1. Introduction
The ability of some metal compounds to cause cancers in exposed workers has been known for a long time, with early documented cases dating back to the 19th century. Massive growth of manufacturing and other economic activities in the major industrialized countries has been accompanied by parallel increases in the large-scale consumption of nonferrous metals, some of which are now recognized human carcinogens. High-volume utilization and poor practices in the disposal of metal-containing waste products created numerous sources of heavy environmental contamination, including some of the largest toxic sites known as Superfund sites in the US. Toxic metals represent the ultimate form of persistent environmental pollutants because they are chemically and biologically indestructible. Despite well-recognized carcinogenic potentials of such toxic metals as chromium and nickel and metalloid arsenic, the molecular mechanisms underlying their cell-transforming ability remain poorly understood. Carcinogenic metals are typically weak mutagens and with the exception of chromium, they do not form DNA adducts which represent a key initiating event in the cancer-inducing activity of organic carcinogens. A long-held view that elevated production of reactive oxygen species is the main pathway in metal carcinogenicity is clearly at odds with data on weak or no mutagenicity of most metals. This review will summarize the most recent development in the field of metal carcinogenicity and cocarcinogenicity with special emphasis on the roles of activated signaling pathways, epigenetic changes and DNA repair processes.
2. Nickel
2.1. Human exposure and carcinogenicity
Nickel(II) is a toxic and carcinogenic metal (1). It is used in modern industry with other metals to form alloys to produce coins, jewelry, and stainless steel as well as for nickel plating and manufacturing Ni-Cd batteries. Among new applications it is important to note its role as a catalyst for production of carbon nanoparticles. This new technology increases consumption and respectively contamination with nickel compounds. Workers are exposed at different stages of processing of nickel-containing products. The most important route of human exposure to nickel is inhalation. This exposure has long been known to cause acute respiratory symptoms, ranging from mild irritation and inflammation of respiratory system to bronchitis, pulmonary fibrosis, asthma and pulmonary edema (2). Additionally, nickel exposure also may cause cardiovascular and kidney diseases, as well as allergic dermatitis. However, nickel carcinogenic activity represents the most serious concerns. Nickel(II) exerts its carcinogenic activity most likely through nongenotoxic mechanisms. The toxicity and carcinogenicity of Ni(II) depends on its intracellular dose that, in turn, is a function of physicochemical properties of particular nickel compounds, their ability to enter the cell and/or to dissolve within the cell. Because of a fast clearance from the exposed tissues, which limits cellular uptake, water-soluble Ni(II) compounds possess lower toxic and carcinogenic potential as compared to semi-soluble compounds such as nickel subsulfide (1). Possible mechanisms of nickel carcinogenesis have been discussed in a number of comprehensive reviews (1, 3–6).
Epidemiological studies have clearly implicated Ni(II) compounds as human carcinogens based upon an increased mortality from respiratory tract malignancies in refinery workers chronically exposed to nickel-containing dusts and fumes (7)(7, 8). Other health effects of inhalation exposure to soluble and insoluble nickel compounds are reported in a number of recent publications (2, 4, 8–12). Acute lung injury following nickel exposure was demonstrated in mice and rats (13–16). In various animal models, chronic exposure to nickel compounds induces tumors at virtually any site of administration (1, 3). Nickel compounds efficiently transform rodent and human cells in vitro (17–20). Based on these observations, the International Agency for Research on Cancer (IARC) evaluated the carcinogenicity of nickel in 1990 (21). All Ni(II) compounds were recognized as human carcinogens (Group 1) and metallic nickel is classified as possibly carcinogenic to humans (Group 2B) (21). The purpose of this review is to reevaluate existing hypotheses based on recently obtained data.
2.2. Genetic and epigenetic changes
Although various potentially mutagenic DNA lesions have been shown to occur following nickel exposure, the actual mutagenic activity of nickel compounds observed in most of the mutational systems examined thus far from Salmonella to mammalian cells in vitro has been low (22–25). Thus, it may be suggested that nickel-induced mutagenic activity is not the primary cause in nickel-induced carcinogenesis. Indeed, the experiments with the SHE system have provided confirmatory evidence that cell immortalization can occur as an indirect consequence of carcinogen exposure following an induced high frequency change in the treated population, rather than through direct targeted mutagenesis ((26). Additionally, no increase of ouabain-resistant or 6-thioguanine–resistant colonies has been found in human diploid fibroblasts even at concentrations of Ni3S2 that increased the frequency of anchorage-independence by 200-fold (17). These data implicate epigenetic changes as primary events in nickel carcinogenesis which may include changes in the histone acetylation, methylation or ubiquitylation levels, structural changes and/or alterations in DNA methylation as well as the activation or suppression of a number of transcription factors (27–33).
Changes in DNA methylation leading to the inactivation of gene expression following the exposure to nickel compounds were initially found using the transgenic E. coli gpt gene in Chinese hamster G12 cells as a model (29). Although the mechanisms by which nickel induces DNA hypermethylation in cultured cells are presently unknown, a possible model includes the ability of nickel to substitute for magnesium, increase chromatin condensation and trigger de novo DNA methylation (29). Changes in DNA methylation can also be observed in vivo in nickel-induced tumors. The injection of nickel sulfide into wild type C57BL/6 mice, as well as a mouse heterozygous for the tumor suppressor gene, p53 produced malignant histiocytomas in all mice (34). The hypermethylation of the promoter of the tumor suppressor gene p16 was observed in all tumors. Fhit is another tumor suppressor gene silenced by nickel exposure both in vitro and in vivo (35). Fhit is a tumor suppressor gene whose expression is frequently reduced or lost in tumors and pre-malignant lesions. A decrease of up to > 90% in FHIT protein levels was observed in 22 local sarcomas (mostly fibrosarcomas) induced by i.m. injection of nickel subsulfide in C57BL/6 and MT+ (C57BL/6 overexpressing metallothionein) mice, as compared with normal muscles. The lack of FHIT protein coincided with the absence of the Fhit-mRNA transcript in these tumors.
In addition to gene silencing by DNA methylation, the suppressive effects of nickel on histone H4 acetylation in vitro in both yeast and mammalian cells have been reported (30, 33). Acetylation of lysine 12 and 16 in yeast was more strongly affected than lysine 5 and 8 and it was proposed that nickel binding to histidine 18 in histone H4 may be responsible for this effect (36). The loss of histone acetylation and DNA methylation worked together in gpt gene silencing in G12 transgenic cell line by nickel (32, 37). In human lung cells exposed to soluble nickel compounds three major changes in histone modifications were observed: (i) loss of acetylation of H2A, H2B, H3 and H4; (ii) increases of H3K9 dimethylation; and (iii) substantial increases in the ubiquitylation of H2A and H2B (28, 30–33). The acetylation of the core histone N-terminal “tail” domains is recognized as a highly conserved mechanism for regulating chromatin functional states. Biochemical data supports a correlation between histone acetylation and gene activation, suggesting that histone acetylation acts to enhance the access of transcription-associated proteins to DNA. Conversely, histone methylation results in more compacted chromatin and gene silencing. If gene silencing mediated by histone modification plays a role in nickel-induced cell transformation, then the reactivation of these genes may reverse this effect. Indeed, recent experiments showed that the exposure of nickel-transformed cells to the histone deacetylase inhibitor trichostatin A (TSA) resulted in the appearance of significant number of revertants measured by their inability to grow in soft agar (38). Moreover, pretreatment of cells with TSA inhibited the ability of nickel to transform mouse PW, or human HOS cells to anchorage-independent growth. Low levels of histone acetylation in nickel-exposed cells may result from low levels of acetyl CoA, which is a universal donor of acetyl group. This may occur because the conversion of pyruvate into acetyl-CoA is blocked by the enzyme pyruvate dehydrogenase kinase (Fig. 1). Taken together these data suggest that epigenetic changes probably are more important for nickel-induced toxic and carcinogenic effects than mutational changes.
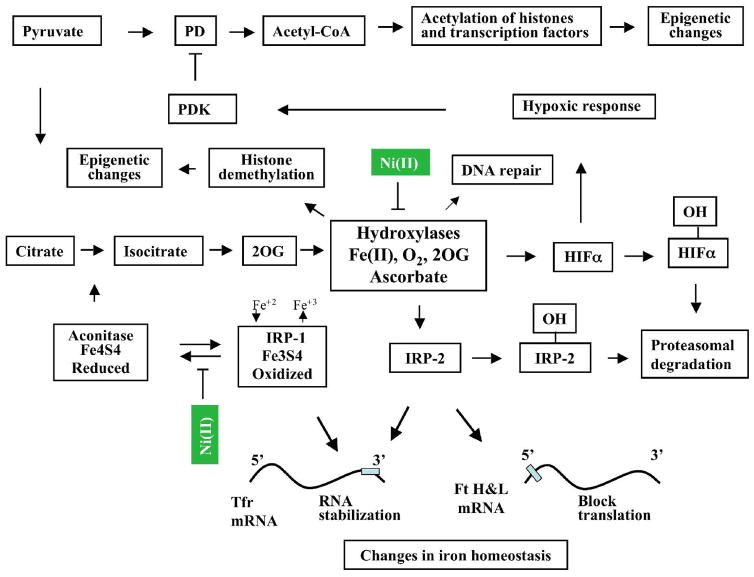
Figure 1.
Schematic representation of major cellular interactions of Ni(II) derived from water-insoluble or water-soluble nickel compounds. Primary intracellular effect of Ni(II) is iron oxidation in iron-containing hydroxylases and iron-sulfur clusters. This occurs in part due to the depletion of intracellular ascorbate, which is a major iron reductant in cells. Iron oxidation in iron-containing hydroxylases results in the inhibition of their activity. Inhibition of HIF-prolyl hydroxylases 1–3 and HIF-asparaginyl hydroxyalse leads to HIFαs accumulation and activation of hypoxia-inducible genes (hypoxic response). Pyruvate dehydrogenase kinase (PDK) is one of the hypoxia-inducible genes. It inhibits pyruvate dehydrogenase (PD), which is critical for the conversion of pyruvate to acetyl-CoA and maintaining of acetyl-CoA levels. Since acetyl-CoA is a donor of acetyl group the decrease in the levels of acetyl-CoA will affect acetylation of histones and other transcription factors. Inhibition of other hydroxylases should lead to the loss of IRP-2 hydroxylation and IRP-2 stabilization. This along with oxidation of iron in IRP-1 result in transferrin receptor (Tfr) RNA stabilization and blocks ferritin heavy and light chain (Ft H&L) translation. Since transferrin receptor and ferritin are two major proteins in iron metabolism, this will affect intracellular iron homeostasis. Additionally, oxidation of iron in IRP-1 results in inactivation of aconitase. This in turn should inhibit tricarboxylic acid cycle and affect levels of 2 oxoglutarate (2OG), a co-factor of hydroxylases. Recently discovered iron-containing, 2OG-dependent hydroxylases (enzymes of the JMJD2 family), which can remove methyl groups from H3-K9me3 and H3-K36me3 also could be inhibited by Ni(II). This will impair accessibility of DNA for transcription factors and, as the result, affect gene expression.
2.3. Activation of hypoxic signaling
Nickel is not an essential metal ion in mammalian cells. Therefore, no specific proteins involved in uptake, intracellular distribution or storage of nickel ions are known that may be commonly up-regulated by nickel exposure. Thus, no characteristic to nickel exposure pattern of gene expression was expected. Remarkably, nickel exposure produces rather specific pattern of gene expression, which is similar to the response to hypoxia (39–41). The mechanism involves the inhibition of the HIF prolyl- and asparaginyl-hydroxylases, the stabilization of the HIFα proteins and stimulation of HIF-1-dependent transcription (39). The HIF-1 transcription factor is a HIF-1α/HIF-1β (ARNT) dimer, which is formed in response to low oxygen tension in the cells. The α subunit is the regulatory component of the HIF-1 complex and is unique to the hypoxic response. Under normoxic conditions this protein is virtually undetectable in most cells, but can accumulate following exposure to proteasomal inhibitors, such as lactacystin or MG-132 (42). Accumulation of the HIFα subunit in the presence of hypoxia or nickel implies that proteasomal degradation of the protein is impaired by these exposures.
Hydroxylation of proline residues 402 and 564 in the presence of oxygen in the oxygen-dependent degradation domain (ODD) of HIF-1α leads to its interaction with the von Hippel Lindau (pVHL) tumor suppressor protein, a part of the ubiquitin-ligase complex (43–45). This results in ubiquitylation and rapid proteosomal degradation of HIF-1α. The structural basis for specific interaction of HIFα and VHL is provided by the introduction of hydroxyl group at the proline C4-position. The oxygen of this hydroxyl group facilitates hydrogen bonding with VHL’s Ser-111 and His-115 residues. This is sufficient to enable discrimination between non-hydroxylated and hydroxylated HIF-1α protein (44–48). In addition to proline hydroxylation, asparagine 803 hydroxylation, which allows for complex formation between HIF-1α and the CBP and p300 transcriptional co-activators, regulates transactivation of HIF-dependent genes (49, 50).
Nickel was found to be a strong inducer of HIF-1α protein and activator of HIF-dependent transcription (39, 51, 52). Since Ni(II) is similar to Fe(II), it was suggested that Ni(II) could change iron metabolism and either replace Fe(II) in the hydroxylases or interfere with Fe(II) uptake (53, 54). These data were mainly supported by the finding that ferritin disappeared and IRP1 was activated in nickel-exposed cells (55–57). However, the oxidation of iron in iron-sulfur clusters could produce the same result (Fig. 1). Modest changes in intracellular iron levels in nickel-exposed cells support this notion (55). Alternatively, it was suggested that reactive oxygen species (ROS) may play an important role in HIF-1α stabilization and metals, which are known to generate free radicals in cells, may stabilize HIF-1α through this mechanism (58). However, some data indicate that Ni(II) and perhaps other metals do not require free ROS generation for HIF-1α stabilization (59, 60). Recently, we showed that the depletion of intracellular ascorbate by Ni(II) may lead to the inhibition of prolyl hydroxylases (39, 61). These enzymes are members of the Fe(II)-, 2-oxoglutarate (2OG)-, and ascorbate-dependent family of dioxygenases. Ascorbate plays an important role in the reduction of enzyme-bound iron, which is vital for maintaining hydroxylases activity. The active role of ascorbate in the hydroxylase reactions may explain the controversy about the role of ROS in HIF-1 activation and reconcile previously obtained data. Indeed, ROS may deplete through oxidation a variety of reducing molecules. However, since only ascorbate is capable of maintaining iron in the reduced state its presence is critical for hydroxylase activity. Ni(II) can deplete intracellular ascorbate via oxidation and/or by inhibiting ascorbate uptake by cells (52, 61–63). This results in the inactivation of the hydroxylases and produces a phenotype observed in hypoxic cells or in cells with mutated VHL (61, 63). Thus, exposure of cells to Ni(II) most likely results in oxidation of intracellular iron followed by the induction of HIF-1 and activation expression of hypoxia-inducible genes (39).
The activation of hypoxic signaling pathway and switch of cellular metabolism to a state that mimics permanent hypoxia may be a part of nickel-induced carcinogenesis (39, 64, 65). Hypoxia is common in tumors because tumor body is growing faster than the blood vessels growing into it. It may promote tumor progression via activation of genes coding for proteins enabling cells to overcome nutritive deprivation and to escape from the hostile metabolic microenvironment (66). Stimulation of angiogenesis through up-regulation of VEGF and other growth factors involved in building new blood vessels is also important part of survival program. Additionally, cellular responses to hypoxic stress include inhibition of cell proliferation and, when cell damage is irreversible, apoptosis. Therefore, imitation of the state of hypoxia by nickel may provide the conditions for the selection of cells that have altered energy metabolism, changed growth control or/and become resistant to apoptosis. The activation of HIF-1 transcription factor and modification of histones may represent a molecular basis for cellular adaptation in growing tumor. The selection theory seems to explain low mutagenic, but high transforming activity of nickel compounds (26). However, one may suggest that for successful cell transformation an additional mutagenic (DNA damage) event is required.
2.4. Ni(II) as a cocarcinogen
Nickel compounds were shown to act synergistically with many mutagenic carcinogens in enhancing cell transformation both in vitro and in vivo (67–69). Single i.p. injection of nickel(II) acetate followed by subsequent dosing with sodium barbital in drinking water produced renal cortical adenocarcinomas, some of them metastatic to lung, liver, and spleen (70). These tumors occurred only in co-exposed rats. Growing body of evidence pointed out that nickel enhances the cytotoxicity and genotoxicity of DNA-damaging agents through inhibition of DNA repair. Thus, exposure to particulate black NiO and soluble NiCl2 affected removal of DNA adducts formed by benzo[a]pyrene in human lung cells (69, 71). Nickel inhibits repair of O6-methylguanine and N7-methylguanine induced by treatment with N-methyl-N-nitrosourea in Chinese hamster ovary cells (72). Nickel blocks the removal of cyclobutane pyrimidine dimers produced by UV light exposure in HeLa cells (73).
More recently, a new class of DNA repair enzymes has been found. It includes alkyl DNA dioxygenases, such as the Escherichia coli AlkB dioxygenase and its two human homologues, ABH2 and ABH3, which facilitate a novel mechanism of DNA repair (74). In the presence of oxygen these enzymes can specifically hydroxylate alkyl groups on 1-methyladenine and 3-methylcytosine (75). The requirement of iron for the reaction as well as inhibition of hydroxylase activity in crude cell extracts by iron chelators suggested that these enzymes are iron- and ascorbate-dependent. The activity of these enzymes, similarly to protein hydroxylases depends on iron oxidation status and may be inhibited by nickel exposure. These results indicate that the nucleotide and base excision repair pathway is affected by water-soluble and particulate nickel compounds and provide further evidence that DNA repair inhibition may be one of the mechanisms involved in nickel cocarcinogenic activity.
In addition to inhibition of DNA repair, epigenetic changes induced by nickel compounds should be taken into consideration. The induction of cytosine methylation and histone deacetylation may lead to the inherited inactivation of expression of senescence/tumor suppressor gene(s) and additionally contribute to the carcinogenic mechanism (29, 30). It is noteworthy to emphasize that in animals nickel-induced carcinogenesis is known to be tissue, strain and species-dependent (1). This suggests that genetic predispositions, including variations in the expression of genes involved in the metabolism of antioxidants, most likely glutathione and vitamin C, in different species and strains of animals, may also play an important role in nickel carcinogenesis (76). It is conceivable that similar genetic predispositions take place in human populations.
In conclusion, carcinogenic nickel produces significant alterations in cellular metabolism which include, but not limited by stimulation of glycolytic activity, alteration of iron homeostasis, depletion of ascorbate, and hypoxic stress. These effects of nickel exposure are summarized in Fig. 1. It is clear that such metabolic alterations will lead to the modulation of gene expression (epigenetic changes). A co-exposure with genotoxic carcinogens may exacerbate nickel effects.
3. Arsenic
3.1. Human exposure and carcinogenicity
Arsenic is an environmental contaminant, which can be found in the soil, water and airborne particles as the result of both natural and human activities (77, 78). Epidemiological studies have confirmed that exposure to arsenic and its compounds can have adverse effects on human health. Inhaling arsenic can cause lung carcinomas, while ingestion in food or water, can provoke skin, respiratory system, liver and bladder tumors, as well as diabetes, cardiovascular and neurological diseases (77). Humans are clearly more sensitive to inorganic arsenic carcinogenesis than animals. In rodents it had proven difficult to induce tumors after inorganic arsenic exposure as a single agent, making it problematic, in the absence of animal model, to study mechanisms of arsenic carcinogenesis (79). Exposure to arsenic occurs generally in the form of either arsenite (AsIII) or arsenate (AsV). The increase in cancer risk observed in epidemiological studies is attributed mainly to the exposure to inorganic arsenite, which is more toxic than arsenate (80, 81). This may be due to a better cellular uptake of arsenite, which, at equimolar concentration, is accumulated in many cell types much faster as compared with arsenate. Inside the cell arsenate may be reduced to arsenite. Glutathione seems to play an important role in arsenate reduction and detoxification through conjugation (82) (Figure 2). After ingestion arsenic is rapidly excreted, primarily through the urine mainly in the form methylated arsenic metabolites (83). These methylated metabolites are produced in vivo by conjugation using S-adenosylmethionine as the methyl donor (82, 84). The methyltransferases involved in the process are not yet characterized. Methylated arsenic metabolites include pentavalent or trivalent monomethylated (MMA) and dimethylated (DMA). The levels of S-adenosylmethionine are important in arsenic metabolism since a low intake of dietary methyl groups, i.e. low dietary content of methionine or folate deficiency, results in lower arsenic methylation (85). Since methylated species are excreted much faster than inorganic arsenic species, it is conceivable that methylation represents a part of detoxification program.
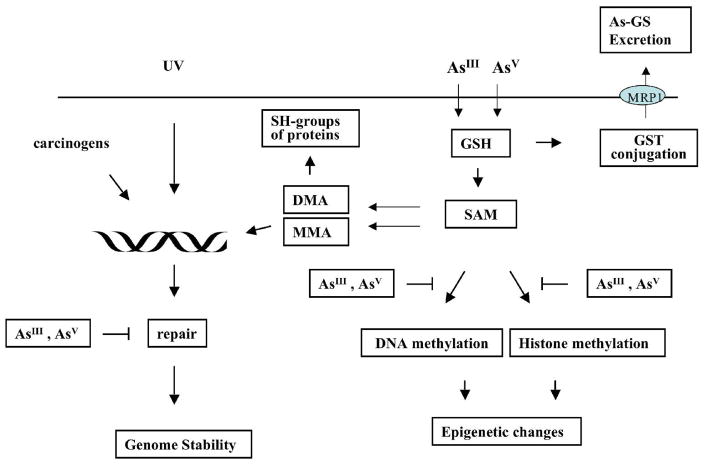
Figure 2.
Schematic representation of major cellular interactions of tri- and pentavalent arsenic. Both forms, tri- or pentavalent arsenic can enter cells. Inside the cells, pentavalent arsenate can be reduced by glutathione to trivalent arsenite and/or GSH-As can be methylated to pentavalent or trivalent monomethylated (MMA) or dimethylated (DMA) arsenic. DMAIII and MMAIII could react with sulfhydryl groups of some proteins. This will result in modification of protein function and retention of arsenic inside the cell. Arsenic methylation can deplete S-adenosylmethionine, which serves as a universal donor of methyl group. As the result, DNA or histone methylation pattern may be changed. GSH-As complexes can be excreted from cells by the MRP1 transporter. MMA or DMA along with other carcinogens or with UV radiation can induce DNA damage. The repair of this damage may also be inhibited by arsenic.
Pentavalent arsenicals were not shown to be a carcinogenic risk to humans at typical environmental exposures. However, dimethylarsinic acid (DMAV) is carcinogenic at high doses to the rat urinary bladder, but not in mice (86). The carcinogenic mode of action involves cytotoxicity followed by regenerative cell proliferation. The cytotoxicity is due to formation of a reactive metabolite, most likely dimethylarsinous acid (DMAIII), which causes oxidative damage and/or binds with critical urothelial sulfhydryl groups (86). Thus, these data indicate that methylated forms of arsenic may be potentially more dangerous, than non-methylated ones. This, however, will require further investigations. It is noteworthy, that arsenicals administered to the rat may also bind to a specific cysteine in the hemoglobin alpha chain as DMA(III), regardless of the arsenical being administered. Hemoglobin binding is responsible for the greater accumulation of arsenic species in rat blood than in human or mouse blood. The significance of this modification is not clear, however, it is conceivable that this may be the reason that the rat being one of the few arsenic carcinogenic animal models available at present.
3.2. Increased cellular proliferation
In cell and animal models arsenite and perhaps some methylated metabolites may activate signal transduction pathways which enhance cell proliferation, reduce antiproliferative signaling or inhibit differentiation, and override checkpoints controlling cell division after genotoxic insult (87, 88). In animals treated with arsenite, hyperplasia is seen in the urinary bladder epithelium and in skin (89, 90). In human keratinocytes increased mRNA transcripts and secretion of keratinocyte growth factors, including granulocyte macrophage-colony stimulating factor (GM-CSF) and transforming growth factor-α (TGFα) were observed (91). In a human uroepithelial cell line arsenic activates EGFR and ERK in ligand-independent manner, which does not involve autophosphorylation Tyr(1173) (92). c-Src activity is also induced by arsenic and is a prerequisite for the EGFR and ERK activation. Activation of growth factor receptors regulates G1 phase cyclins and associated cyclin-dependent kinases (cdks). Cyclin D1 is one of cyclins up-regulated by arsenite exposure (93, 94). Cyclin D1 has a very short half life and protein level depends on protein phosphorylation by a number of kinases including extracellular signal-related kinases (ERKs), phosphotidylinositol 3-kinase (PI3K) and IKK. Initially, in human fibroblasts it was found that long-term and low dose (but not short-term, high dose) exposure to arsenite resulted in increased expression of cyclin D1 (93). However, it was later shown that even 12 hrs exposure to low dose arsenite caused cyclin D1 up-regulation in human keratinocytes (94). Low concentrations of arsenite also disrupt p53 function, which results in down-regulation of p21 in response to genotoxic stress (93). Murine fibroblasts chronically exposed to low concentrations of arsenite show increased proliferative response to epidermal growth factor (EGF) and increased expression of c-myc and E2F-1 (positive growth regulators) (95). Thus, exposure to arsenite results in the stimulation of cell cycle progression, especially G1-S transition. Down-regulation of p53 function suggests that in the presence of arsenic cells may enter cell cycle with unrepaired DNA lesions.
3.3. Apoptotic effects of arsenite exposure and NF-kB signaling pathway
Arsenic trioxide is an effective treatment for acute promyelocytic leukemia (APL) (96). The APL patients resistant to all-trans retinoic acid and other types of chemotherapy can still respond to arsenic trioxide. In vitro studies showed that at micromolar concentration arsenic trioxide triggers APL cell apoptosis. Lower doses of arsenic were shown to induce differentiation of the leukemic cells. Both apoptosis and cell differentiation are important factors of arsenic therapeutic effect. Another type of malignancy which is often resistant to chemotherapy is an adult T-cell leukemia/lymphoma (ATL). Recent data showed that combined exposure of ATL cells to arsenic and IFN-α has dramatic synergistic effects on both cell cycle arrest and induction of apoptosis in these cells (97). The apoptotic effect of arsenic was caused by an up-regulation of IkB-α, resulting in a sharp decrease in DNA binding of NF-kB complexes and suppression of NF-kB target genes due to the cytoplasmic retention of RelA (97). Similarly to the effect on lymphocytes sodium arsenite downregulates NF-kB activity by inhibiting phosphorylation and subsequent degradation of IkB-α in Caco-2 cells (98). The stabilization of IkB-α by sodium arsenite did not require reactive oxygen species formation (98). Thus, therapeutic effect of arsenic is mediated through down-regulation of an important antiapoptotic transcription factor NF-kB. The effect of arsenite on NF-kB was extensively studied in vitro. The experimental findings, however, are contradictory. In some instances, the exposure to arsenite up-regulated NF-kB (99–101). In others, the exposure to arsenite down-regulated NF-kB (102, 103). This controversy could be explained by the use of different cell models as well as by different time and doses of treatment. Thus, low dose and short-term of treatment with sodium arsenite could activate NF-kB DNA binding, whereas chronic exposure to 0.1 or 0.5 μM As(III) decreased NF-kB DNA binding activity (104).
3.4. Genetic and epigenetic changes
Arsenic is known to induce deletion mutations and chromosomal alterations such as aberrations, aneuploidy, and sister-chromatid exchanges, but not point mutations (105). In spite of its low mutagenic activity, arsenic has high transforming activity (106). Among genetic changes it is also important to note that arsenic exposure can cause gene amplification. Thus, two arsenic salts, sodium arsenite and sodium arsenate, were shown to induce a high frequency of methotrexate-resistant 3T6 cells (107). The resistance was due to the amplification of the dihydrofolate reductase gene. The ability of arsenic to induce gene amplification may relate to its carcinogenic effects in humans since amplification of oncogenes is observed in many human tumors. Large body of evidence has been accumulated over the last ten years that epigenetic changes are important in arsenic carcinogenesis. Thus, exposure to arsenic can induce both DNA hypomethylation and hypermethylation. DNA methylation changes are typically observed in cancer, in which global methylation is reduced, but some gene-specific promoter methylation is increased (108). The exposure of human lung adenocarcinoma A549 cells to arsenite results in the increased cytosine methylation in the p53 promoter (109). Both hypo- and hypermethylation of different genes was found in human kidney cells treated with arsenite in vitro (110). Global DNA hypomethylation and evidence of depressed levels of S-adenosyl-methionine and decreased DNA methyltransferase activity were found in rat liver epithelial cell line following chronic exposure to low levels of arsenic (111). Chronic oral exposure of A/J mice to inorganic arsenate which results in appearance of lung tumors causes decrease or loss of p16INK4a and RASSF1A expression in these tumors as compared to that in non-tumor lung tissues from both control and inorganic AsV-exposed mice (112). This reduced or lost expression was the result of hypermethylation of these genes. Significant DNA hypermethylation of promoter region of p53 gene was observed in DNA of arsenic-exposed people compared to control subjects (113). This hypermethylation showed a dose-response relationship. Further, hypermethylation of p53 gene was also observed in arsenic-induced skin cancer patients compared to subjects having skin cancer unrelated to arsenic, though not at significant level. However, a small subgroup of cases showed hypomethylation with high arsenic exposure. Significant hypermethylation of gene p16 was also observed in cases of arsenicosis exposed to high level of arsenic. Thus, the ability of arsenic to alter DNA methylation in humans may be important in carcinogenesis. Unlike nickel, exposure to arsenite does not cause methylation and silencing of the transgenic E. coli gpt gene in Chinese hamster G12 cells, which indicates that these two carcinogenic metals are acting through different pathways (29).
3.5. Metabolic changes
Arsenite was shown to inhibit pyruvate dehydrogenase (PD) activity through binding to vicinal dithiols in pure enzyme and tissue extract. However, more recently it was shown that arsenite by producing ROS may cause PD oxidation which inactivates an enzyme (114). This can occur at a much lower concentration than that needed for direct binding of arsenite to the critical thiols. The ROS produced by arsenite may up-regulate HIF-1 and downstream VEGF in normoxic H134 and OVCAR-3 cells (115). Pretreatment with the ROS inhibitors catalase and mannitol attenuated arsenite-induced ROS production, but did not affect induction of VEGF mRNA and HIF-1α protein. In contrast, pretreatment with the thiol antioxidants glutathione or N-acetylcysteine completely abrogated both effects, whereas a potentiation was observed by depletion of intracellular glutathione. Further studies, however, did not confirm transcriptional up-regulation of hypoxic genes. Thus, sodium arsenite did not activate a HIF-1-dependent reporter gene in OVCAR-3 cells, indicating that functional HIF-1 was not induced. In agreement with this hypothesis, up-regulation of VEGF mRNA was not reduced in HIF-1alpha (−/−) mouse fibroblast cell lines. Altogether, these data suggest that not HIF-1, but rather p38, mediates induction of VEGF mRNA expression by sodium arsenite (116).
Arsenic reduction and methylation is aimed to detoxify and excrete obtained products from cells. Indeed in human urine, the major metabolites of inorganic arsenicals such as arsenite and arsenate are MMAV and DMAV. However, in rat bile, the major metabolites of inorganic AsIII have been reported to be arsenic–glutathione (As-GSH) complexes. As-GSH complexes can be transported from cells via multidrug resistance-associated protein (MRP) family (117, 118) (Figure 2). Consistent with this notion, C57BL/6 mice, which have higher expression level of MRP1, but not other ABC transporters, were shown to be more resistant than BALB/c mice to sodium arsenic induced renal injury (119). Complex relationship between glutathionylation and methylation of arsenic was investigated recently. It was suggested that As-GSH complexes are substrates for arsenic methyltransferase, therefore all observed products are components of arsenic metabolic pathway (82, 120).
3.6. Arsenic as a cocarcinogen
At low non-mutagenic concentrations arsenite can enhance the mutagenicity of other carcinogens, probably by interfering with DNA repair (79). Arsenite enhances the mutagenicity and/or clastogenicity of UV, N-methyl-N-nitrosourea (MNU), diepoxybutane, X-rays, and methylmethane sulfonate in mammalian cells (121–124). Arsenic inhibits repair of DNA adducts caused by benzopyrene in rats (125). In mice arsenite can enhance the carcinogenicity of ultraviolet radiation (UV) (126, 127). Combined exposure of 10 mg/L sodium arsenite in drinking water with 1.7 KJ/m(2) solar UVR 3 times weekly after 26 weeks resulted in a 2.4-fold increase in skin tumor yield compared with mice given UV alone. No tumors appeared in any organs in control mice or in mice given arsenite alone. The molecular mechanism for tumor formation involves reduction in the repair rate of photoadducts and inhibition of apoptosis (128, 129).
Recently, it has been shown that a short period of maternal exposure to inorganic arsenic in the drinking water results in multi-tissue carcinogenesis in the adult offspring (130). For example, prenatally exposed female C3H offspring showed dose-related increases in ovarian tumors and lung carcinoma and in proliferative lesions (tumors plus preneoplastic hyperplasia) of the uterus and oviduct. In addition, prenatal arsenic plus postnatal exposure to the tumor promoter, 12-O-tetradecanoyl phorbol-13-acetate (TPA) in C3H mice produced excess lung tumors in both sexes and liver tumors in females. In CD1 mice additional postnatal treatment with diethylstilbestrol or tamoxifen after prenatal arsenic exposure induces urinary bladder transitional cell proliferative lesions, including carcinoma and papilloma, and enhances the carcinogenic response in the liver of both sexes. These data provide convincing evidence that arsenic is a transplacental carcinogen and cocarcinogen in mice with the ability to target tissues of potential human relevance, such as the urinary bladder, lung and liver.
In conclusion, arsenic is acting as a nongenotoxic carcinogen mainly via alterations in DNA methylation (Fig. 2). It cannot be excluded that arsenic may also cause alterations in histone methylation, although this has not been shown yet. Because of its inhibitory effects on DNA repair, arsenic acts as a very efficient cocarcinogen.
4. Chromium
4.1. Human exposure and carcinogenicity
Cr and its compounds have a long history of industrial uses in manufacturing of a large number of high-volume products, such as stainless steel and pressure-treated wood. Occupational exposure to Cr is found among about half a million industrial workers in the US and several million worldwide (131, 132). Environmental exposure likely impacts dozens of million people drinking Cr-containing water, residing in the vicinity of numerous toxic sites and chemical manufactures and other industrial users. One example of widespread exposure is the presence of significant contamination with hexavalent Cr in approximately 30% of the drinking water sources in California1. The presence of Cr in urban particulate matter and emissions from automobile catalytic converters leads to exposure by very large segments of populations in densely populated areas.
Although Cr can exist in several valence states, the most commonly encountered products contain this metal in the +6, +3 and 0 oxidative forms (133). Cr(0) is usually present in its metallic form, which typically occurs in the alloys with other metals, particularly Fe and Co. Welding and other strongly oxidizing conditions convert Cr(0) to Cr(III) and Cr(VI). Cr(III) is thermodynamically stable and it is the final oxidative form found in all biological systems. Depending on the nature of the counter ion, the solubility of Cr(VI) compounds various from very high (salts with alkali metals) to moderate (salts of Ca, Mg, Sr, Zn) to very low (barium and lead salts). The highest exposure to Cr(VI) occurs in chromate manufacturing, chrome plating, ferrochrome production and stainless steel welding. Welders employed in construction and small car repair shops are at particular risk of heavy exposure because of the absence or practical difficulties in the installation of exhaust systems removing Cr(VI)-containing fumes from the breathing area.
Occupational exposure to Cr(VI) compounds, but not other oxidative forms of Cr, is a well-documented cause of respiratory cancers (21, 134, 135). Cr(VI)-associated neoplasms are typically located in the lung, but risk of nasal cancers is also significantly increased (136–138). Contrary to some very optimistic views that Cr(VI) carcinogenesis is caused only by massive exposures and therefore, it is no longer a concern (139), recent epidemiological and risk assessment studies have actually found as much as 25% lifetime risk of dying of lung cancer under 52 μg/m3 permissible exposure limit (135, 140). This standard originally adapted by OSHA in 1971 was lowered 10-fold to 5 μg/m3 in 2006 (132), but even the new standard is expected to result in additional 10–45 deaths per 1000 exposed workers. In parallel with epidemiological findings, recent studies in cells cultured under more biologically relevant conditions found a much greater potential for Cr(VI) to cause chromosomal damage and mutations (141) than it was previously thought.
Although a frequently referenced review by IARC in 1990 (21) found stronger evidence for carcinogenicity of less soluble chromates, specifically for salts with moderate solubility, the follow-up epidemiological studies clearly showed that human exposure to soluble chromates also significantly increased risk of lung cancer (142, 143). The ability of all types of cells to take up, metabolize and form genotoxic Cr-DNA damage from Cr(VI) (section 4.2) lends major mechanistic support for the view that Cr(VI) can induce malignancies at sites outside the respiratory system (144, 145). A recently released draft of the NTP report2 on testing of dichromate in drinking water contains clear evidence of its carcinogenicity in the oral cavity and small intestine. While NTP studies have not found evidence for systemic cancers following ingestion of dichromate, exposed animals showed clear signs of toxicity in the liver and other internal organs, indicating the ability of toxic Cr(VI) to avoid detoxification in the GI tract and enter systemic circulation. Sustained elevation of Cr levels in red blood cells of human volunteers following ingestion of Cr(VI)-laced water (146) is also consistent with absorption of significant amounts of Cr(VI) into the blood (131).
Insoluble salts of lead and barium chromates were negative in the implantation model of lung carcinogenesis (147), and epidemiological data for their carcinogenicity are also not as strong as for other chromates (134). However, considering that soluble chromates were also negative in the implantation model but are now confirmed as carcinogenic, it is prudent to consider all chromates as equally carcinogenic.
Squamous cell carcinoma is the most common form of lung malignancies in chromate workers and the majority of tumors were located in the central part of the lung (148). Interestingly, Cr(VI) exposure was associated with the development of multiple tumors in several subjects, pointing to the potential existence of individual susceptibility factors in Cr-induced carcinogenesis. Squamous cell carcinoma appears to develop from dysplastic lesions over a relatively short period of time of about one year (149). The location of lung tumors corresponds to the sites of Cr accumulation, with bronchial bifurcations exhibiting the highest Cr levels in ex-workers who had been exposed to soluble chromates (150). Long-term retention of Cr in the bronchial tissue primarily occurred in the stroma (151), probably reflecting a faster clearance of Cr from the more rapidly renewable bronchial epithelia. Sedimentation and inertial impaction of typical chromate particles with 1–3 μm in diameter were likely the main reasons for the high deposition of Cr in the areas of bifurcations. Suppressed mucus flow delaying effective clearance of chromate particles could be another contributing factor, since chronic exposure to respiratory toxicants causes loss of cilia near bifurcations (150). Lung deposition profiles of Cr from particles of lower solubility chromates are currently unknown but they are expected to show the same pattern of ‘hotspots”. Although the majority of lung cancers were found among chromate workers who smoked (135, 148), this may simply reflect a fact that the majority of workers were smokers. Smoking does not affect Cr accumulation in the lung (148) and chromate exposure was clearly established as an independent risk factor for lung cancer (135). Molecular features of chromate and smoking-associated cancers are very different (section 4.4), arguing for the smoking-unrelated origin of the majority of chromate malignancies.
4.2. Cr(VI) metabolism and DNA damage
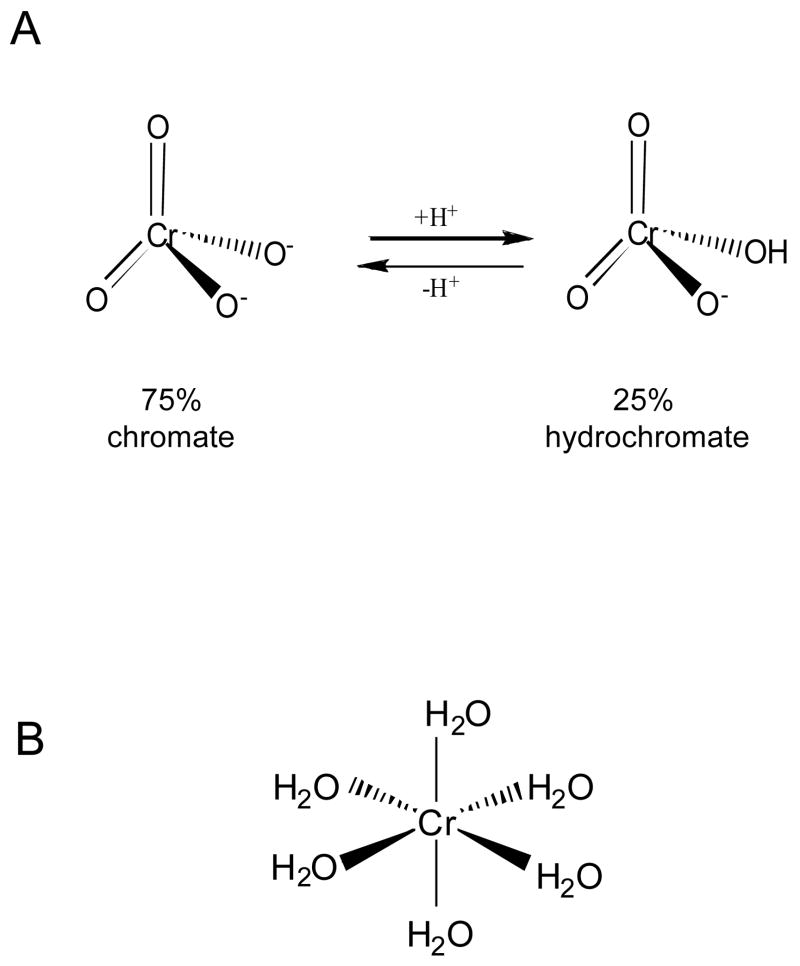
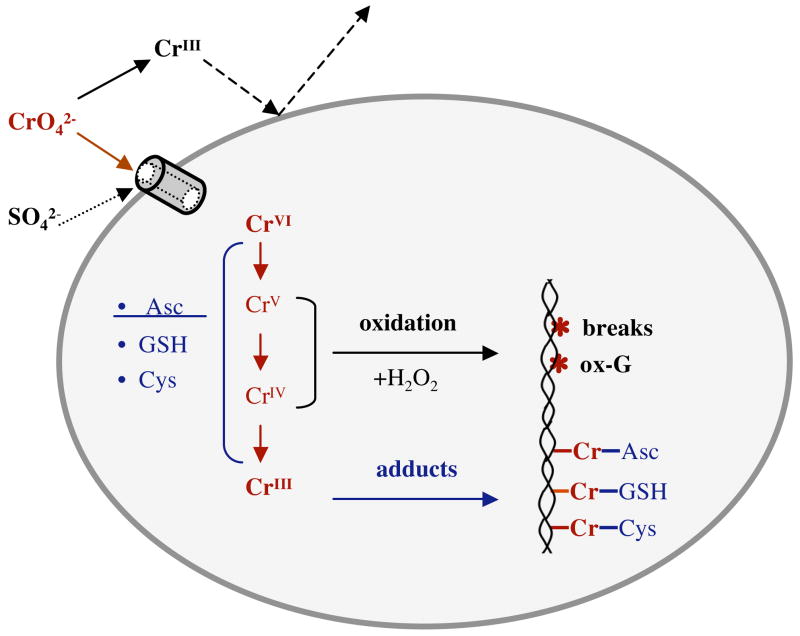
At neutral pH Cr(VI) exists as a mixture of chromate (CrO42−) or hydrochromate (HCrO4−) anions with the approximate ratio of 3:1 (133) (Fig. 3). Chromates are isostructural with physiological sulfate and phosphate ions and because of this molecular mimicry, Cr(VI) readily enters cells through the sulfate channels (152). Human and other mammalian cells are capable of massive accumulation of Cr(VI), with cellular levels 10–20 times above those outside the cell already within 3 hrs (141, 153). A 24-hr long incubation can lead to 100-fold or higher accumulations (154). Cr(VI) is a pro-carcinogen that by itself is completely unreactive toward DNA under physiological pH and temperature. In the biological systems, however, Cr(VI) undergoes a series of reduction reactions yielding thermodynamically stable Cr(III). When this occurs extracellularly, reduction acts as the detoxification process due to the production of poorly permeable Cr(III) complexes (Fig. 4). Inside the cell, Cr(VI) reduction is the activation event that is responsible for the generation of genotoxic damage and other forms of toxicity. Unlike the majority of other human pro-carcinogen, Cr(VI) metabolism in mammalian cells does not require any enzymes and relies on the direct electron transfer from nonprotein thiols, such as glutathione and cysteine, and ascorbate (133). Studies of chromate reduction in tissue homogenates (155–157) and measurements of reduction rates in defined reaction mixtures (158, 159) all showed that ascorbate was the dominant biological reducer of Cr(VI) accounting for about 90% of its metabolism in cells in vivo. In contrast to millimolar levels of ascorbate in cells in vivo (for example, 1.3 mM in human lung, Ref. 160), cultured human and nonhepatic cells from other species contained at best about 50–60 μM but usually much lower ascorbate concentrations (61, 141, 153, 159) and they rely on thiols for Cr(VI) reduction (133). Thus, unless cellular ascorbate levels are restored to normal, typical cell cultures provide a nonphysiological model of Cr(VI) metabolism, which has recently been found to underestimate genotoxic and mutagenic abilities of Cr(VI) (141). Even delivery of as low as 80 μM ascorbate into A549 cells was found to have a strong effect on the yield of intermediate reaction products (161).
Figure 3.
Structures of tetrahedral Cr(VI) and octahedral Cr(III) complexes. A – Chromate (CrO42−) and hydrochromate (HCrO4−) are the main aqueous forms of Cr(VI) at neutral pH. B – Octahedral arrangement of H2O groups in hexacoordinate complexes of Cr(III). At neutral pH Cr(H2O)63+ complex undergoes rapid hydrolysis producing a mixture of mononuclear and polynuclear species containing hydroxo ligands (133).
Figure 4.
Major steps in uptake, metabolism and formation of DNA damage by Cr(VI).
The end-product of Cr(VI) metabolism in all biological systems is always Cr(III) (162–164), but the reduction process can also generate variable amounts of Cr(V), Cr(IV) and organic radicals depending on the reducer and the ratio of reactants (165–167). Under conditions of ≥2-fold molar excess of the reducer, reactions of Cr(VI) with ascorbate generate Cr(IV) as the first Cr intermediate (166–168). The presence of Cr(V) was only detectable when high concentrations of the reactants were used and ascorbate was present at non-physiological 1:1 or lower ratio to Cr(VI). The formation of Cr(V) in the mixtures containing ascorbate concentrations that were insufficient to complete Cr(VI) reduction probably resulted from the secondary reactions of Cr(IV) (CrVI + CrIV → 2CrV and 2CrIV → CrV + CrIII). While the presence of small amounts of short-lived Cr(V) at higher than 2-fold ratio of ascorbate to Cr(VI) cannot be excluded due to the technical limitations of the employed spin resonance spectroscopy approaches, it is doubtful that environmental levels of Cr(VI) will be sufficient to produce significant quantities of Cr(V) in cells with millimolar ascorbate concentrations. The first step in reduction of Cr(VI) by physiological concentrations of cysteine proceeds primarily through one electron transfer (169), which explains a strong signal for Cr(V) in Cr(VI)-cysteine reactions (170). The initial electron transfer reaction in the glutathione-driven reductions is predominanatly a two-electron process (171) but the production of Cr(V)-glutathione species is also readily detectable (165, 170, 172). The final product of Cr(VI) metabolism, Cr(III), forms stable coordination complexes with nucleic acids and proteins. In vitro reduction reactions also generate large amounts of Cr(III) complexes containing two molecules of the unoxidized reducer (173, 174). Studies in Cr(VI)-treated cells and in vitro reduction reactions produced evidence for the formation of several types of DNA damage, including strand breaks and various Cr-DNA adducts.
Cr-DNA adducts
Small Cr-DNA adducts are the most abundant form of Cr(VI)-induced genetic lesions in mammalian cells (133) and they were found to be responsible for all mutagenic damage generated during Cr(VI) reduction with cysteine (175) and ascorbate (158). The majority (50–75%) of adducts generated during in vitro Cr(VI) reductions are binary Cr-DNA complexes (159, 176). Binary adducts are only weakly mutagenic (158, 177) and their presence in cells is uncertain due to the presence of numerous Cr(III)-binding small molecules. Ternary Cr-DNA adducts can be disrupted during DNA isolation (159), which produces binary adducts and further complicates the assessment of the real levels of these small adducts. The predominant form of Cr-DNA complexes in cells are ternary adducts (crosslinks) which include Cr(III) atom bridging DNA and small cellular molecule (L-Cr-DNA). Four major forms of ternary adducts are glutathione-Cr-DNA, cysteine-Cr-DNA, histidine-Cr-DNA and ascorbate-Cr-DNA complexes (159, 178, 179). All ternary adducts were much more mutagenic than binary adducts and ascorbate-Cr-DNA crosslinks were the most potent pre-mutagenic Cr-DNA modifications (158, 177). Ternary adducts are formed through attack of DNA by preformed ligand-Cr(III) complexes (180). Binary adducts can also be generated in the direct reaction of newly formed Cr(III) with DNA (175, 176, 180) but the possibility that a fraction of binary adducts results from the reaction of intermediate Cr forms, particularly Cr(IV), cannot be excluded. Cr(V) complexes exhibit little or no direct binding to DNA (181, 182) and the presence of Cr(V) in Cr(VI) reduction reactions is not required for the formation of Cr-DNA adducts in vitro (158, 159) or in cells (141, 153). The primary site of attachment for all Cr(III) adducts is the phosphate group (180, 183), but induction of G/C-targeted mutagenic events by Cr-DNA modifications (175, 177) has also led to the suggestion that the mutagenic forms of adducts are probably Cr(III) microchelates involving phosphate group and N7 position of G (175). Thus, there are apparently two classes of Cr-DNA phosphate adducts: the majority (about 90%) are nonmutagenic monofunctional Cr-phosphate complexes while the minority are mutagenic phosphate-N7dG microchelates. Both types of adducts are substrates for nucleotide excision repair (NER) in human (184) and hamster (185) cells, as evidenced by persistence of total adducts and increased toxicity and mutagenicity of Cr-DNA damage in NER-deficient cells. A recent assignment of Cr-dG binding to NGG sequences (186), which was based on the mapping of DNA nicks made by bacterial UvrABC exinuclease in Cr-adducted DNA, is consistent with the sequence specificity of Cr-adduct mutagenesis (177). The discrepancy in the reported nucleotide specificity of Cr-DNA binding determined by UvrABC mapping (186) and other approaches (177, 180) can be related to the ability of UvrABC to recognize only a fraction of DNA adducts with the largest degree of duplex distortion. Preferential binding of Cr(III) to the phosphate backbone leads to only minor 1–2° distortions in the DNA duplexes (187).
DNA-protein and DNA interstrand crosslinks
The formation of DNA-protein crosslinks (DPC) by Cr(VI) is well established in various biological systems (188, 189) and in the in vitro reactions (190). The overall yield of DPC in cells was estimated to be less than 1% of all Cr-DNA adducts but it could be significantly higher in vitro (133). The availability of sensitive methodologies led to the frequent use of DPC measurements as a biomarker of Cr(VI) exposure in humans (190, 192) and aquatic species (193). The biological significance and repair of Cr-induced DPC remain largely unknown, however, a very large size of these lesions would likely represent a major obstacle for replication and transcription processes. The presence of interstrand DNA crosslinks have been detected only under certain in vitro conditions (176, 194, 195) and based on the severe steric restrictions for intercalation of octahedral Cr(III) complexes, it was argued that interstrand crosslinks were probably produced by Cr(III) oligomers (133). The presence of oligomeric Cr(III) forms is very unlikely inside the nucleus and the lack of Cr(VI) hypersensitivity in crosslink repair-deficient ERCC4(XPF)-null CHO cells (185) provided a strong argument that DNA crossliking is probably an in vitro phenomenon arising under conditions of high Cr(III) and low ligand concentrations. If formed, interstrand crosslink would represent a potent block for cellular DNA replication.
DNA breaks
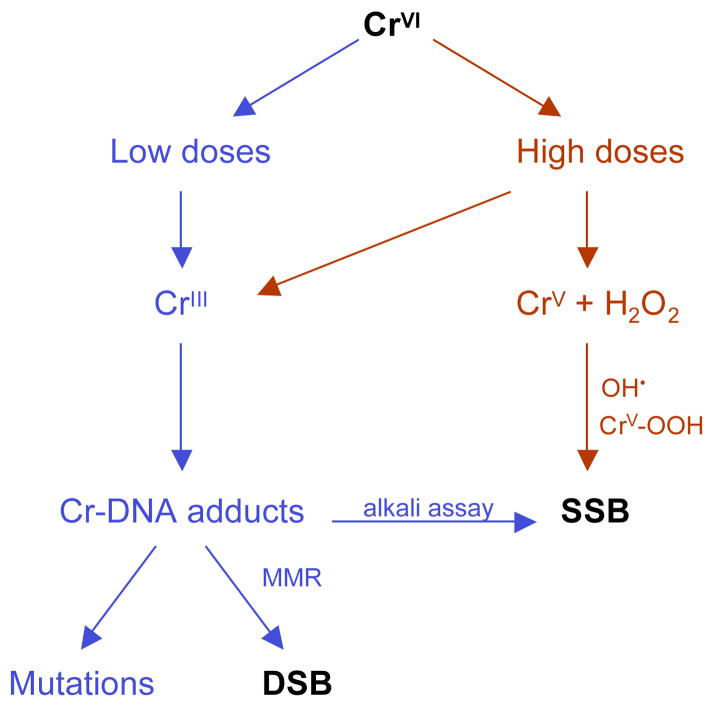
The presence of single-strand breaks (SSB) in chromate-treated cells in culture and in animal tissues has been reported in several studies (196–199) that used standard detection assays for quantitation of these DNA lesions. While the results of these and related studies showed clear positive responses, the reliance of all of the employed methodologies (alkali elution, alkaline unwinding assay, alkaline single cell electrophoresis) on DNA unwinding in strongly alkali conditions raises a major concern whether the recorded data measured frank SSB or breaks that were caused by the alkaline assay conditions. Cr-DNA phosphate adducts (176), similarly to other modifications of DNA phosphate groups, make phosphodiester linkages unstable under alkaline conditions causing breaks. In fact, this property of alkylphosphotriesters has long been used for their quantitation in cellular DNA (200). Another complication in the detection of SSB is a rapid excision of Cr-DNA adducts by NER (184), which in human cells generates about 50,000 excision events/min following exposure to 2–5 μM Cr(VI). Assessment of the formation of toxic SSB by genetic approaches relying on the comparison of Cr(VI) toxicity in SSB-repair deficient (XRCC1−/−) and proficient (XRCC1+) isogenic EM9-CHO cells (154) showed that toxic SSB were formed at Cr(VI) doses causing more than 50% clonogenic lethality and accumulation >0.5 mM cellular Cr. The production of SSB was inhibited by the addition of catalase, iron chelators or by elevation of glutathione levels. These results along with the oxygen-dependence of SSB induction in glutathione-chromate mixtures (201) led to the suggestion that SSB were caused by oxidizing species generated in the reaction of Cr(V) with H2O2 (154). The ability of Cr(V) to act as a catalyst in Fenton-like reactions with H2O2 has been well established (181, 202). The source of H2O2 in vitro has been traced to Fe contamination, particularly in the stock preparations of the reducers (154, 158, 176). In Cr-treated cells, increased production of H2O2 could be a result of mitochondrial damage or elevated activity of NADPH oxidases. Since ascorbate-driven reductions generate minimal if any Cr(V) at biologically relevant conditions (166–168), then the formation of SSB in ascorbate-supplemented cells should be very low. In support of this suggestion, in vitro reactions of Cr(VI) with 0.2 and 1 mM ascorbate concentrations found no significant DNA strand breakage (158, 203), but experiments in human cells with restored physiological levels of ascorbate are certainly needed to directly examine this question.
Cytogenetic studies in Cr(VI)-exposed cells in culture and in vivo have long reported findings, such as increased frequencies of chromosomal breaks (204, 205) and micronuclei (206), consistent with the induction of DNA double-strand breaks (DSB). However, it is only very recently a more direct evidence for the formation of DSB in Cr(VI)-treated human cells has been obtained (141, 207, 208). DSB were produced via an indirect mechanism, which required passage of cells through S-phase and participation of mismatch repair proteins. A more detailed discussion of this phenomenon is presented in section 4.3. Figure 5 summarizes the major pathways leading to the production of SSB and DSB by Cr(VI).
Figure 5.
Direct and indirect mechanisms in the generation of single-strand (SSB) and double-strand (DSB) DNA breaks by Cr(VI).
DNA base damage
Damage to the base component of DNA can involve either loss of bases (i.e. production of abasic sites) or chemical modifications with the retention of altered bases in DNA duplexes. In vitro studies of Cr(VI) reductions with ascorbate and glutathione showed that the formation of abasic sites closely mirrored the yield of SSB and required the same reactive species (201, 209). Similarly to SSB, when iron-free reaction conditions were used, no abasic sites were observed (158, 176). Given the parallel production of both types of backbone damage, cellular conditions permitting the induction of SSB should also lead to the concomitant production of abasic sites. No direct measurements of abasic sites in Cr(VI)-treated human cells have yet been done.
Administration of Cr(VI) to animals with different tissue levels of ascorbate failed to induce the formation of 8-oxoG (210), which is the most widely used indicator of the oxidative insult on DNA. Similarly, GC/MS analyses of DNA modified in the presence of Cr(VI) and ascorbate or glutathione have not found 8-oxoG or other base oxidation products (211). Consistent with the absence of oxidative base damage, replication of Cr(VI)/reducer-treated plasmids in human cells generated no mutagenic events when Cr(III)-DNA binding was prevented or disrupted (158, 175, 203). However, Slade et al. (212) have recently reported that Cr(VI) reduction by ascorbate yielded significant amounts of spiroiminodihydantoin, which is one of the advanced oxidation products of guanine. Thus, it appears that guanine oxidation can occur during Cr(VI) reduction but its main product was different than it is typically expected for the oxidant-producing reactions. Apart from potentially significant differences in the reaction conditions, the discrepancy between the lack of mutagenic responses in shuttle-vector plasmids treated with Cr(VI)-ascorbate in phosphate buffer (158, 203) and the production of spiroiminodihydantoin in PBS buffer (212) may be related to either a weak mutagenicity of this lesion or its rapid repair in human cells. Determination of spiroiminodihydantoin and other advanced oxidation products of guanine in ascorbate-complemented human cells would be very useful to clarify their importance in genotoxic effects of Cr(VI).
4.3. Genomic instability, toxicity and Cr(VI) carcinogenesis
Cr(VI)-associated carcinogenesis differs from malignant processes involved in smoking-induced lung tumors by its very low frequency of mutational inactivation of p53 (213). A small number of p53 mutations found in chromate-induced lung cancers included base substitutions at A/T pairs and double missense mutations. This spectrum of mutagenic events is more consistent with the mutator phenotype of tumor cells since Cr(VI)-induced mutations do not occur at A/T bases pairs. In all biological systems examined to date, G/C pairs were the predominant targets of Cr(VI) mutagenesis (158, 175, 177, 214, 215). Thus, Cr(VI)-activated malignant process proceeds through a completely different pathway despite that fact the majority of lung cancers were found among chromate workers who were smokers (135).
One interesting feature of Cr(VI)-associated cancers was the presence of microsatellite instability (216) that indicates a complete loss of functional mismatch repair (MMR) (217, 218). In Cr-induced cancers, microsatellite instability was associated with the loss of expression of MLH1 (219), which is one of the essential MMR proteins. The absence of MMR leads to the inability of cells to correct replication errors and these cells exhibit 100-times and higher mutation rates at their chromosomal genes, but the frequency of mutagenic events is even greater in the areas of simple nucleotide repeats known as microsatellites. Thus, chromate-associated cancer cells express mutator phenotype caused by the loss of the major mutation avoidance system – MMR. Once cells inactivated MMR, the subsequent acquisition of mutations in the critical growth-controlling genes is greatly accelerated since these cells maintain high rates of random mutagenesis and no longer need continuous exposure to Cr(VI) for additional mutagenic events. Then the question arises how and why Cr(VI) selectively leads to the appearance of this specific form of genomic instability, which is uncommon for other lung carcinogens. The answer to this question appears to lie in the active role that MMR plays in toxic and genotoxic effects of Cr(VI).
MMR-deficient mouse and human cells have recently been found to be resistant to apoptosis and clonogenic lethality of Cr(VI) in human and mouse cells (153, 208). The absence of MMR also eliminated the ability of Cr-DNA adducts to inhibit cellular replication of Cr-modified vectors and strongly suppressed the induction of DSB in Cr(VI)-treated cells (141, 208). Thus, MMR acted as the aberrant repair process generating genotoxic damage rather than eliminating it. Cr-resistant phenotype was induced by the loss of any of the four main MMR proteins (PMS2, MLH1, MSH2 and MSH6) (153, 208), indicating that the entire MMR complex was required for the processing of Cr-DNA adducts into highly toxic DSB. The damage-promoting effects of MMR extended to the full range of Cr(VI) concentrations from very low nontoxic (<1 μM) to highly toxic doses (>90% clonogenic lethality). The potentiating effects of MMR were most strongly pronounced in cells supplemented with physiological levels of ascorbate (141, 153). Apoptotic responses and clonogenic lethality induced by Cr(VI) in human cells with normal or low ascorbate concentrations did not require the involvement of p53 (153, 208). In addition to the promotion of chromosomal breaks via activation of abnormal MMR, ascorbate was also a very potent enhancer of mutagenic activity of Cr(VI) (141). Ascorbate-Cr-DNA adduct is a unique form of DNA damage induced during Cr(VI) metabolism by vitamin C (159) and they were highly mutagenic during replication of shuttle-vectors in human cells (158). MMR-promoted DSB were preferentially found in G2 phase of cell cycle, irrespective of doses, post-exposure time and type of cells (141, 208). G2-specificity of DSB production was caused by the requirement for Cr-damaged DNA to pass through S-phase in order for MMR system to activate aberrant processing (141). A combination of these findings led to the model that highly mutagenic adducts, such as ascorbate-Cr-DNA crosslinks, induce mismatches during replication of damaged DNA and these compounds lesions (mismatches at the site of Cr adducts) then lead to abnormal MMR (141). In this scenario, premutagenic adducts induce mutations and also promote larger chromosomal abnormalities (deletions, translocations) resulting from the error-prone repair of DSB through a nonhomologous end-joining process. Exposure of human cells to Cr(VI) is known to induce a series of gross chromosomal alterations, particularly in telomerase-negative primary cells (220).
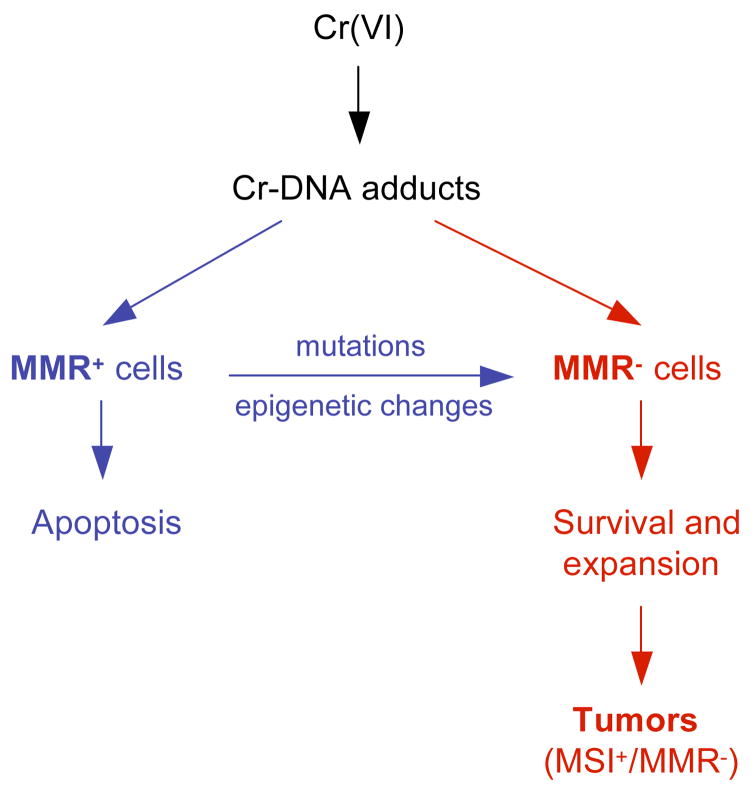
The observed tolerance of Cr(VI) by MMR-deficient cells and the absence of MMR in chromate-induced lung cancers (216, 219) led to the formulation of the selection model of Cr(VI) carcinogenesis (208, 221) (Fig. 6). This model postulates that chronic exposure to toxic doses of Cr(VI) results in the selective outgrowth of resistant clones that lack MMR. Once a population of these cells emerged, the subsequent exposure to Cr(VI) may no longer be necessary for generation of additional mutations needed for the further progression of initiated cells because MMR-null cells have very high rates of spontaneous mutagenesis. Since p53 plays no significant role in toxicity of Cr(VI) at biologically relevant doses (153, 208), there is no selective pressure to inactivate this tumor-suppressor and this could be a main reason why Cr-induced tumors retained wild-type p53. Overall, Cr(VI) carcinogenesis can be envisioned as a deadly combination of Cr-DNA damage processed by MMR into chromosomal abnormalities at low Cr(VI) doses and elimination of cells with intact mutation avoidance mechanism at higher doses. Paradoxically, intracellular ascorbate is a very potent stimulator of both processes leading to genomic instability in Cr(VI)-exposed cells (141, 153).
Figure 6.
Selection model of Cr(VI) carcinogenesis. Resistance of mismatch repair-deficient (MMR−) cells allows their survival and expansion during repetitive exposures to toxic doses of Cr(VI). MMR− cells can arise spontaneously and can be caused by mutagenic Cr-DNA damage. Very high rates of spontaneous mutagenesis in MMR− cells lead to accelerated acquisition of the necessary mutations in the critical cancer-controlling genes and resulting tumors exhibit microsatellite instability (MSI+ phenotype).
4.4. Cr(VI) as a cocarcinogen
While there are well-documented situations of human exposure to Cr(VI) as a single agent, such as encountered in chromate production, the majority of other occupational and probably all environmental exposures are actually co-exposures with other carcinogens. Two examples of common co-exposures are stainless steel welders and Cr(VI)-exposed workers who are also smokers. The possibility of cocarcinogenesis has been discussed for a long time but the underlying considerations were largely theoretical and only recently chromate cocarcinogenesis has been demonstrated in animal studies and the likely underlying mechanistic basis has emerged. Two reports from Costa and colleagues (222, 223) provided strong experimental data demonstrating that Cr(VI) can act as a potent cocarcinogen for UV-induced skin tumors. In both studies, the presence of Cr(VI) in drinking water caused dose-dependent increases in the frequency of skin tumors in UV-irradiated hairless mice. Cr(VI) alone produced no tumors, indicating that it acted a strong enhancer of UV-initiated tumorigenesis. Supplementation with vitamin E or selenomethionine had no effect on Cr(VI)-mediated enhancement of skin carcinogenesis (223), suggesting that cocarcinogenic effects were not oxidant-mediated. The same regiment of antioxidants was very effective in suppressing As(III)-potentiated skin carcinogenesis in UV-irradiated hairless mice (224), demonstrating that Cr(VI) and As(III) enhanced UV-induced tumorigenesis via different mechanisms. While the evidence for Cr-UV cocarcinogenesis in mouse skin is very clear (222, 223), whether Cr(VI) reached skin cells through systemic distribution after ingestion of Cr(VI)-laced water or skin was exposed to Cr(VI) externally remains uncertain.
The inability to repair UV-induced DNA damage leads to dramatically increased risk of skin cancer and is the cause of xeroderma pigmentosum syndrome (225). Thus, one likely target of Cr-UV synergism could be interference of Cr(VI) with NER of pyrimidine dimers. Cr-DNA adducts are very good substrates for human NER (184). Thus, the presence of Cr-DNA adducts in UV-irradiated keratinocytes can be expected to divert NER machinery to repair of relatively weakly mutagenic Cr-DNA phosphate modifications, which would increase persistence of more mutagenic UV-DNA damage. It should be noted that exposures to even mildly or non-toxic Cr(VI) concentrations produce very high frequencies of Cr-DNA adducts (141, 184), which requires a prolonged involvement of many NER complexes to remove DNA-bound Cr complexes. For example, a 3-hr treatment of primary human IMR90 cells with 2 μM Cr(VI), a dose corresponding to a current federal standard for Cr in drinking water, caused as much as 107 Cr-DNA adducts/cell (184). Based on the repair rate of t1/2= 8.2 hr in these cells, a full engagement of cellular NER would still leave about 106 Cr lesions/genome at 24 hr post-exposure (184). The reported comutagenicity of Cr(VI) and UV (226), which offers further support for the biological plausibility of Cr-UV cocarcinogenesis, can be explained by the same mechanism involving the competition for NER factors.
Tobacco smoking and Cr(VI) exposure represent another potential case for synergistic tumorigenesis. Smoking is very common among chromate-exposed workers and DNA adducts formed by polycyclic aromatic hydrocarbons, which are one of the main groups of tobacco-derived mutagens, are repaired by NER (227). Tang and co-workers (228, 229) have found pre-exposure to Cr(VI) indeed led to a significantly slower repair of BPDE-DNA adducts, which was accompanied by increased cytotoxicity and mutagenesis of BPDE. Co-exposure of repair-deficient cells produced no synergism, thus establishing NER as the key target of enhancement of BPDE genotoxicity by Cr(VI). Interestingly, Cr(VI) appears to cause a selective increase in the number of BPDE adducts at the mutational “hotspots” of p53 in smoking-induced lung cancer: codons 248, 273 and 282 (228). These sites are likely to be repair “coldspots”, which would make them particularly sensitive to decreased NER due to a competition with Cr-DNA adducts. Although the majority of lung cancer cases among Cr(VI)-exposed workers were smokers (135), the interaction between smoking and Cr(VI) remained statistically uncertain due to the small number of cancer cases among nonsmokers. Despite good parallels between UV-Cr and BPDE-Cr comutagenesis, smoking-induced cancers have a more complex molecular etiology due to the presence of many carcinogens some of which produce DNA damage that is not substrate for NER (for example, formaldehyde or nitrosamines, Ref. 230, 231). Cr(VI) and smoking-induced cancers also have different spectra of inactivated tumor-suppressors. While p53 mutations caused by activated polycyclic aromatic hydrocarbons and other mutagens are very common in tobacco-associated lung cancers (232), the majority of lung cancers in chromate workers retained wild-type p53 and instead inactivated expression of MLH1 mismatch repair protein (213, 216, 219). These major molecular differences provide further support for epidemiological findings that despite its potential for cocarcinogenesis, Cr(VI) can act as a potent lung carcinogen by itself (135).
Acknowledgments
Research by K.S. is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. A.Z. acknowledges support by NIEHS grants ES008786, ES012915 and P42 ES013660. The authors thank Dr. K. Kasprzak for the critical reading of the manuscript.
Footnotes
NTP Technical Report on the toxicology and carcinogenesis studies of sodium dichromate dihydrate in F344/N rats and B6C3F1 mice. NIH Publication # 07-5887, 2007.
References
- 1.Kasprzak KS, Sunderman FW, Salnikow K. Nickel carcinogenesis. Mutat Res. 2003;533:67–97. doi: 10.1016/j.mrfmmm.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Morgan LG, Usher V. Health problems associated with nickel refining and use. Ann Occup Hyg. 1994;38:189–198. doi: 10.1093/annhyg/38.2.189. [DOI] [PubMed] [Google Scholar]
- 3.Sunderman FW., Jr Carcinogenicity of nickel compounds in animals. IARC Sci Publ. 1984:127–142. [PubMed] [Google Scholar]
- 4.Sutherland JE, Costa M. Epigenetics and the environment. Ann N Y Acad Sci. 2003;983:151–160. doi: 10.1111/j.1749-6632.2003.tb05970.x. [DOI] [PubMed] [Google Scholar]
- 5.Lu H, Shi X, Costa M, Huang C. Carcinogenic effect of nickel compounds. Mol Cell Biochem. 2005;279:45–67. doi: 10.1007/s11010-005-8215-2. [DOI] [PubMed] [Google Scholar]
- 6.Denkhaus E, Salnikow K. Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol. 2002;42:35–56. doi: 10.1016/s1040-8428(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 7.Doll R, Morgan LG, Speizer FE. Cancers of the lung and nasal sinuses in nickel workers. Br J Cancer. 1970;24:623–632. doi: 10.1038/bjc.1970.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IARC. Report of the International Committee on Nickel Carcinogenesis in Man. Scand J Work Environ Health. 1990;16:1–82. doi: 10.5271/sjweh.1813. [DOI] [PubMed] [Google Scholar]
- 9.Haber LT, Erdreicht L, Diamond GL, Maier AM, Ratney R, Zhao Q, Dourson ML. Hazard identification and dose response of inhaled nickel-soluble salts. Regul Toxicol Pharmacol. 2000;31:210–230. doi: 10.1006/rtph.2000.1377. [DOI] [PubMed] [Google Scholar]
- 10.Grimsrud TK, Berge SR, Haldorsen T, Andersen A. Exposure to different forms of nickel and risk of lung cancer. Am J Epidemiol. 2002;156:1123–1132. doi: 10.1093/aje/kwf165. [DOI] [PubMed] [Google Scholar]
- 11.Antonini JM, Taylor MD, Zimmer AT, Roberts JR. Pulmonary responses to welding fumes: role of metal constituents. J Toxicol Environ Health A. 2004;67:233–249. doi: 10.1080/15287390490266909. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher P, Pacheco K, Newman LS. Inorganic dust pneumonias: the metal-related parenchymal disorders. Environ Health Perspect. 2000;108(Suppl 4):685–696. doi: 10.1289/ehp.00108s4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunnick JK, Benson JM, Hobbs CH, Hahn FF, Cheng YS, Eidson AF. Comparative toxicity of nickel oxide, nickel sulfate hexahydrate, and nickel subsulfide after 12 days of inhalation exposure to F344/N rats and B6C3F1 mice. Toxicology. 1988;50:145–156. doi: 10.1016/0300-483x(88)90087-x. [DOI] [PubMed] [Google Scholar]
- 14.Dunnick JK, Elwell MR, Benson JM, Hobbs CH, Hahn FF, Haly PJ, Cheng YS, Eidson AF. Lung toxicity after 13-week inhalation exposure to nickel oxide, nickel subsulfide, or nickel sulfate hexahydrate in F344/N rats and B6C3F1 mice. Fund Appl Toxicol. 1989;12:584–594. doi: 10.1016/0272-0590(89)90031-6. [DOI] [PubMed] [Google Scholar]
- 15.Dunnick JK, Elwell MR, Radovsky AE, Benson JM, Hahn FF, Nikula KJ, Barr EB, Hobbs CH. Comparative carcinogenic effects of nickel subsulfide, nickel oxide, or nickel sulfate hexahydrate chronic exposures in the lung. Cancer Res. 1995;55:5251–5256. [PubMed] [Google Scholar]
- 16.Benson JM, Cheng YS, Eidson AF, Hahn FF, Henderson RF, Pickrell JA. Pulmonary toxicity of nickel subsulfide in F344/N rats exposed for 1–22 days. Toxicology. 1995;103:9–22. doi: 10.1016/0300-483x(95)03098-z. [DOI] [PubMed] [Google Scholar]
- 17.Biedermann KA, Landolph JR. Induction of anchorage independence in human diploid foreskin fibroblasts by carcinogenic metal salts. Cancer Res. 1987;47:3815–3823. [PubMed] [Google Scholar]
- 18.Tveito G, Hansteen IL, Dalen H, Haugen A. Immortalization of normal human kidney epithelial cells by nickel(II) Cancer Res. 1989;49:1829–1835. [PubMed] [Google Scholar]
- 19.Patierno SR, Dirscherl LA, Xu J. Transformation of rat tracheal epithelial cells to immortal growth variants by particulate and soluble nickel compounds. Mutat Res. 1993;300:179–193. doi: 10.1016/0165-1218(93)90049-j. [DOI] [PubMed] [Google Scholar]
- 20.Rani AS, Qu DQ, Sidhu MK, Panagakos F, Shah V, Klein KM, Brown N, Pathak S, Kumar S. Transformation of immortal, non-tumorigenic osteoblast-like human osteosarcoma cells to the tumorigenic phenotype by nickel sulfate. Carcinogenesis. 1993;14:947–953. doi: 10.1093/carcin/14.5.947. [DOI] [PubMed] [Google Scholar]
- 21.International Agency for Research on Cancer. Chromium, nickel and welding. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 49, World Health Organization; Lyon, France. 1990. [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher GG, Rossetto FE, Turnbull JD, Nieboer E. Toxicity, uptake, and mutagenicity of particulate and soluble nickel compounds. Environ Health Perspect. 1994;102(Suppl 3):69–79. doi: 10.1289/ehp.94102s369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biggart NW, Costa M. Assessment of the uptake and mutagenicity of nickel chloride in salmonella tester strains. Mutat Res. 1986;175:209–215. doi: 10.1016/0165-7992(86)90056-4. [DOI] [PubMed] [Google Scholar]
- 24.Arrouijal FZ, Hildebrand HF, Vophi H, Marzin D. Genotoxic activity of nickel subsulphide alpha-Ni3S2. Mutagenesis. 1990;5:583–589. doi: 10.1093/mutage/5.6.583. [DOI] [PubMed] [Google Scholar]
- 25.Kargacin B, Klein CB, Costa M. Mutagenic responses of nickel oxides and nickel sulfides in Chinese hamster V79 cell lines at the xanthine-guanine phosphoribosyl transferase locus. Mutat Res. 1993;300:63–72. doi: 10.1016/0165-1218(93)90141-y. [DOI] [PubMed] [Google Scholar]
- 26.Trott DA, Cuthbert AP, Overell RW, Russo I, Newbold RF. Mechanisms involved in the immortalization of mammalian cells by ionizing radiation and chemical carcinogens. Carcinogenesis. 1995;16:193–204. doi: 10.1093/carcin/16.2.193. [DOI] [PubMed] [Google Scholar]
- 27.Karaczyn AA, Golebiowski F, Kasprzak KS. Truncation, deamidation, and oxidation of histone H2B in cells cultured with nickel(II) Chem Res Toxicol. 2005;18:1934–1942. doi: 10.1021/tx050122a. [DOI] [PubMed] [Google Scholar]
- 28.Karaczyn AA, Golebiowski F, Kasprzak KS. Ni(II) affects ubiquitination of core histones H2B and H2A. Exp Cell Res. 2006;312:3252–3259. doi: 10.1016/j.yexcr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Lee YW, Klein CB, Kargacin B, Salnikow K, Kitahara J, Dowjat K, Zhitkovich A, Christie NT, Costa M. Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: a new model for epigenetic carcinogens. Mol Cell Biol. 1995;15:2547–2557. doi: 10.1128/mcb.15.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broday L, Peng W, Kuo MH, Salnikow K, Zoroddu M, Costa M. Nickel compounds are novel inhibitors of histone H4 acetylation. Cancer Res. 2000;60:238–241. [PubMed] [Google Scholar]
- 31.Chen H, Ke Q, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol Cell Biol. 2006;26:3728–3737. doi: 10.1128/MCB.26.10.3728-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke Q, Davidson T, Chen H, Kluz T, Costa M. Alterations of histone modifications and transgene silencing by nickel chloride. Carcinogenesis. 2006;27:1481–1488. doi: 10.1093/carcin/bgl004. [DOI] [PubMed] [Google Scholar]
- 33.Golebiowski F, Kasprzak KS. Inhibition of core histones acetylation by carcinogenic nickel(II) Mol Cell Biochem. 2005;279:133–139. doi: 10.1007/s11010-005-8285-1. [DOI] [PubMed] [Google Scholar]
- 34.Govindarajan B, Klafter R, Miller MS, Mansur C, Mizesko M, Bai X, LaMontagne K, Jr, Arbiser JL. Reactive oxygen-induced carcinogenesis causes hypermethylation of p16(Ink4a) and activation of MAP kinase. Mol Med. 2002;8:1–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Kowara R, Salnikow K, Diwan BA, Bare RM, Waalkes MP, Kasprzak KS. Reduced Fhit protein expression in nickel-transformed mouse cells and in nickel-induced murine sarcomas. Mol Cell Biochem. 2004;255:195–202. doi: 10.1023/b:mcbi.0000007275.22785.91. [DOI] [PubMed] [Google Scholar]
- 36.Zoroddu MA, Schinocca L, Kowalik-Jankowska T, Kozlowski H, Salnikow K, Costa M. Molecular mechanisms in nickel carcinogenesis: modeling Ni(II) binding site in histone H4. Environ Health Perspect. 2002;110(Suppl 5):719–723. doi: 10.1289/ehp.02110s5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Kluz T, Zhang P, Chen HB, Costa M. Analysis of specific lysine histone H3 and H4 acetylation and methylation status in clones of cells with a gene silenced by nickel exposure. Toxicol Appl Pharm. 2003;190:272–277. doi: 10.1016/s0041-008x(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Salnikow K, Kluz T, Chen LC, Su WC, Costa M. Inhibition and reversal of nickel-induced transformation by the histone deacetylase inhibitor trichostatin A. Toxicol Appl Pharm. 2003;192:201–211. doi: 10.1016/s0041-008x(03)00280-1. [DOI] [PubMed] [Google Scholar]
- 39.Maxwell P, Salnikow K. HIF-1: an oxygen and metal responsive transcription factor. Cancer Biol Ther. 2004;3:29–35. doi: 10.4161/cbt.3.1.547. [DOI] [PubMed] [Google Scholar]
- 40.Salnikow K, Blagosklonny MV, Ryan H, Johnson R, Costa M. Carcinogenic nickel induces genes involved with hypoxic stress. Cancer Res. 2000;60:38–41. [PubMed] [Google Scholar]
- 41.Salnikow K, Davidson T, Kluz T, Chen H, Zhou D, Costa M. GeneChip analysis of signaling pathways effected by nickel. J Environ Monit. 2003;5:206–209. doi: 10.1039/b210262p. [DOI] [PubMed] [Google Scholar]
- 42.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 44.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 45.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 46.Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, Jones EY. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 47.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Jr, Pavletich NP. Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 49.Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, Eck MJ. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci U S A. 2002;99:5367–5372. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salnikow K, An WG, Melillo G, Blagosklonny MV, Costa M. Nickel-induced transformation shifts the balance between HIF-1 and p53 transcription factors. Carcinogenesis. 1999;20:1819–1823. doi: 10.1093/carcin/20.9.1819. [DOI] [PubMed] [Google Scholar]
- 52.Kaczmarek M, Timofeeva OA, Karaczyn A, Malyguine A, Kasprzak KS, Salnikow K. The role of ascorbate in the modulation of HIF-1alpha protein and HIF-dependent transcription by chromium(VI) and nickel(II) Free Radic Biol Med. 2007;42:1246–1257. doi: 10.1016/j.freeradbiomed.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson T, Chen H, Garrick MD, D’Angelo G, Costa M. Soluble nickel interferes with cellular iron homeostasis. Mol Cell Biochem. 2005;279:157–162. doi: 10.1007/s11010-005-8288-y. [DOI] [PubMed] [Google Scholar]
- 54.Davidson TL, Chen H, Di Toro DM, D’Angelo G, Costa M. Soluble nickel inhibits HIF-prolylhydroxylases creating persistent hypoxic signaling in A549 cells. Mol Carcinog. 2006;45:479–489. doi: 10.1002/mc.20176. [DOI] [PubMed] [Google Scholar]
- 55.Salnikow K, Li X, Lippmann M. Effect of nickel and iron co-exposure on human lung cells. Toxicol Appl Pharmacol. 2004;196:258–265. doi: 10.1016/j.taap.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Chen H, Davidson T, Singleton S, Garrick MD, Costa M. Nickel decreases cellular iron level and converts cytosolic aconitase to iron-regulatory protein 1 in A549 cells. Toxicol Appl Pharmacol. 2005;206:275–287. doi: 10.1016/j.taap.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Kang GS, Li Q, Chen H, Costa M. Effect of metal ions on HIF-1alpha and Fe homeostasis in human A549 cells. Mutat Res. 2006;610:48–55. doi: 10.1016/j.mrgentox.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrew AS, Klei LR, Barchowsky A. Nickel requires hypoxia-inducible factor-1 alpha, not redox signaling, to induce plasminogen activator inhibitor-1. Am J Physiol Lung Cell Mol Physiol. 2001;281:L607–615. doi: 10.1152/ajplung.2001.281.3.L607. [DOI] [PubMed] [Google Scholar]
- 60.Salnikow K, Su W, Blagosklonny MV, Costa M. Carcinogenic metals induce hypoxia-inducible factor-stimulated transcription by reactive oxygen species-independent mechanism. Cancer Res. 2000;60:3375–3378. [PubMed] [Google Scholar]
- 61.Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J Biol Chem. 2004;279:40337–40344. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- 62.Karaczyn A, Ivanov S, Reynolds M, Zhitkovich A, Kasprzak KS, Salnikow K. Ascorbate depletion mediates up-regulation of hypoxia-associated proteins by cell density and nickel. J Cell Biochem. 2006;97:1025–1035. doi: 10.1002/jcb.20705. [DOI] [PubMed] [Google Scholar]
- 63.Salnikow K, Kasprzak KS. Ascorbate depletion: a critical step in nickel carcinogenesis? Environ Health Perspect. 2005;113:577–584. doi: 10.1289/ehp.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salnikow K, Davidson T, Zhang Q, Chen LC, Su W, Costa M. The involvement of hypoxia-inducible transcription factor-1-dependent pathway in nickel carcinogenesis. Cancer Res. 2003;63:3524–3530. [PubMed] [Google Scholar]
- 65.Costa M, Davidson TL, Chen H, Ke Q, Zhang P, Yan Y, Huang C, Kluz T. Nickel carcinogenesis: epigenetics and hypoxia signaling. Mutat Res. 2005;592:79–88. doi: 10.1016/j.mrfmmm.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Vaupel P, Mayer A. Hypoxia and anemia: effects on tumor biology and treatment resistance. Transfus Clin Biol. 2005;12:5–10. doi: 10.1016/j.tracli.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Rivedal E, Sanner T. Synergistic effect on morphological transformation of hamster embryo cells by nickel sulphate and benz[a]pyrene. Cancer Lett. 1980;8:203–208. doi: 10.1016/0304-3835(80)90002-6. [DOI] [PubMed] [Google Scholar]
- 68.Rivedal E, Sanner T. Metal salts as promoters of in vitro morphological transformation of hamster embryo cells initiated by benzo(a)pyrene. Cancer Res. 1981;41:2950–2953. [PubMed] [Google Scholar]
- 69.Schwerdtle T, Seidel A, Hartwig A. Effect of soluble and particulate nickel compounds on the formation and repair of stable benzo[a]pyrene DNA adducts in human lung cells. Carcinogenesis. 2002;23:47–53. doi: 10.1093/carcin/23.1.47. [DOI] [PubMed] [Google Scholar]
- 70.Kasprzak KS, Diwan BA, Konishi N, Misra M, Rice JM. Initiation by nickel acetate and promotion by sodium barbital of renal cortical epithelial tumors in male F344 rats. Carcinogenesis. 1990;11:647–652. doi: 10.1093/carcin/11.4.647. [DOI] [PubMed] [Google Scholar]
- 71.Hu W, Feng Z, Tang MS. Nickel (II) enhances benzo[a]pyrene diol epoxide-induced mutagenesis through inhibition of nucleotide excision repair in human cells: a possible mechanism for nickel (II)-induced carcinogenesis. Carcinogenesis. 2004;25:455–462. doi: 10.1093/carcin/bgh012. [DOI] [PubMed] [Google Scholar]
- 72.Iwitzki F, Schlepegrell R, Eichhorn U, Kaina B, Beyersmann D, Hartwig A. Nickel(II) inhibits the repair of O6-methylguanine in mammalian cells. Arch Toxicol. 1998;72:681–689. doi: 10.1007/s002040050561. [DOI] [PubMed] [Google Scholar]
- 73.Hartwig A, Mullenders LH, Schlepegrell R, Kasten U, Beyersmann D. Nickel(II) interferes with the incision step in nucleotide excision repair in mammalian cells. Cancer Res. 1994;54:4045–4051. [PubMed] [Google Scholar]
- 74.Sedgwick B. Repairing DNA-methylation damage. Nat Rev Mol Cell Biol. 2004;5:148–157. doi: 10.1038/nrm1312. [DOI] [PubMed] [Google Scholar]
- 75.Duncan T, Trewick SC, Koivisto P, Bates PA, Lindahl T, Sedgwick B. Reversal of DNA alkylation damage by two human dioxygenases. Proc Natl Acad Sci U S A. 2002;99:16660–16665. doi: 10.1073/pnas.262589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodriguez RE, Misra M, Diwan BA, Riggs CW, Kasprzak KS. Relative susceptibilities of C57BL/6, (C57BL/6 × C3H/He)F1, and C3H/He mice to acute toxicity and carcinogenicity of nickel subsulfide. Toxicology. 1996;107:131–140. doi: 10.1016/0300-483x(95)03251-a. [DOI] [PubMed] [Google Scholar]
- 77.Tchounwou PB, Centeno JA, Patlolla AK. Arsenic toxicity, mutagenesis, and carcinogenesis - a health risk assessment and management approach. Mol Cell Biochem. 2004;255:47–55. doi: 10.1023/b:mcbi.0000007260.32981.b9. [DOI] [PubMed] [Google Scholar]
- 78.Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and systemic health effects associated with arsenic exposure - a critical review. Toxicol Pathol. 2003;31:575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- 79.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533:37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Lerman SA, Clarkson TW, Gerson RJ. Arsenic uptake and metabolism by liver cells is dependent on arsenic oxidation state. Chem-Biol Interact. 1983;45:401–406. doi: 10.1016/0009-2797(83)90087-x. [DOI] [PubMed] [Google Scholar]
- 81.Bertolero F, Pozzi G, Sabbioni E, Saffiotti U. Cellular uptake and metabolic reduction of pentavalent to trivalent arsenic as determinants of cytotoxicity and morphological transformation. Carcinogenesis. 1987;8:803–808. doi: 10.1093/carcin/8.6.803. [DOI] [PubMed] [Google Scholar]
- 82.Aposhian HV, Aposhian MM. Arsenic toxicology: five questions. Chem Res Toxicol. 2006;19:1–15. doi: 10.1021/tx050106d. [DOI] [PubMed] [Google Scholar]
- 83.Marafante E, Vahter M, Norin H, Envall J, Sandstrom M, Christakopoulos A, Ryhage R. Biotransformation of dimethylarsinic acid in mouse, hamster and man. J Appl Toxicol. 1987;7:111–117. doi: 10.1002/jat.2550070207. [DOI] [PubMed] [Google Scholar]
- 84.Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD. Methylated arsenicals: the implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit Rev Toxicol. 2006;36:99–133. doi: 10.1080/10408440500534230. [DOI] [PubMed] [Google Scholar]
- 85.Vahter M, Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett. 1987;37:41–46. doi: 10.1016/0378-4274(87)90165-2. [DOI] [PubMed] [Google Scholar]
- 86.Cohen SM, Ohnishi T, Arnold LL, Le XC. Arsenic-induced bladder cancer in an animal model. Toxicol Appl Pharmacol. 2007;222:258–263. doi: 10.1016/j.taap.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 87.Simeonova PP, Luster MI. Mechanisms of arsenic carcinogenicity: genetic or epigenetic mechanisms? J Environ Pathol Toxicol Oncol. 2000;9:281–286. [PubMed] [Google Scholar]
- 88.Salnikow K, Cohen MD. Backing into Cancer: Effects of Arsenic on Cell Differentiation. Toxicol Sci. 2002;65:161–163. doi: 10.1093/toxsci/65.2.161. [DOI] [PubMed] [Google Scholar]
- 89.Germolec DR, Spalding J, Boorman GA, Wilmer JL, Yoshida T, Simeonova PP, Bruccoleri A, Kayama F, Gaido K, Tennant R, Burleson F, Dong W, Lang RW, Luster MI. Arsenic can mediate skin neoplasia by chronic stimulation of keratinocyte-derived growth factors. Mutat Res. 1997;386:209–218. doi: 10.1016/s1383-5742(97)00006-9. [DOI] [PubMed] [Google Scholar]
- 90.Simeonova PP, Wang S, Toriuma W, Kommineni V, Matheson J, Unimye N, Kayama F, Harki D, Ding M, Vallyathan V, Luster MI. Arsenic mediates cell proliferation and gene expression in the bladder epithelium: association with activating protein-1 transactivation. Cancer Res. 2000;60:3445–3453. [PubMed] [Google Scholar]
- 91.Germolec DR, Yoshida T, Gaido K, Wilmer JL, Simeonova PP, Kayama F, Burleson F, Dong W, Lange RW, Luster MI. Arsenic induces overexpression of growth factors in human keratinocytes. Toxicol Appl Pharmacol. 1996;141:308–318. doi: 10.1006/taap.1996.0288. [DOI] [PubMed] [Google Scholar]
- 92.Simeonova PP, Wang S, Hulderman T, Luster MI. c-Src-dependent activation of the epidermal growth factor receptor and mitogen-activated protein kinase pathway by arsenic. Role in carcinogenesis J Biol Chem. 2002;277:2945–2950. doi: 10.1074/jbc.M109136200. [DOI] [PubMed] [Google Scholar]
- 93.Vogt BL, Rossman TG. Effects of arsenite on p53, p21 and cyclin D expression in normal human fibroblasts -- a possible mechanism for arsenite’s comutagenicity. Mutat Res. 2001;478:159–168. doi: 10.1016/s0027-5107(01)00137-3. [DOI] [PubMed] [Google Scholar]
- 94.Ouyang W, Ma Q, Li J, Zhang D, Liu ZG, Rustgi AK, Huang C. Cyclin D1 induction through IkappaB kinase beta/nuclear factor-kappaB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res. 2005;65:9287–9293. doi: 10.1158/0008-5472.CAN-05-0469. [DOI] [PubMed] [Google Scholar]
- 95.Trouba KJ, Wauson EM, Vorce RL. Sodium Arsenite-Induced Dysregulation of Proteins Involved in Proliferative Signaling. Toxicology and Applied Pharmacology. 2000;164:161–170. doi: 10.1006/taap.1999.8873. [DOI] [PubMed] [Google Scholar]
- 96.Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen SJ, Chen Z. Arsenic trioxide, a therapeutic agent for APL. Oncogene. 2001;20:7146–7153. doi: 10.1038/sj.onc.1204762. [DOI] [PubMed] [Google Scholar]
- 97.Kfoury Y, Nasr R, Hermine O, de The H, Bazarbachi A. Proapoptotic regimes for HTLV-I-transformed cells: targeting Tax and the NF-kappaB pathway. Cell Death Differ. 2005;12(Suppl 1):871–877. doi: 10.1038/sj.cdd.4401624. [DOI] [PubMed] [Google Scholar]
- 98.Hershko DD, Robb BW, Hungness ES, Luo G, Hasselgren PO. Arsenite stabilizes IkappaBalpha and prevents NF-kappaB activation in IL-1 beta-stimulated Caco-2 cells independent of the heat shock response. J Cell Biochem. 2002;84:687–698. doi: 10.1002/jcb.10083. [DOI] [PubMed] [Google Scholar]
- 99.Chen F, Zhang Z, Leonard SS, Shi X. Contrasting roles of NF-kappaB and JNK in arsenite-induced p53-independent expression of GADD45alpha. Oncogene. 2001;20:3585–3589. doi: 10.1038/sj.onc.1204442. [DOI] [PubMed] [Google Scholar]
- 100.Jaspers I, Samet JM, Reed W. Arsenite exposure of cultured airway epithelial cells activates kappaB-dependent interleukin-8 gene expression in the absence of nuclear factor-kappaB nuclear translocation. J Biol Chem. 1999;274:31025–31033. doi: 10.1074/jbc.274.43.31025. [DOI] [PubMed] [Google Scholar]
- 101.Ouyang W, Li J, Ma Q, Huang C. Essential roles of PI-3K/Akt/IKKbeta/NFkappaB pathway in cyclin D1 induction by arsenite in JB6 Cl41 cells. Carcinogenesis. 2006;27:864–873. doi: 10.1093/carcin/bgi321. [DOI] [PubMed] [Google Scholar]
- 102.Mathieu J, Besancon F. Clinically tolerable concentrations of arsenic trioxide induce p53-independent cell death and repress NF-kappa B activation in Ewing sarcoma cells. Int J Cancer. 2006;119:1723–1727. doi: 10.1002/ijc.21970. [DOI] [PubMed] [Google Scholar]
- 103.Park MJ, Lee JY, Kwak HJ, Park CM, Lee HC, Woo SH, Jin HO, Han CJ, An S, Lee SH, Chung HY, Park IC, Hong SI, Rhee CH. Arsenic trioxide (As2O3) inhibits invasion of HT1080 human fibrosarcoma cells: role of nuclear factor-kappaB and reactive oxygen species. J Cell Biochem. 2005;95:955–969. doi: 10.1002/jcb.20452. [DOI] [PubMed] [Google Scholar]
- 104.Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP-1 and NF-kappaB DNA binding activity and related gene expression. Toxicol Lett. 2002;133:33–45. doi: 10.1016/s0378-4274(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 105.(NRC), N. R. C. S. o. A. i. D. W. Arsenic in Drinking Water. National Academy Press; Washington, DC: 1999. [Google Scholar]
- 106.Barrett JC, Lamb PW, Wang TC, Lee TC. Mechanisms of arsenic-induced cell transformation. Biol Trace Elem Res. 1989;21:421–429. doi: 10.1007/BF02917284. [DOI] [PubMed] [Google Scholar]
- 107.Lee TC, Tanaka N, Lamb PW, Gilmer TM, Barrett JC. Induction of gene amplification by arsenic. Science. 1988;241:79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- 108.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 109.Mass MJ, Wang L. Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutat Res. 1997;386:263–277. doi: 10.1016/s1383-5742(97)00008-2. [DOI] [PubMed] [Google Scholar]
- 110.Zhong CX, Mass MJ. Both hypomethylation and hypermethylation of DNA associated with arsenite exposure in cultures of human cells identified by methylation-sensitive arbitrarily-primed PCR. Toxicol Lett. 2001;122:223–234. doi: 10.1016/s0378-4274(01)00365-4. [DOI] [PubMed] [Google Scholar]
- 111.Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad USA. 1997;94:10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol Sci. 2006;91:372–381. doi: 10.1093/toxsci/kfj159. [DOI] [PubMed] [Google Scholar]
- 113.Chanda S, Dasgupta UB, GuhaMazumder D, Gupta M, Chaudhuri U, Lahiri S, Das S, Ghosh N, Chatterjee D. DNA hypermethylation of promoter of gene p53 and p16 in arsenic-exposed people with and without malignancy. Toxicol Sci. 2006;89:431–437. doi: 10.1093/toxsci/kfj030. [DOI] [PubMed] [Google Scholar]
- 114.Samikkannu T, Chen CH, Yih LH, Wang AS, Lin SY, Chen TC, Jan KY. Reactive oxygen species are involved in arsenic trioxide inhibition of pyruvate dehydrogenase activity. Chem Res Toxicol. 2003;16:409–414. doi: 10.1021/tx025615j. [DOI] [PubMed] [Google Scholar]
- 115.Duyndam MC, Hulscher TM, Fontijn D, Pinedo HM, Boven E. Induction of vascular endothelial growth factor expression and hypoxia-inducible factor 1alpha protein by the oxidative stressor arsenite. J Biol Chem. 2001;276:48066–48076. doi: 10.1074/jbc.M106282200. [DOI] [PubMed] [Google Scholar]
- 116.Duyndam MC, Hulscher ST, van der Wall E, Pinedo HM, Boven E. Evidence for a role of p38 kinase in hypoxia-inducible factor 1-independent induction of vascular endothelial growth factor expression by sodium arsenite. J Biol Chem. 2003;278:6885–6895. doi: 10.1074/jbc.M206320200. [DOI] [PubMed] [Google Scholar]
- 117.Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol. 2001;60:302–309. doi: 10.1124/mol.60.2.302. [DOI] [PubMed] [Google Scholar]
- 118.Vernhet L, Allain N, Bardiau C, Anger JP, Fardel O. Differential sensitivities of MRP1-overexpressing lung tumor cells to cytotoxic metals. Toxicology. 2000;142:127–134. doi: 10.1016/s0300-483x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 119.Kimura A, Ishida Y, Wada T, Yokoyama H, Mukaida N, Kondo T. MRP-1 expression levels determine strain-specific susceptibility to sodium arsenic-induced renal injury between C57BL/6 and BALB/c mice. Toxicol Appl Pharmacol. 2005;203:53–61. doi: 10.1016/j.taap.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 120.Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol. 2005;79:183–191. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- 121.Li JH, Rossman TG. Mechanism of comutagenesis of sodium arsenite with n-methyl-n-nitrosourea. Biol Trace Elem Res. 1989;21:373–381. doi: 10.1007/BF02917278. [DOI] [PubMed] [Google Scholar]
- 122.Li JH, Rossman TG. Comutagenesis of sodium arsenite with ultraviolet radiation in Chinese hamster V79 cells. Biol Met. 1991;4:197–200. doi: 10.1007/BF01141180. [DOI] [PubMed] [Google Scholar]
- 123.Lee TC, Huang RY, Jan KY. Sodium arsenite enhances the cytotoxicity, clastogenicity, and 6-thioguanine-resistant mutagenicity of ultraviolet light in Chinese hamster ovary cells. Mutat Res. 1985;148:83–89. doi: 10.1016/0027-5107(85)90210-6. [DOI] [PubMed] [Google Scholar]
- 124.Wiencke JK, Yager JW. Specificity of arsenite in potentiating cytogenetic damage induced by the DNA crosslinking agent diepoxybutane. Environ Mol Mutagen. 1992;19:195–200. doi: 10.1002/em.2850190303. [DOI] [PubMed] [Google Scholar]
- 125.Tran HP, Prakash AS, Barnard R, Chiswell B, Ng JC. Arsenic inhibits the repair of DNA damage induced by benzo(a)pyrene. Toxicol Lett. 2002;133:59–67. doi: 10.1016/s0378-4274(02)00088-7. [DOI] [PubMed] [Google Scholar]
- 126.Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite cocarcinogenesis: an animal model derived from genetic toxicology studies. Environ Health Perspect. 2002;110(Suppl 5):749–752. doi: 10.1289/ehp.02110s5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol Appl P harmacol. 2004;198:394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 128.Wu F, Burns FJ, Zhang R, Uddin AN, Rossman TG. Arsenite-induced alterations of DNA photodamage repair and apoptosis after solar-simulation UVR in mouse keratinocytes in vitro. Environ Health Perspect. 2005;113:983–986. doi: 10.1289/ehp.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He YY, Pi J, Huang JL, Diwan BA, Waalkes MP, Chignell CF. Chronic UVA irradiation of human HaCaT keratinocytes induces malignant transformation associated with acquired apoptotic resistance. Oncogene. 2006;25:3680–3688. doi: 10.1038/sj.onc.1209384. [DOI] [PubMed] [Google Scholar]
- 130.Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol. 2007;222:271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhitkovich A. Chromium: exposure, toxicity and biomonitoring approaches. In: Wilson SH, Suk WA, editors. Biomarkers of Environmentally Associated Disease: Technologies, Concepts, and Perspectives. CRC Press LLC; New York: 2002. pp. 269–287. [Google Scholar]
- 132.Occupational Safety and Health Administration (OSHA), Department of Labor. Occupational exposure to hexavalent chromium. Final rule Fed Regist. 2006;71:10099–10385. [PubMed] [Google Scholar]
- 133.Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem Res Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- 134.Langard S. One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am J Ind Med. 1990;17:189–215. doi: 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- 135.Gibb HJ, Lees PS, Pinsky PF, Rooneym BC. Lung cancer among workers in chromium chemical production. Am J Ind Med. 2000;38:115–126. doi: 10.1002/1097-0274(200008)38:2<115::aid-ajim1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 136.Davies JM, Easton DF, Bidstrup PL. Mortality from respiratory cancer and other causes in United Kingdom chromate production workers. Br J Ind Med. 1991;48:299–313. doi: 10.1136/oem.48.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Satoh N, Fukuda S, Takizawa M, Furuta Y, Kashiwamura M, Inuyama Y. Chromium-induced carcinoma in the nasal region. A report of four cases. Rhinology. 1994;32:47–50. [PubMed] [Google Scholar]
- 138.Sunderman FW., Jr Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann Clin Lab Sci. 2001;31:3–24. [PubMed] [Google Scholar]
- 139.De Flora S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis. 2000;21:533–541. doi: 10.1093/carcin/21.4.533. [DOI] [PubMed] [Google Scholar]
- 140.Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PS. Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal. 2004;24:1099–1108. doi: 10.1111/j.0272-4332.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 141.Reynolds M, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res. 2007;35:465–476. doi: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mancuso TF. Chromium as an industrial carcinogen: Part I. Am J Ind Med. 1997;31:1291–39. doi: 10.1002/(sici)1097-0274(199702)31:2<129::aid-ajim1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 143.Sorahan T, Burges DC, Hamilton L, Harrington JM. Lung cancer mortality in nickel/chromium platers, 1946–95. Occup Environ Med. 1998;55:236–242. doi: 10.1136/oem.55.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Costa M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit Rev Toxicol. 1997;27:431–442. doi: 10.3109/10408449709078442. [DOI] [PubMed] [Google Scholar]
- 145.Costa M, Klein CB. Toxicity and carcinogenicity of chromium compounds in humans. Crit Rev Toxicol. 2006;36:155–163. doi: 10.1080/10408440500534032. [DOI] [PubMed] [Google Scholar]
- 146.Kerger BD, Finley BL, Corbett GE, Dodge DG, Paustenbach DJ. Ingestion of chromium(VI) in drinking water by human volunteers: Absorption, distribution, and excretion of single and repeated doses. J Tox Environ Health. 1997;50:67–95. doi: 10.1080/009841097160618. [DOI] [PubMed] [Google Scholar]
- 147.Levy LS, Martin PA, Bidstrup PL. Investigation of the potential carcinogenicity of a range of chromium containing materials on rat lung. Br J Ind Med. 1986;43:243–256. doi: 10.1136/oem.43.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. Characteristics of chromate workers’ cancers, chromium lung deposition and precancerous bronchial lesions: an autopsy study. Br J Cancer. 1994;70:160–166. doi: 10.1038/bjc.1994.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Satoh Y, Ishikawa Y, Nakagawa K, Hirano T, Tsuchiya E. A follow-up study of progression from dysplasia to squamous cell carcinoma with immunohistochemical examination of p53 protein overexpression in the bronchi of ex-chromate workers. Br J Cancer. 1997;75:678–683. doi: 10.1038/bjc.1997.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. “Hot spots” of chromium accumulation at bifurcations of chromate workers’ bronchi. Cancer Res. 1994;54:2342–2346. [PubMed] [Google Scholar]
- 151.Kondo K, Takahashi Y, Ishikawa S, Uchihara H, Hirose Y, Yoshizawa K, Tsuyuguchi M, Takizawa H, Miyoshi T, Sakiyama S, Monden Y. Microscopic analysis of chromium accumulation in the bronchi and lung of chromate workers. Cancer. 2003;98:2420–2429. doi: 10.1002/cncr.11818. [DOI] [PubMed] [Google Scholar]
- 152.Alexander J, Aaseth J. Uptake of chromate in human red blood cells and isolated rat liver cells: the role of the anion carrier. Analyst. 1995;120:931–933. doi: 10.1039/an9952000931. [DOI] [PubMed] [Google Scholar]
- 153.Reynolds M, Zhitkovich A. Cellular vitamin C increases chromate toxicity via a death program requiring mismatch repair but not p53. Carcinogenesis. 2007;28:1613–1620. doi: 10.1093/carcin/bgm031. [DOI] [PubMed] [Google Scholar]
- 154.Messer J, Reynolds M, Stoddard L, Zhitkovich A. Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radic Biol Med. 2006;40:1981–1992. doi: 10.1016/j.freeradbiomed.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 155.Suzuki Y, Fukuda K. Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch Toxicol. 1990;64:169–176. doi: 10.1007/BF02010721. [DOI] [PubMed] [Google Scholar]
- 156.Standeven AM, Wetterhahn KE. Ascorbate is the principal reductant of chromium (VI) in rat liver and kidney ultrafiltrates. Carcinogenesis. 1991;12:1733–1737. doi: 10.1093/carcin/12.9.1733. [DOI] [PubMed] [Google Scholar]
- 157.Standeven AM, Wetterhahn KE. Ascorbate is the principal reductant of chromium(VI) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis. 1992;13:1319–1324. doi: 10.1093/carcin/13.8.1319. [DOI] [PubMed] [Google Scholar]
- 158.Quievryn G, Peterson E, Messer J, Zhitkovich A. Genotoxicity and mutagenicity of chromium(VI)/ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry. 2003;42:1062–1070. doi: 10.1021/bi0271547. [DOI] [PubMed] [Google Scholar]
- 159.Quievryn G, Messer J, Zhitkovich A. Carcinogenic chromium(VI) induces cross-linking of vitamin C to DNA in vitro and in human lung A549 cells. Biochemistry. 2002;41:3156–3167. doi: 10.1021/bi011942z. [DOI] [PubMed] [Google Scholar]
- 160.Slade R, Stead AG, Graham JA, Hatch GE. Comparison of lung antioxidant levels in humans and laboratory animals. Am Rev Respir Dis. 1985;131:742–746. doi: 10.1164/arrd.1985.131.5.742. [DOI] [PubMed] [Google Scholar]
- 161.Martin BD, Schoenhard JA, Hwang JM, Sugden KD. Ascorbate is a pro-oxidant in chromium-treated human lung cells. Mutat Res. 2006;610:74–84. doi: 10.1016/j.mrgentox.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 162.Dillon CT, Lay PA, Cholewa M, Legge GJ, Bonin AM, Collins TJ, Kostka KL, Shea-McCarthy G. Microprobe X-ray absorption spectroscopic determination of the oxidation state of intracellular chromium following exposure of V79 Chinese hamster lung cells to genotoxic chromium complexes. Chem Res Toxicol. 1997;10:533–535. doi: 10.1021/tx970010m. [DOI] [PubMed] [Google Scholar]
- 163.Ortega R, Fayard B, Salome M, Deves G, Susini J. Chromium oxidation state imaging in mammalian cells exposed in vitro to soluble or particulate chromate compounds. Chem Res Toxicol. 2005;18:1512–1519. doi: 10.1021/tx049735y. [DOI] [PubMed] [Google Scholar]
- 164.Levina A, Harris HH, Lay PA. X-ray absorption and EPR spectroscopic studies of the biotransformations of chromium(VI) in mammalian cells. Is chromodulin an artifact of isolation methods? J Am Chem Soc. 2007;129:1065–1075. doi: 10.1021/ja063792r. [DOI] [PubMed] [Google Scholar]
- 165.Bose RN, Moghaddas S, Gelerinter E. Long-lived chromium(IV), chromium(V) metabolites in the chromium(VI)-glutathione reaction: NMR, ESR, HPLC, and kinetic characterization. Inorg Chem. 1992;31:1987–1994. [Google Scholar]
- 166.Stearns DM, Wetterhahn KE. Reaction of Cr(VI) with ascorbate produces chromium(V), chromium(IV), and carbon-based radicals. Chem Res Toxicol. 1994;7:219–230. doi: 10.1021/tx00038a016. [DOI] [PubMed] [Google Scholar]
- 167.Lay PA, Levina A. Activation of molecular oxygen during the reactions of chromium(VI/V/IV) with biological reductants: implications for chromium-induced genotoxicities. J Am Chem Soc. 1998;120:6704–6714. [Google Scholar]
- 168.Goodgame DML, Joy MA. EPR study of the Cr(V) and radical species produced in the reduction of Cr(VI) by ascorbate. Inorg Chem Acta. 1987;135:115–118. [Google Scholar]
- 169.Quievryn G, Goulart M, Messer J, Zhitkovich A. Reduction of Cr(VI) by cysteine: significance in human lymphocytes and formation of DNA damage in reactions with variable reduction rates. Mol Cell Biochem. 2001;222:107–118. [PubMed] [Google Scholar]
- 170.Borges KM, Boswell JS, Liebross RH, Wetterhahn KE. Activation of chromium(VI) by thiols results in chromium(V) formation, chromium binding to DNA and altered DNA conformation. Carcinogenesis. 1991;12:551–561. doi: 10.1093/carcin/12.4.551. [DOI] [PubMed] [Google Scholar]
- 171.O’Brien P, Wang G, Wyatt PB. Studies on the kinetics of the reduction of chromate by glutathione and related thiols. Polyhedron. 1992;11:3211–3216. [Google Scholar]
- 172.Levina A, Zhang L, Lay PA. Structure and reactivity of a chromium(v) glutathione complex. Inorg Chem. 2003;42:767–784. doi: 10.1021/ic020621o. [DOI] [PubMed] [Google Scholar]
- 173.Cieslak-Golonka M, Raczko M, Staszak Z. Synthesis, spectroscopic and magnetic studies of chromium(III) complexes isolated from in vitro reduction of the chromium(VI) ion with the main cellular reductants ascorbic acid, cysteine and glutathione. Tetrahedron. 1992;19:2549–2555. [Google Scholar]
- 174.Cieslak-Golonka M. Toxic and mutagenic effects of chromium(VI). A review. Polyhedron. 1995;15:3667–3689. [Google Scholar]
- 175.Zhitkovich A, Song Y, Quievryn G, Voitkun V. Non-oxidative mechanisms are responsible for the induction of mutagenesis by reduction of Cr(VI) with cysteine: role of ternary DNA adducts in Cr(III)-dependent mutagenesis. Biochemistry. 2001;40:549–560. doi: 10.1021/bi0015459. [DOI] [PubMed] [Google Scholar]
- 176.Zhitkovich A, Messer J, Shrager S. Reductive metabolism of Cr(VI) by cysteine leads to the formation of binary and ternary Cr-DNA adducts in the absence of oxidative DNA damage. Chem Res Toxicol. 2000;13:1114–1124. doi: 10.1021/tx0001169. [DOI] [PubMed] [Google Scholar]
- 177.Voitkun V, Zhitkovich A, Costa M. Cr(III)-mediated crosslinks of glutathione or amino acids to the DNA phosphate backbone are mutagenic in human cells. Nucleic Acids Res. 1998;26:2024–2030. doi: 10.1093/nar/26.8.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Voitkun V, Zhitkovich A, Costa M. Complexing of amino acids to DNA by chromate in intact cells. Environ Health Persp. 1994;102:251–255. doi: 10.1289/ehp.94102s3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhitkovich A, Voitkun V, Costa M. Glutathione and free amino acids form stable adducts with DNA following exposure of intact mammalian cells to chromate. Carcinogenesis. 1995;16:907–913. doi: 10.1093/carcin/16.4.907. [DOI] [PubMed] [Google Scholar]
- 180.Zhitkovich A, Voitkun V, Costa M. Formation of the amino acid–DNA complexes by hexavalent and trivalent chromium in vitro: importance of trivalent chromium and the phosphate group. Biochemistry. 1996;35:7275–7282. doi: 10.1021/bi960147w. [DOI] [PubMed] [Google Scholar]
- 181.Molyneux MJ, Davies MJ. Direct evidence for hydroxyl radical-induced damage to nucleic acids by chromium(VI)-derived species: implications for chromium carcinogenesis. Carcinogenesis. 1995;16:875–882. doi: 10.1093/carcin/16.4.875. [DOI] [PubMed] [Google Scholar]
- 182.Levina A, Lay PA, Dixon NE. Disproportionation of a model chromium(V) complex causes extensive chromium(III)-DNA binding in vitro. Chem Res Toxicol. 2001;14:946–950. doi: 10.1021/tx010077g. [DOI] [PubMed] [Google Scholar]
- 183.Montrel’ MM, Shabarchina LI, Pletneva TV, ErshovIu A. IR-spectroscopic study of the interaction of chromium salts with native DNA. Biofizika. 1993;38:636–643. [PubMed] [Google Scholar]
- 184.Reynolds M, Peterson E, Quievryn G, Zhitkovich A. Human nucleotide excision repair efficiently removes chromium-DNA phosphate adducts and protects cells against chromate toxicity. J Biol Chem. 2004;279:30419–30424. doi: 10.1074/jbc.M402486200. [DOI] [PubMed] [Google Scholar]
- 185.O’Brien TJ, Brooks BR, Patierno SR. Nucleotide excision repair functions in the removal of chromium-induced DNA damage in mammalian cells. Mol Cell Biochem. 2005;279:85–95. doi: 10.1007/s11010-005-8225-0. [DOI] [PubMed] [Google Scholar]
- 186.Arakawa H, Wu F, Costa M, Rom W, Tang MS. Sequence specificity of Cr(III)-DNA adduct formation in the p53 gene: NGG sequences are preferential adduct-forming sites. Carcinogenesis. 2006;27:639–645. doi: 10.1093/carcin/bgi249. [DOI] [PubMed] [Google Scholar]
- 187.Blankert SA, Coryell VH, Picard BT, Wolf KK, Lomas RE, Stearns DM. Characterization of nonmutagenic Cr(III)-DNA interactions. Chem Res Toxicol. 2003;16:847–854. doi: 10.1021/tx034007g. [DOI] [PubMed] [Google Scholar]
- 188.Costa M. DNA-protein complexes induced by chromate and other carcinogens. Environ Health Perspect. 1991;92:45–52. doi: 10.1289/ehp.919245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhitkovich A, Voitkun V, Kluz T, Costa M. Utilization of DNA-protein crosslinks as a biomarker of chromium exposure. Environ Health Persp. 1998;106:969–974. doi: 10.1289/ehp.98106s4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Salnikow K, Zhitkovich A, Costa M. Analysis of the binding sites of chromium to DNA and protein in vitro and in intact cells. Carcinogenesis. 1992;13:2341–2346. doi: 10.1093/carcin/13.12.2341. [DOI] [PubMed] [Google Scholar]
- 191.Costa M, Zhitkovich A, Toniolo P. DNA-protein crosslinks in welders: molecular implications. Cancer Res. 1993;53:460–463. [PubMed] [Google Scholar]
- 192.Taioli E, Kinney P, Zhitkovich A, Fulton H, Voitkun V, Cosma G, Frenkel K, Toniolo P, Garte S, Costa M. Application of reliability models to studies of biomarker validation. Environ Health Persp. 1994;102:306–309. doi: 10.1289/ehp.94102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kuykendall JR, Miller KL, Mellinger KN, Cain AV. Waterborne and dietary hexavalent chromium exposure causes DNA-protein crosslink (DPX) formation in erythrocytes of largemouth bass (Micropterus salmoides) Aquat Toxicol. 2006;78:27–31. doi: 10.1016/j.aquatox.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 194.Bridgewater LC, Manning FC, Patierno SR. Base-specific arrest of in vitro DNA replication by carcinogenic chromium: relationship to DNA interstrand crosslinking. Carcinogenesis. 1994;15:2421–2427. doi: 10.1093/carcin/15.11.2421. [DOI] [PubMed] [Google Scholar]
- 195.O’Brien T, Mandel HG, Pritchard DE, Patierno SR. Critical role of chromium (Cr)-DNA interactions in the formation of Cr-induced polymerase arresting lesions. Biochemistry. 2002;41:12529–12537. doi: 10.1021/bi020452j. [DOI] [PubMed] [Google Scholar]
- 196.Sugiyama M, Wang XW, Costa M. Comparison of DNA lesions and cytotoxicity induced by calcium chromate in human, mouse, and hamster cell lines. Cancer Res. 1986;46:4547–4551. [PubMed] [Google Scholar]
- 197.Gao M, Binks SP, Chipman JK, Levy LS, Braithwaite RA, Brown SS. Induction of DNA strand breaks in peripheral lymphocytes by soluble chromium compounds. Hum Exp Toxicol. 1992;11:77–82. doi: 10.1177/096032719201100203. [DOI] [PubMed] [Google Scholar]
- 198.Hodges NJ, Adam B, Lee AJ, Cross HJ, Chipman JK. Induction of DNA-strand breaks in human peripheral blood lymphocytes and A549 lung cells by sodium dichromate: association with 8-oxo-2-deoxyguanosine formation and inter-individual variability. Mutagenesis. 2001;16:467–474. doi: 10.1093/mutage/16.6.467. [DOI] [PubMed] [Google Scholar]
- 199.Ueno S, Kashimoto T, Susa N, Furukawa Y, Ishii M, Yokoi K, Yasuno M, Sasaki YF, Ueda J, Nishimura Y, Sugiyama M. Detection of dichromate(VI)-induced DNA strand breaks and formation of paramagnetic chromium in multiple mouse organs. Toxicol Appl Pharmacol. 2001;170:56–62. doi: 10.1006/taap.2000.9081. [DOI] [PubMed] [Google Scholar]
- 200.Abbondandolo A, Dogliotti E, Lohman PH, Berends F. Molecular dosimetry of DNA damage caused by alkylation. I Single-strand breaks induced by ethylating agents in cultured mammalian cells in relation to survival. Mutat Res. 1982;92:361–377. doi: 10.1016/0027-5107(82)90236-6. [DOI] [PubMed] [Google Scholar]
- 201.Casadevall M, Kortenkamp A. The formation of both apurinic/apyrimidinic sites and single-strand breaks by chromate and glutathione arises from attack by the same single reactive species and is dependent on molecular oxygen. Carcinogenesis. 1995;16:805–809. doi: 10.1093/carcin/16.4.805. [DOI] [PubMed] [Google Scholar]
- 202.Shi XG, Sun XL, Gannett PM, Dalal NS. Deferoxamine inhibition of Cr(V)-mediated radical generation and deoxyguanine hydroxylation: ESR and HPLC evidence. Arch Biochem Biophys. 1992;293:281–286. doi: 10.1016/0003-9861(92)90396-e. [DOI] [PubMed] [Google Scholar]
- 203.Quievryn G, Messer J, Zhitkovich A. Lower mutagenicity but higher stability of Cr-DNA adducts formed during gradual chromate activation with ascorbate. Carcinogenesis. 2006;27:2316–2321. doi: 10.1093/carcin/bgl076. [DOI] [PubMed] [Google Scholar]
- 204.Sen P, Conway K, Costa M. Comparison of the localization of chromosome damage induced by calcium chromate and nickel compounds. Cancer Res. 1987;47:2142–2147. [PubMed] [Google Scholar]
- 205.Wise JP, Leonard JC, Patierno SR. Clastogenicity of lead chromate particles in hamster and human cells. Mutat Res. 1992;278:69–79. doi: 10.1016/0165-1218(92)90287-a. [DOI] [PubMed] [Google Scholar]
- 206.Witt KL, Knapton A, Wehr CM, Hook GJ, Mirsalis J, Shelby MD, MacGregor JT. Micronucleated erythrocyte frequency in peripheral blood of B6C3F(1) mice from short-term, prechronic, and chronic studies of the NTP carcinogenesis bioassay program. Environ Mol Mutagen. 2000;36:163–194. [PubMed] [Google Scholar]
- 207.Ha L, Ceryak S, Patierno SR. Generation of S phase-dependent DNA double-strand breaks by Cr(VI) exposure: involvement of ATM in Cr(VI) induction of gamma-H2AX. Carcinogenesis. 2004;25:2265–2274. doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- 208.Peterson-Roth E, Reynolds M, Quievryn G, Zhitkovich A. Mismatch repair proteins are activators of toxic responses to chromium-DNA damage. Mol Cell Biol. 2005;25:3596–3607. doi: 10.1128/MCB.25.9.3596-3607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Casadevall M, Kortenkamp A. The generation of apurinic/apyrimidinic sites in isolated DNA during the reduction of chromate by glutathione. Carcinogenesis. 1994;15:407–409. doi: 10.1093/carcin/15.2.407. [DOI] [PubMed] [Google Scholar]
- 210.Yuann JM, Liu KJ, Hamilton JW, Wetterhahn KE. In vivo effects of ascorbate and glutathione on the uptake of chromium, formation of chromium(V), chromium-DNA binding and 8-hydroxy-2′-deoxyguanosine in liver and kidney of osteogenic disorder shionogi rats following treatment with chromium(VI) Carcinogenesis. 1999;20:1267–75. doi: 10.1093/carcin/20.7.1267. [DOI] [PubMed] [Google Scholar]
- 211.Casadevall M, da Cruz Fresco P, Kortenkamp A. Chromium(VI)-mediated DNA damage: oxidative pathways resulting in the formation of DNA breaks and abasic sites. Chem Biol Interact. 1999;123:117–132. doi: 10.1016/s0009-2797(99)00128-3. [DOI] [PubMed] [Google Scholar]
- 212.Slade PG, Hailer MK, Martin BD, Sugden KD. Guanine-specific oxidation of double-stranded DNA by Cr(VI) and ascorbic acid forms spiroiminodihydantoin and 8-oxo-2′-deoxyguanosine. Chem Res Toxicol. 2005;18:1140–1149. doi: 10.1021/tx050033y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kondo K, Hino N, Sasa M, Kamamura Y, Sakiyama S, Tsuyuguchi M, Hashimoto M, Uyama T, Monden Y. Mutations of the p53 gene in human lung cancer from chromate-exposed workers. Biochem Biophys Res Commun. 1997;239:95–100. doi: 10.1006/bbrc.1997.7425. [DOI] [PubMed] [Google Scholar]
- 214.Chen J, Thilly WG. Mutational spectrum of chromium(VI) in human cells. Mutat Res. 1994;323:21–27. doi: 10.1016/0165-7992(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 215.Cheng L, Sonntag DM, de Boer J, Dixon K. Chromium(VI)-induced mutagenesis in the lungs of big blue transgenic mice. J Environ Pathol Toxicol Oncol. 2000;19:239–249. [PubMed] [Google Scholar]
- 216.Hirose T, Kondo K, Takahashi Y, Ishikura H, Fujino H, Tsuyuguchi M, Hashimoto M, Yokose T, Mukai K, Kodama T, Monden Y. Frequent microsatellite instability in lung cancer from chromate-exposed workers. Mol Carcinog. 2002;33:172–180. doi: 10.1002/mc.10035. [DOI] [PubMed] [Google Scholar]
- 217.Kunkel TA, Erie DA. DNA mismatch re pair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 218.Modrich P. Mechanisms in eukaryotic mismatch repair. J Biol Chem. 2006;281:30305–30309. doi: 10.1074/jbc.R600022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Takahashi Y, Kondo K, Hirose T, Nakagawa H, Tsuyuguchi M, Hashimoto M, Sano T, Ochiai A, Monden Y. Microsatellite instability and protein expression of the DNA mismatch repair gene, hMLH1, of lung cancer in chromate-exposed workers. Mol Carcinog. 2005;42:150–158. doi: 10.1002/mc.20073. [DOI] [PubMed] [Google Scholar]
- 220.Glaviano A, Nayak V, Cabuy E, Baird DM, Yin Z, Newson R, Ladon D, Rubio MA, Slijepcevic P, Lyng F, Mothersill C, Case CP. Effects on hTERT on metal ion-induced genomic instability. Oncogene. 2006;25:3424–3435. doi: 10.1038/sj.onc.1209399. [DOI] [PubMed] [Google Scholar]
- 221.Zhitkovich A, Peterson -Roth E, Reynolds M. Killing of chromium-damaged cells by mismatch repair and its relevance to carcinogenesis. Cell Cycle. 2005;4:1050–1052. [PubMed] [Google Scholar]
- 222.Davidson T, Kluz T, Burns F, Rossman T, Zhang Q, Uddin A, Nadas A, Costa M. Exposure to chromium (VI) in the drinking water increases susceptibility to UV-induced skin tumors in hairless mice. Toxicol Appl Pharmacol. 2004;196:431–437. doi: 10.1016/j.taap.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 223.Uddin AN, Burns FJ, Rossman TG, Chen H, Kluz T, Costa M. Dietary chromium and nickel enhance UV-carcinogenesis in skin of hairless mice. Toxicol Appl Pharmacol. 2007;221:329–338. doi: 10.1016/j.taap.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 224.Uddin AN, Burns FJ, Rossman TG. Vitamin E and organoselenium prevent the cocarcinogenic activity of arsenite with solar UVR in mouse skin. Carcinogenesis. 2005;26:2179–2186. doi: 10.1093/carcin/bgi180. [DOI] [PubMed] [Google Scholar]
- 225.Cleaver JE. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nature Rev Cancer. 2005;5:564–573. doi: 10.1038/nrc1652. [DOI] [PubMed] [Google Scholar]
- 226.Hartwig A, Beyersmann D. Comutagenicity and inhibition of DNA repair by metal ions in mammalian cells. Biol Trace Elem Res. 1989;21:359–365. doi: 10.1007/BF02917276. [DOI] [PubMed] [Google Scholar]
- 227.Friedberg EC. How nucleotide excision repair protects against cancer. Nat Rev Cancer. 2001;1:22–33. doi: 10.1038/35094000. [DOI] [PubMed] [Google Scholar]
- 228.Feng Z, Hu W, Rom WN, Costa M, Tang MS. Chromium(VI) exposure enhances polycyclic aromatic hydrocarbon-DNA binding at the p53 gene in human lung cells. Carcinogenesis. 2003;24:771–778. doi: 10.1093/carcin/bgg012. [DOI] [PubMed] [Google Scholar]
- 229.Hu W, Feng Z, Tang MS. Chromium(VI) enhances (+/−)-anti-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene-induced cytotoxicity and mutagenicity in mammalian cells through its inhibitory effect on nucleotide excision repair. Biochemistry. 2004;43:14282–14289. doi: 10.1021/bi048560o. [DOI] [PubMed] [Google Scholar]
- 230.Quievryn G, Zhitkovich A. Loss of DNA-protein crosslinks from formaldehyde-exposed cells occurs through spontaneous hydrolysis and an active repair process linked to proteasome function. Carcinogenesis. 2000;21:1573–1580. [PubMed] [Google Scholar]
- 231.Mijal RS, Thomson NM, Fleischer NL, Pauly GT, Moschel RC, Kanugula S, Fang Q, Pegg AE, Peterson LA. The repair of the tobacco specific nitrosamine derived adduct O6-[4-Oxo-4-(3-pyridyl)butyl]guanine by O6-alkylguanine-DNA alkyltransferase variants. Chem Res Toxicol. 2004;17:424–434. doi: 10.1021/tx0342417. [DOI] [PubMed] [Google Scholar]
- 232.Pfeifer GP, Denissenko MF, Olivier M, Tre tyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]