatograph readily separates α2 from β2, and it was hoped that this process could remove a large amount of the non cross-linked protein. A typical FPLC trace (Fig 1) eluted with the indicated NaCl gradient was subdivided into six regions (I–VI). SDS-PAGE gel (Fig 1, inset) showed that region I contains free α, regions II–V contain variable mixtures of α-[14C]-BP-β and free α and [14C]-BP-β(V365C) and region VI contains predominantly [14C]-BP-β(V365C). These results were unexpected and interesting. Instead of separation of α-β from α2 and β2, the chromatogram suggests that α-β forms a complex with free α and β, presumably due to the tight dimerization of β2. This (α-β)αβ complex appears to have increased subunit affinity relative to the non cross-linked subunits (supporting information, Fig S1). The ratio of α-β:α:β is 1:3:2 in region III and 1:3:6 in region IV (ratios are the average of 4 determinations). The α-β thus retards α2 and causes β2 to elute earlier than β2 in the control. This observation could provide a strategy for crystallization of the elusive α2β2 complex. Importantly, this chromatography typically removes ~50–55% of the protein, the goal of the experiment.
Fig 1.
Purification of the photo cross-linked mixture by anion exchange chromatography (POROS 10 HQ) in 50 mM Tris pH 7.6 with the indicated NaCl gradient. The regions I–VI are shown by vertical lines (from left to right). (Inset) SDS-PAGE gel of region I–VI.
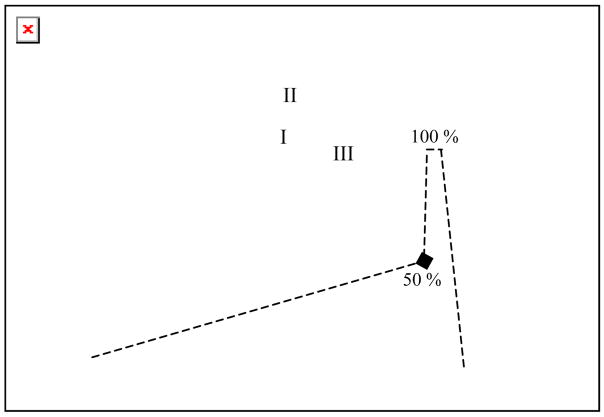
The partially purified mixture (regions II–V) was pooled, alkylated by standard procedures with iodoacetamide, dialyzed into 2M urea and digested with trypsin. The peptide mixture was then analyzed by reverse phase HPLC with a Jupiter® C18 column using the indicated gradient (Fig 2). Fractions of 1 mL were collected and analyzed for radioactivity.
Fig 2.
Purification of the peptides generated by trypsin digestion of the photo cross-linked mixture with reverse phase (Jupitor®) HPLC in 0.1% TFA/H2O with the indicated gradient (---) of 0.1% TFA/CH3CN. The radioactivity of each fraction (1 mL, -■-) is overlaid on the absorbance (—).
Three distinct radioactive regions with retention times of 49 (I), 52 (II) and 68 (III) min were observed superimposed on a radioactive background that was present throughout the chromatogram. Previous crystallographic and NMR studies have demonstrated the flexibility of C-terminal tail of β2.19 When complexed with α2, however, one would have expected this C terminus to be structured and consequently little non-specific cross-linking. Thus, the basis for the background is not understood, but appears to be associated with the long photolysis time (supporting information, Fig S2) and the weak dissociation constant between the subunits.12–14 In order to identify the interaction sites, each of the three regions (I–III) was pooled and rechromatographed twice: once in CH3CN/ammonium acetate at pH 6.8 and finally in CH3CN/TFA/H2O supporting information, Fig S3–S5).
Peak II was found to be similar to a control in which [14C]-BP-β2 (V365C) alone was photolyzed, and digested with trypsin and chromatographed. Edman sequencing of the resulting peptide revealed S329NPIP (Table 1), the expected proteolytic fragment from unreacted [14C]-BP-β2 (V365C). Interestingly, peak I, not observed in photolysis of [14C]-BP-β2 (V365C) alone, gave the same peptide sequence (S329NPIP). No additional amino acid sequence with comparable signal intensity was observed in Edman analysis, which suggested that peak I also resulted from the unreacted [14C]-BP-β2(V365C). One possible origin of the peak I could be an inter subunit cross-link between β1 and β2 (of β2). However, the molecular weight of the resulting peptide would be twice that in peak II and would be expected to have a longer retention time on HPLC analysis. Alternatively, if the C-terminal tail cross-linked to itself (intra subunit cross-linking), a cyclic peptide would result which might explain the shorter retention time of peak I.
Table 1.
The Edman analysis of peptides from peaks I–III and unreacted [14C]-BP−β2(V365C).
| Fractions | Cycles
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| [14C]-BP− β(V365C) | S | N | P | I | P |
| Peak I | S | N | P | I | P |
| Peak II | S | N | P | I | P |
| Peak III | S, A | N, V | P, E | I, L | P, F |
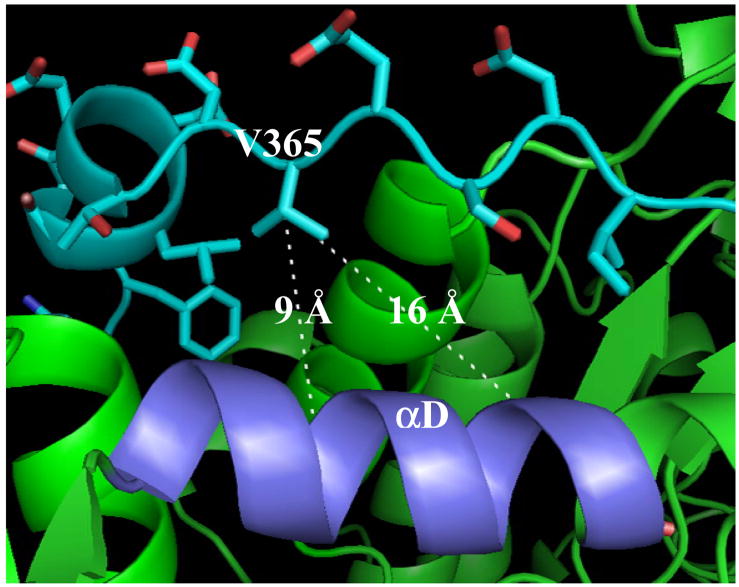
The peptide isolated from region III gave two amino acids in each cycle of Edman sequencing: SNPIP and AVELF (Table 1). While SNPIP originates from the C-terminal tail of [14C]-BP-β2(V365C), AVELF is the proteolytic fragment resulting from cleavage of α2 at position 395. Analysis of the crystal structure of α27 in complex with 20 amino acid peptide to the C-terminus of β2 shows that A395VELF is present in helix αD (Fig 3). The detailed analysis of the structure shows that the residue V365 is pointing directly towards αD. Since the Edman sequencing extended to F399, the first five residues of αD, cross-linking must occur within peptide S400LMMQER406. The distance between V365, and S400 and T409 are ~9 Å and ~16 Å (Fig 3), respectively. The distance between –CH2− of the BP analog attached directly to the thiol of C365 and the ketone of the BP involved in hydrogen atom abstraction is ~9 Å, which would bring S400LMMQER406 within the reactive volume of BP.20 The cross-linking data provide the most direct evidence thus far that the crystal structure of the peptide (356–375) of β2 and α2 is an excellent model for subunit docking. 7,25
Fig 3.
The crystal structure of α2 (green) in complex with C-terminal tail (cyan) of β2. 7 The orientation and distances of V365 with respect to S400LMMQERAST409 (αD, light blue) are shown.
As noted above, BP has been attached to 15 positions within the C-terminus of β2 (341–375) including Y356 which plays an essential role in radical propagation between the two subunits.9, 10, 21 Since residues 341–375 are disordered in β2, cross-linking information throughout this region would be of great value in thinking about a model for subunit interactions. The cross-linking efficiencies, however, within this tail are generally low (~9%), requiring enrichment of the peptide(s) of interest to achieve purification. To facilitate this enrichment, we are currently incorporating p-propargyloxyphenylalanine into β2 using a methodology pioneered by Schultz et al. 22, 23 p-Propargyloxyphenylalanine can be used in a 3+2 cycloaddtion reaction with biotinylated azide for affinity purification by streptavidin beads. 24
A method to purify cross-linked peptides of interest in conjunction with radiolabeling should allow us to map interaction site(s) in the presence of different substrate (ADP, CDP, UDP and GDP) and/or effector (dGTP, ATP, dATP, TTP) pairs. Our recent studies with fluorescent probes within this tail region of β2 have shown that the Kd for subunit interactions vary up to 50 fold.12 The differences in structure may be detectable by this cross-linking method. Additionally, it is conceivable that Y356 in β2 moves during the radical propagation step between the subunits with different substrate and/or effector pairs. The availability of [14C]-BPI in conjunction with a method to rapidly pull out labeled peptides could be useful in understanding the amazing gymnastics responsible for substrate specificity communicated over ~40–50 Å within the active RNR complex.7, 25
Supplementary Material
Materials, methods and Fig S1–S5 have been published as supporting information.
Acknowledgments
This work was supported by the NIH grant (GM 29595) to JoAnne Stubbe. A. Quamrul Hassan is an Anna Fuller fellow funded by David H. Koch Institute for Integrative Cancer Research at MIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference and notes
- 1.Stubbe J, van der Donk WA. Chem Rev. 1998;98(2):705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- 2.Jordan A, Reichard P. Annu Rev Biochem. 1998;67:71–98. doi: 10.1146/annurev.biochem.67.1.71. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Lohman GJS, Stubbe J. Proc Natl Acad Sci USA. 2007;104(36):14324–14329. doi: 10.1073/pnas.0706803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown NC, Reichard P. J Mol Biol. 1969;46(1):25–38. doi: 10.1016/0022-2836(69)90055-2. [DOI] [PubMed] [Google Scholar]
- 5.Thelander L. J Biol Chem. 1973;248(13):4591–4601. [PubMed] [Google Scholar]
- 6.Sjöberg BM, Reichard P, Gräslund A, Ehrenberg A. J Biol Chem. 1977;252(2):536–541. [PubMed] [Google Scholar]
- 7.Uhlin U, Eklund H. Nature. 1994;370(6490):533–9. doi: 10.1038/370533a0. [DOI] [PubMed] [Google Scholar]
- 8.A structure of α2β2 complex of a class Ib RNR from Salmonella typhimurium has recently been reported at 4.0 Å resolution (ref 17). One of the two β monomers reveals its C-terminal peptide binding near αD, while the second β monomer is dangling in a position, likely to be inactive in nucleotide reduction based on our PELDOR experiments (ref 15, 16). The C-terminus of the S. typhimurium β binds in the same region as is observed for E. coli α2-peptide complex. The corresponding residue to V365 (T321) is not, however, visible.
- 9.Seyedsayamdost MR, Stubbe J. J Am Chem Soc. 2006;128(8):2522–2523. doi: 10.1021/ja057776q. [DOI] [PubMed] [Google Scholar]
- 10.Seyedsayamdost MR, Stubbe J. J Am Chem Soc. 2007;129(8):2226–2227. doi: 10.1021/ja0685607. [DOI] [PubMed] [Google Scholar]
- 11.Cooperman BS. Biopolymers. 2003;71(2):117–131. doi: 10.1002/bip.10397. [DOI] [PubMed] [Google Scholar]
- 12.Hassan AQ, Wang Y, Plate L, Stubbe J. Submitted to Biochemistry. doi: 10.1021/bi8012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Climent I, Sjöberg BM, Huang CY. Biochemistry. 1992;31(20):4801–4807. doi: 10.1021/bi00135a009. [DOI] [PubMed] [Google Scholar]
- 14.Climent I, Sjöberg BM, Huang CY. Biochemistry. 1991;30(21):5164–5171. doi: 10.1021/bi00235a008. [DOI] [PubMed] [Google Scholar]
- 15.Seyedsayamdost MR, Chan CTY, Mugnaini V, Stubbe J, Bennati M. J Am Chem Soc. 2007;129:15748–15749. doi: 10.1021/ja076459b. [DOI] [PubMed] [Google Scholar]
- 16.Bennati M, Robblee JH, Mugnaini V, Stubbe J, Freed JH, Borbat P. J Am Chem Soc. 2005;127(43):15014–15015. doi: 10.1021/ja054991y. [DOI] [PubMed] [Google Scholar]
- 17.Uppsten M, Farnegardh M, Domkin V, Uhlin U. J Mol Biol. 2006;359(2):365–77. doi: 10.1016/j.jmb.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Wahlstrom JL, Randall MA, Lawson JD, Lyons DE, Siems WF, Crouch GJ, Barr R, Facemyer KC, Cremo CR. J Biol Chem. 2003;278(7):5123–5131. doi: 10.1074/jbc.M206963200. [DOI] [PubMed] [Google Scholar]
- 19.Lycksell PO, Sahlin M. FEBS Lett. 1995;368(3):441–444. doi: 10.1016/0014-5793(95)00706-f. [DOI] [PubMed] [Google Scholar]
- 20.Dorman G, Prestwich GD. Biochemistry. 1994;33(19):5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 21.Seyedsayamdost MR, Yee CS, Reece SY, Nocera DG, Stubbe J. J Am Chem Soc. 2006;128(5):1562–1568. doi: 10.1021/ja055927j. [DOI] [PubMed] [Google Scholar]
- 22.Deiters A, Schultz PG. Bioorg & Med Chem Lett. 2005;15(5):1521–1524. doi: 10.1016/j.bmcl.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 23.Seyedsayamdost MR, Xie J, Chan CTY, Schultz PG, Stubbe J. J Am Chem Soc. 2007;129:15060–15071. doi: 10.1021/ja076043y. [DOI] [PubMed] [Google Scholar]
- 24.Finn MG, Kolb HC, Fokin VV, Sharpless KB. Prog Chem. 2008;20:1–4. [Google Scholar]
- 25.Eriksson M, Uhlin U, Ramaswamy S, Ekberg M, Regnstrom K, Sjöberg BM, Eklund H. Structure. 1997;5(8):1077–1092. doi: 10.1016/s0969-2126(97)00259-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials, methods and Fig S1–S5 have been published as supporting information.