Abstract
Objectives
In previous work, we have demonstrated that delivery of low concentrations (250 ppm) of carbon monoxide (CO) via inhalation to donor and/or recipients protects transplanted lungs from ischemia-reperfusion injury (IRI) (improved gas exchange, diminished intragraft and systemic inflammation and retention of graft vascular endothelial cell ultrastructure). In this study we examined whether delivery of CO to lung grafts in the preservation solution could protect against lung IRI.
Methods
Orthotopic left lung transplantation was performed in syngeneic Lewis to Lewis rats. Grafts were preserved in UW solution with (CO-UW) or without (control UW) CO at 4°C for 6 hours. CO gas (5% or 100%) was bubbled into UW solution at 4°C for 5 minutes before use.
Results
In controls, IR injury resulted in significant deterioration of graft function and was associated with a massive cellular infiltrate 2 hours after reperfusion. Grafts stored in CO-UW (5%), however, demonstrated significantly better gas exchange and significantly reduced intragraft inflammation (reduced inflammatory mediators and cellular infiltrate). Experiments demonstrated that the protective effects afforded by 100% CO-UW were not as potent as those of 5% CO-UW.
Conclusions
This study demonstrates that 5% CO as an additive to the cold flush/preservation solution can impart potent anti-inflammatory and cytoprotective effects following cold preservation and transplantation of lung grafts. Such ex vivo treatment of lung grafts with CO can minimize concerns associated with CO inhalation and may offer opportunity to significantly advance the application of CO in the clinical setting.
Keywords: lung transplantation, ischemia reperfusion injury, cold preservation, carbon monoxide
INTRODUCTION
Lung transplantation (LTx) outcomes have improved significantly over the last two decades and, as a result, lung transplantation is a viable option for many patients with end-stage lung disease. However, while 1-year lung transplant survival rates have significantly improved to >80%, 5-year survival rates remain poor and both short and long term outcomes lag significantly below those of most other solid organ transplants (OPTN; organ procurement and transplantation network).
During the procurement, transport and transplantation process, donor lung grafts are exposed to an obligatory period of cold ischemia with subsequent warm reperfusion, leading to graft injury [i.e. ischemia-reperfusion injury (IRI)]. IRI is recognized as a major determinant of primary graft dysfunction (PGD), which is a major complication in clinical LTx and contributes significantly to early morbidity and mortality.1, 2 Furthermore, the severity of IRI (and PGD) have been associated with the risk of developing bronchiolitis obliterans syndrome (BOS) years later, suggesting that the injury to lung grafts at the time of transplant determines the fate of lung grafts or may increase susceptibility to other events that contribute to subsequent graft dysfunction.3-5 Thus, it is apparent that therapeutic strategies to mitigate IRI of lung allografts could have significant clinical benefit. In addition, the impact of IRI-mitigating strategies could be most profound in the setting of utilization of marginal donors (and perhaps donation after cardiac death donors). There has been an increased reliance on marginal donors to meet the growing demand for organ transplantation and these marginal organs are more susceptible to cold IRI.6, 7
A number of therapeutic modalities have been attempted to attenuate IRI following organ transplantation in both experimental and clinical studies.8, 9 Among these potential therapeutic options, the application of carbon monoxide (CO), a byproduct of the heme oxygenase (HO) system, might be one of the most promising approaches.10 Indeed, we have shown that CO delivered via inhalation at low concentrations to donors and/or recipients mitigates lung IRI associated with lung transplantation in rats.11, 12 In these studies, CO demonstrated potent anti-inflammatory and cytoprotective effects resulting in retention of lung endothelial ultrastructure and improved graft function. Translating this strategy to the clinic may be met with significant hurdles, however, given logistical issues as well as concerns about exposure of healthcare workers to CO during prolonged inhalational therapy as well as concerns regarding the adverse effects of formation of carboxyhemoglobin in recipients. Limiting CO exposure to the graft during the cold ischemic period via saturation of the perfusion and storage solution might be a more clinically applicable strategy and would minimize risk. In this study, we tested the hypothesis that delivery of CO to lung grafts via saturation of the preservation solution used for flush and cold storage would prevent lung IRI. We demonstrate that flushing and storing lung allografts in preservation solution bubbled with CO significantly protects lung grafts from IRI and suggest that this approach may advance the application of CO therapy in the clinical setting of lung transplantation.
MATERIALS AND METHODS
Animals
Inbred male Lewis (LEW, RT11) rats weighing 250 to 300 g were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and maintained in laminar flow cages in a specific pathogen-free animal facility at the University of Pittsburgh. Animals were fed a standard diet and provided water ad libitum. All procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh and the National Research Council’s Guide for the Humane Care and Use of Laboratory Animals.
CO Supplementation of Preservation Solution
For each experiment, a total of 50 mL of University of Wisconsin (UW) solution (Viaspan®; Du Pont, Wilmington, DE) was vigorously bubbled at 4°C for 5 min before use with compressed CO gas mixed in air (5% or 100%, PRAXAIR, Danbury, CT) under sterile condition in a fume hood.13, 14
Rat Lung Transplantation (LTx)
Orthotopic left lung transplantation was performed in LEW recipients from LEW donors utilizing a cuff technique as previously described.11, 12, 15 Briefly, donor rats underwent tracheotomy and were mechanically ventilated with a mixture of 100% oxygen and isoflurane, positive end expiratory pressure (PEEP) of 2 cm H2O, tidal volume of 10 mL/kg and respiratory rate of 70 breaths/minute. Donor animals were heparinized (300 units) and a laparosternotomy was performed. The entire lung in the donor was isolated and excised. Lung grafts were flushed through the main pulmonary artery (20 mL, 20 cmH2O) and stored at 4°C for 6 h with either control UW solution or 5% or 100% CO-bubbled UW solution (CO-UW) in an inflated state with 100% oxygen. Recipient animals were intubated orotracheally and were ventilated on the same settings as donors. A left thoracotomy was performed and the lung graft was implanted using the cuff technique. Sham operated animals underwent anesthesia and a thoracotomy. All recipients survived when they were sacrificed for endpoints.
Experimental Groups
Three groups of transplanted animals and one sham operated group were studied. Animals in group 1 were perfused and stored with control UW (control UW group). In group 2, lung grafts were perfused and stored with 5% CO-UW (5% CO-UW group). In the third group, 100% CO gas was used for bubbling of UW solution (100% CO-UW group).
Lung graft function
The function of lung grafts was assessed by determining the oxygen partial pressure (pO2) (iSTAT Portable Clinical Analyzer; iSTAT Corporation, East Windsor, NJ) of blood drawn from the pulmonary vein of the transplanted lung on a FiO2 of 1.0 while on mechanical ventilation. With this technique, the resultant pO2 is a reflection of only graft gas exchange. (Sham; n=6, control UW; n=7, 5%CO-UW; n=6, 100%CO-UW; n=5)
Stain for infiltrating neutrophils and ED1 positive macrophages
Lung graft tissues procured 2 hours after reperfusion were fixed in 10% formalin, embedded in paraffin and sectioned into 6 μm thickness. Neutrophils in transplanted and contralateral (native) lungs were stained using a naphthol AS-D chloroacetate esterase staining kit (Sigma Diagnostics, St. Louis, MO). The macrophages were stained by immunohistochemistry for ED1 as described previously.11 Positively stained cells were counted in a blinded fashion in 20 high-power fields (400x) per section and expressed as the number of cells per 0.1 mm2. (n=5 for each group)
SYBR Green Real-Time RT-PCR
The effect of CO on IRI-induced proinflammatory gene expression was assessed by SYBR green real time RT-PCR. The time point was determined based on our previous observation that maximum inflammatory mediator expression occurs 2 hours following reperfusion.12 Total RNA was extracted from the lung graft taken 2 hours after reperfusion using the TRIzol reagent (Life Technologies, Inc., Grand Island, NY) according to the manufacturer’s instruction. The mRNA for interleukin (IL)-1β, IL-6, tumor necrosis factor-alpha (TNF-α), inducible nitric oxide synthase (iNOS), cycloxygenase (COX)-2, intracellular adhesion molecule (ICAM)-1 and glyceraldehydes 3-phosphate dehydrogenase (GAPDH) were quantified in duplicate using SYBR Green two-step, real-time RT-PCR, as previously described 16. After removal of potentially contaminating DNA with DNase I (Life Technologies), 3 μg of total RNA from each sample was used for reverse transcription with oligo dT (Life Technologies) and Superscript II (Life Technologies) to generate first-strand cDNA. The PCR reaction mixture was prepared using SYBR green PCR Master Mix (PE Applied Biosystems, Foster City, CA), with the primers being designed according to the literature or published sequences. Thermal cycling conditions were 10 minutes at 95°C to activate the Amplitaq Gold DNA polymerase followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute on an ABI PRISM 7000 Sequence Detection System (PE Applied Biosystems). Expression of each gene was normalized with GAPDH mRNA content. (Sham; n=6, control UW; n=7, 5%CO-UW; n=6, 100%CO-UW; n=5)
Data Analysis
Results are expressed as mean ± SEM. Statistical analysis was performed using the Student’s t-test or analysis of variance where appropriate. A probability level of P<0.05 was considered statistically significant.
RESULTS
Dose dependent solubility of CO in UW solution
The solubility of CO in UW solution at 4°C utilizing various CO concentrations was measured with simplified gas-chromatography using TRI lyzer.13, 14 Bubbling preservation solution with 5% CO for 5 minutes resulted in a CO concentration of 39.1 ± 2.4 μM in solution. The soluble CO concentration increased in a dose dependent manner after bubbling with 50 % or 100 % CO (578.7 ± 45.5 μM and 1025.3 ± 78.9 μM CO, respectively) (Figure. 1A). After bubbling with 100% CO for 5 minutes, the CO concentration in solution was maintained during the cold storage period when the air-phase was removed from the container. In contrast, when UW with CO was allowed to contact air, soluble CO in UW solution was quickly released to the air, and CO levels returned to the basal level within a few hours (Figure 1B). These results demonstrate that once CO is bubbled into the preservation solution, the CO-bubbled solution must be kept in the tightly sealed container with a secured lid without an air layer to maintain a stable CO concentration in solution. Subsequent experiments were performed in this fashion.
Figure 1.
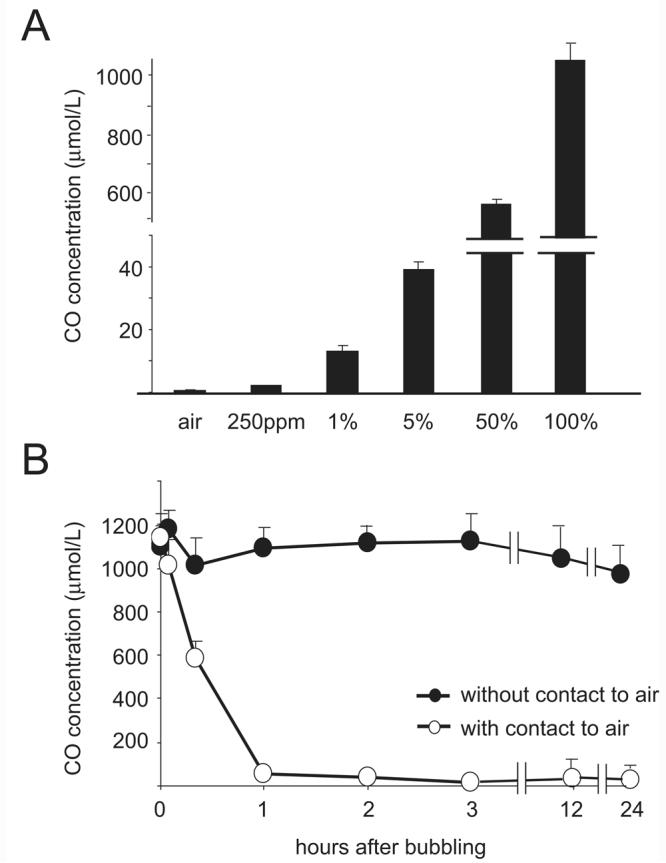
(A) The solubility of CO in UW solution at 4°C after 5 minutes bubbling with different concentrations of CO gas. (n=3 for each concentration) CO solubility rises in a dose dependent manner. (B) Sequential analysis of CO content in UW solution after 5 minutes bubbling with 100% CO gas with or without secured, air-tight lids. CO levels were maintained as long as 24 hours when the container of UW solution was sealed without air-phase (closed circle, n=3 for each time point). However, CO concentrations decreased quickly when CO-bubbled UW was exposed to air. (open circle, n=3)
Flush and storage of lung grafts in 5% CO-UW significantly improves graft function
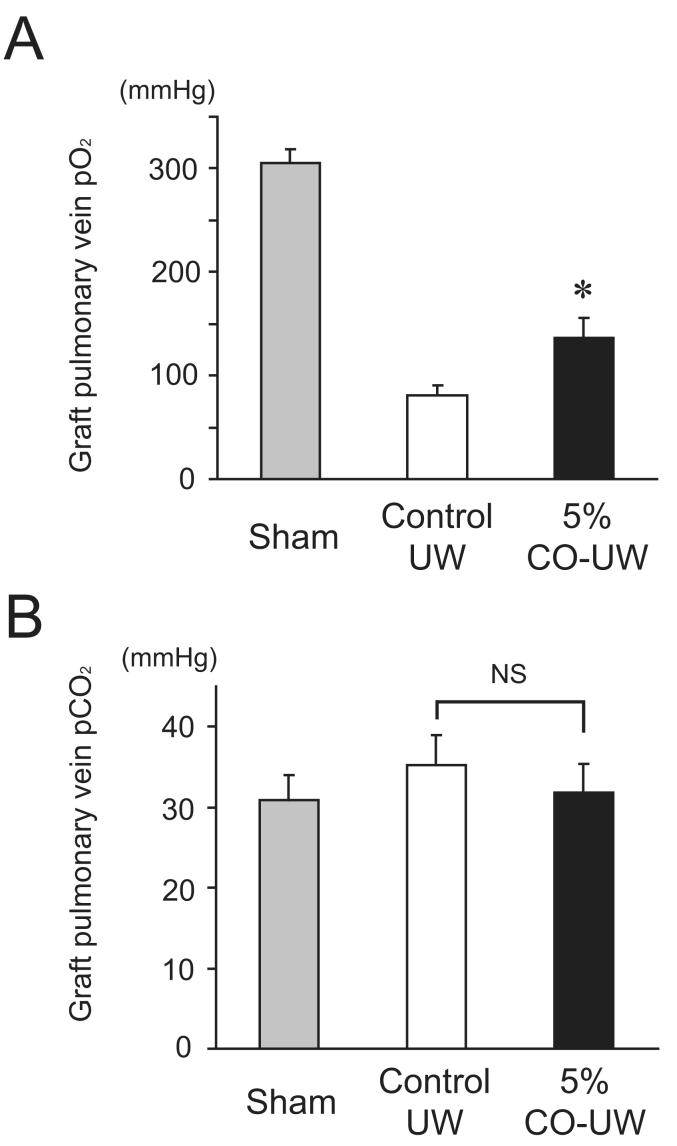
Lung graft function was assessed by determining pO2 levels from the pulmonary vein of grafts at 2 hours after reperfusion. The pO2 values from the graft PV in sham-operated animals were 305.3 ± 13.1 mmHg under mechanical ventilation. Graft function was markedly impaired 2 hours after reperfusion in grafts stored in control UW with pO2 levels that were less than 30% of those in sham operated animals (80.6 ± 9.4 mmHg). Grafts flushed and stored in 5% CO-UW demonstrated significantly improved graft function with graft PV pO2 levels of 146.7 ± 19.8 mmHg (Figure 2A). The pCO2 levels in graft pulmonary vein blood were not significantly different among groups at 2 hours after reperfusion (Sham: 30.9 ± 3.1 mmHg, Control UW group: 35.3 ± 3.6 mmHg, 5% CO-UW group: 31.8 ± 3.5 mmHg) (Figure 2B).
Figure 2.
(A) The pulmonary vein pO2 levels of the sham-operated animals was 305.3 ± 13.1 mmHg. Graft pulmonary vein pO2 of the control UW group was markedly reduced (80.6 ± 9.4 mmHg) at 2 hours after reperfusion. Lung grafts in the 5% CO-UW group showed significantly higher pO2 levels compared to control UW group at 2 hours after reperfusion (5% CO-UW group: 135.9 ± 19.8 mmHg; *p<0.05 vs. control UW group). (B) The pCO2 levels of graft pulmonary veins were not significantly different between groups.
5% CO-UW significantly reduces neutrophil and macrophage infiltration in lung grafts
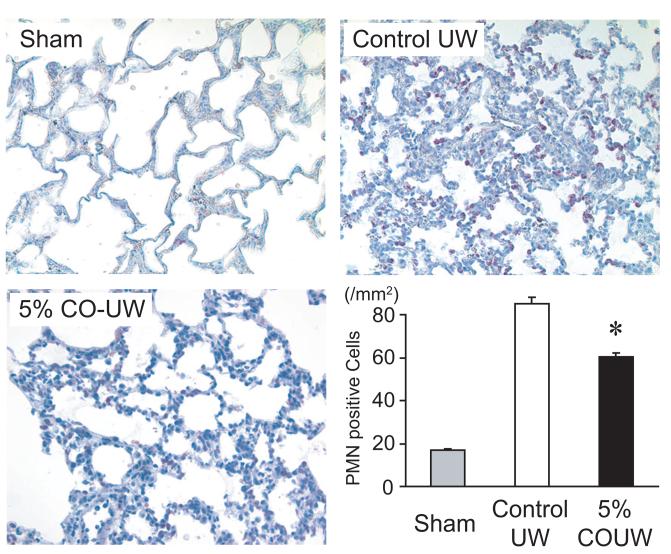
Neutrophil infiltration plays an important role in the inflammatory response of IRI by, among other processes, enhancing graft parenchyma damage by secreting proteolytic enzymes such as elastases17, 18, and physically impairing the microcirculation. Although pulmonary neutrophils were scarce in the sham-operated animals, a number of MPO-positive cells were noted in pulmonary grafts following cold storage in control UW and reperfusion, suggesting that IR injury caused rapid neutrophil infiltration in the grafts. Flush and storage of grafts in CO-UW (5 %) resulted in significantly reduced neutrophil infiltration in the grafts at 2 hours after reperfusion (Figure 3).
Figure 3.
Neutrophil infiltration of lung grafts 2 hours after reperfusion. Prolonged cold storage and reperfusion following transplantation resulted in massive infiltration of MPO positive-neutrophils in control grafts. Flush and storage of lung grafts in UW solution bubbled with 5% CO significantly reduced the number of neutrophils seen in the graft (Original magnification: x400, * p<0.05 vs. control UW).
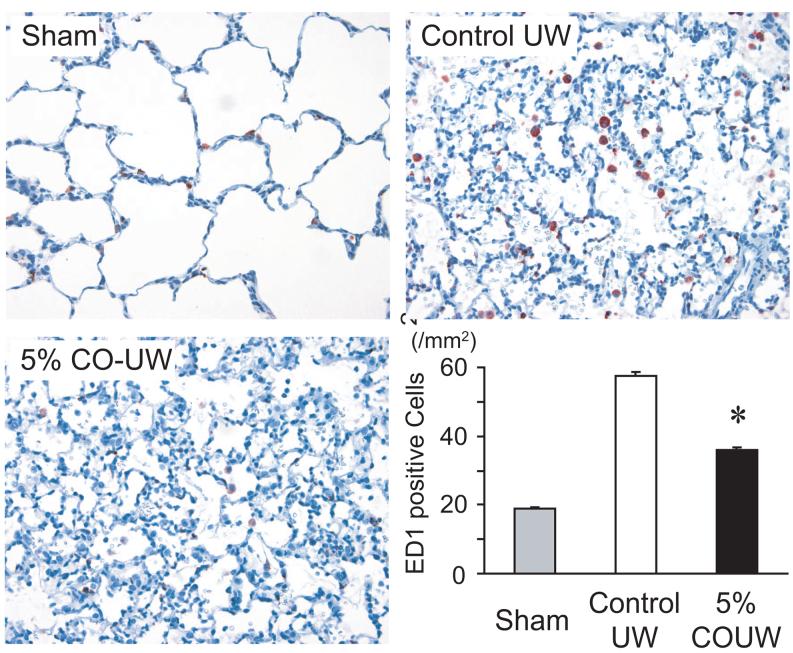
Macrophages activated during the IRI process initiate a cascade of events leading to the activation of the recipient inflammatory system. and play a central role in lung IRI19, 20. The number of ED1+ macrophages in control UW grafts increased to 52.7 ± 1.2 per 0.1 mm2 2 hours after reperfusion from 17.3 ± 0.5 per 0.1 mm2 in sham-operated animals. Lung grafts stored in CO-UW (5%) demonstrated significantly reduced numbers of ED1+ macrophages (32.9 ± 0.8 per 0.1 mm2) (Figure 4).
Figure 4.
ED1+ macrophage recruitment to lung grafts. The number of infiltrating alveolar ED1+ macrophages remarkably increased in control UW at 2 hours after reperfusion. CO-UW (5%) significantly reduced macrophage infiltration (Original magnification: x400, * p<0.05 vs. control UW group).
5% CO-UW significantly reduces expression of mRNA for inflammatory mediators in lung grafts
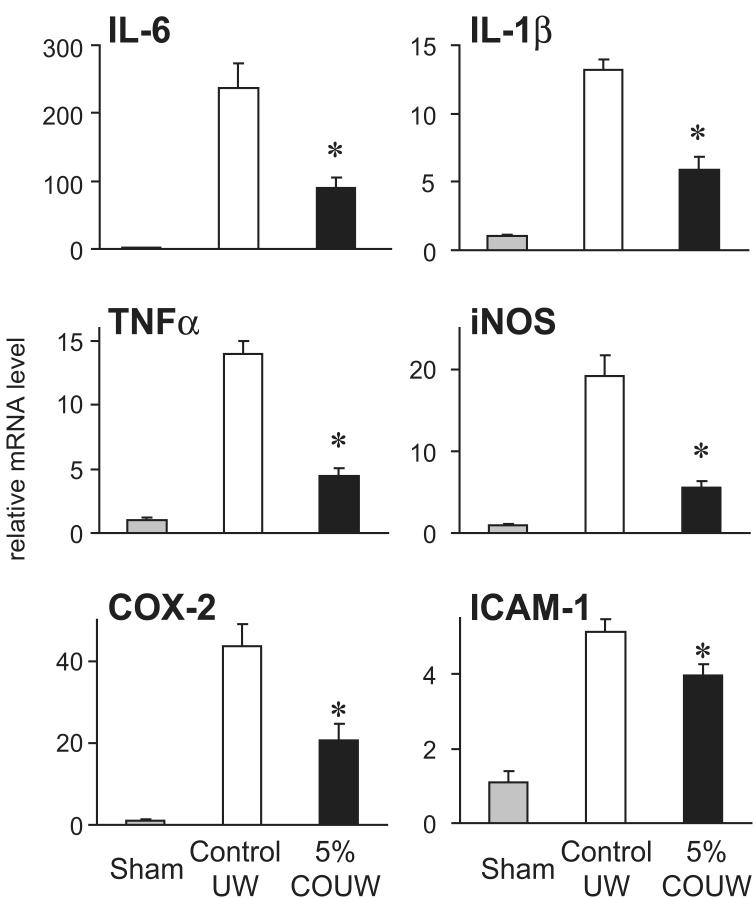
Lung IRI results in the rapid production and release of numerous proinflammatory mediators as well as upregulation of adhesion molecules 21, 22. We evaluated the effect of CO supplementation in the preservation solution on inflammatory mediator expression in lung grafts following IRI using SYBR green real-time RT-PCR. In grafts stored in control UW, mRNA levels for the proinflammatory cytokines IL-6, IL-1β and TNF-α, the stress-induced molecules iNOS and COX-2 and the adhesion molecule ICAM-1 were significantly upregulated within 2 hours following reperfusion. Flush and storage of grafts in 5% CO-UW significantly reduced peak levels of proinflammatory mediator mRNA by 35%-65% compared to the grafts stored in control UW (Figure 5).
Figure 5.
Intragraft mRNA levels of inflammatory mediators. mRNA for the proinflammatory cytokines IL-6, IL-1β and TNF-α, the stress-induced molecules iNOS and COX-2 and the adhesion molecule ICAM-1 were rapidly upregulated within 2 hours after reperfusion in control animals. 5% CO-UW significantly reduced expression of these inflammation-related molecules. (* p<0.05 vs. control UW group)
100% CO-UW does not provide further improvement in graft function following IRI
To evaluate whether treatment with a higher concentration of CO may provide additional graft protection compared to 5% CO, we investigated the effects of supplementation of the preservation solution with 100% CO (resulting in 25 times higher concentration of CO in solution). While flush and storage of grafts with UW solution bubbled with 100% CO improved graft function compared to controls, it did not improve graft function beyond that which was achieved with 5% CO-UW (pO2 levels of graft pulmonary vein stored in 100% CO-UW were 147.8 ± 15.8 mmHg, while those of 5% CO-UW were 146.7 ± 19.8 mmHg) (Figure 6A).
Figure 6.
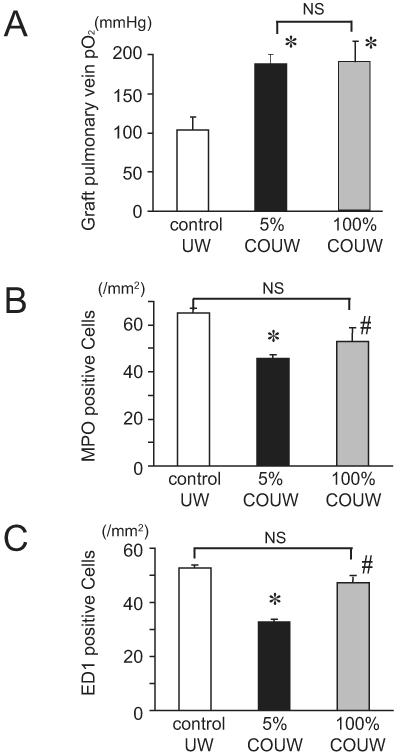
The effects of higher concentration of CO on the pulmonary graft function and cellular infiltration. (A) Graft function, as assessed by graft oxygenation, was no different between grafts flushed and stored with 5% CO-UW compared to 100% CO-UW. Both groups demonstrated improved graft function compared to controls. (B) The number of infiltrating neutrophils was significantly reduced when the grafts were stored in UW with supplementation of 5% CO gas. However, the effects of 100% CO-UW was marginal in preventing neutrophil recruitment (*p<0.05 vs control UW, #p<0.05 vs 5% CO-UW). (C) While 5% CO-UW significantly reduced the number of ED1-positive macrophages in the grafts, 100% CO-UW did not alter macrophage recruitment in the graft lung 2 hours after reperfusion (*p<0.05 vs control UW, #p<0.05 vs 5% CO-UW).
The effects of 100% CO-UW on inflammatory cellular recruitment
Although 5% CO-UW had potent effects on prevention of graft inflammatory cellular infiltration, the number of neurophils in the grafts stored in 100% CO-UW was not different from control UW (Figure 6B). Similarly, the number of ED1 positive macrophages in the grafts stored in 100% CO-UW was 47.1 ± 1.2 per mm2, which was significantly more than those seen in 5% CO-UW (Figure 6C).
Alteration of mRNA levels for inflammatory mediators with 100% CO-UW
The anti-inflammatory effects of 100% CO-UW were also evaluated by determining mRNA expression for inflammatory mediators. As previously demonstrated, 5% CO-UW significantly reduced the peak levels of mRNA expression of inflammatory mediators. The effects of 100% CO-UW were comparable to those of 5% CO-UW in inhibition of iNOS, COX-2 and ICAM-1. In contrast, 100% CO-UW did not reduce mRNA levels of IL-6, IL-1β, and TNF-α compared to those in the grafts stored in control UW (Figure 7).
Figure 7.
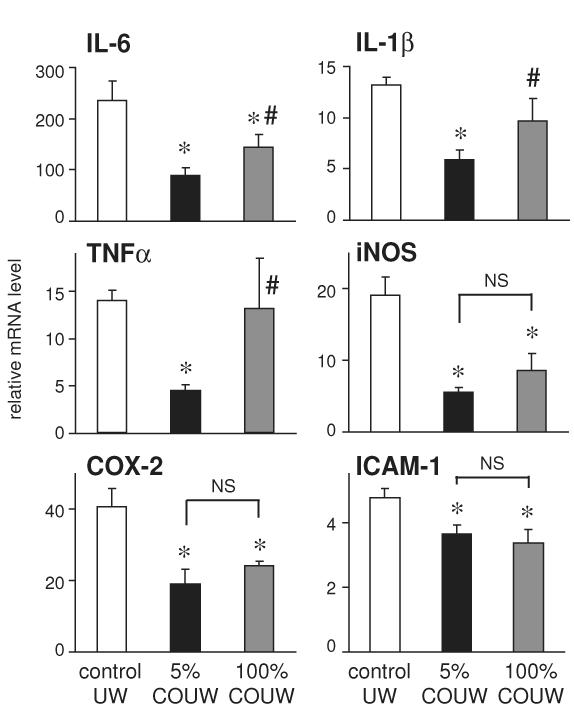
Realtime RT-PCR analysis for inflammatory mediators 2 hours after reperfusion. The upregulation of mRNA for inflammatory mediators were significantly inhibited when lung grafts were perfused and stored in UW solution with 5% CO. The effects of 100% CO had comparable effects in preventing IL-6, iNOS, COX-2 and ICAM-1 mRNA upregulation, however, the anti-inflammatory effects 100% CO was marginal in TNFα and IL-1β mRNA levels (*p<0.05 vs control UW, #p<0.05 vs 5% CO-UW).
DISCUSSION
CO has been increasingly recognized as a pleiotropic molecule with many properties including a role in fundamental cell signaling processes as well as cytoprotective and anti-inflammatory properties that mitigate IR injury. While basal low levels of CO are produced endogenously in mammalian tissues through the action of the enzyme heme oxygenase (HO) on heme, in the setting oxidative stress of transplantation (as well as other scenarios), numerous strategies have been employed to manipulate this system to mitigate IRI in various organ types. The use of chemical HO inducers23, gene therapy to overexpress HO24 and the delivery of CO directly by inhalation 16, 25 or the use of CO releasing molecules26, 27 are therapeutic strategies that have all demonstrated efficacy in experimental transplantation models of IRI. While many or all of these therapeutic approaches may prove clinically feasible, they all create logistical difficulties as well as (in the case of CO inhalation delivery) the potential for exposure of patients and healthcare workers to toxic doses of CO. To simplify delivery of CO and to avoid recipient exposure to higher doses, in the current study we hypothesized and evaluated whether delivery of CO to lung grafts via a CO saturated flush and perfusate solution would mitigate IRI in experimental lung transplantation. Our data demonstrate that flush and cold storage of lung grafts in preservation solution bubbled with 5% CO diminishes IR injury resulting in significantly improved graft function and reduced graft inflammation. Notably, the therapeutic effect observed in this study is comparable to the effect we observed in previous studies where low dose CO (250 ppm) was delivered via inhalation to both lung donors and recipients (beginning pretransplant and continuing posttransplant in recipients). Another important aspect of the current study is the demonstration that CO is rapidly (over hours) released from solution if an air-solution interface exists. Thus, protocols employing this strategy must utilize airtight containers and avoid an air-solution interface.
We have previously shown that storing and flushing intestinal grafts13 and kidney grafts28 in CO-supplemented UW solution prevents graft deterioration associated with IRI. In our previous studies13, 28, we applied 5% CO-UW (achieving a CO concentration of 40.6 μmol/L) based on the results of previous studies using CO-releasing molecules, showing that CO concentrations of 10 to 50 μmol/L in perfusate provided the best results in protection of cardiomyocytes26 and renal vascular system29 against oxidative stress. In this study, we provide evidence that higher concentrations of CO in solution (achieved through bubbling UW solution with 100% CO) do not enhance the protective effects seen with 5% CO gas. This may be an important step for the clinical application of CO as an additive to the preservation solution to determine the most appropriate CO concentration in solution. Our previous work demonstrated that a CO concentration of 2.15 μmol/L (bubbling with 0.1% CO gas) did not improve intestinal cold IRI.13 Based on these results, we will further optimize CO concentration in preservation solution in future experiments to establish an optimized, clinically applicable, effective, and safe ex vivo CO delivery method.
Although the mechanisms involved in the protection afforded by ex vivo CO delivery are not fully elucidated, we postulate one possible mechanism of the binding activity of CO with heme protein. CO possesses a strong affinity to heme moiety of heme proteins and potentially influences the biological behaviors of heme proteins.30, 31 Bysani et al. demonstrated that cytochrome P450, abundant heme proteins in the lung, degrade during IRI and releasing heme.32-34 Free heme released from heme proteins such as cytochrome P450 during IRI not only promotes peroxidation of the lipid membranes of the cells35, 36, but also activate adhesion molecules leading to massive cellular infiltration and increases in vascular permeability, contributing to the pathogenesis of local inflammatory processes.36-39 In addition, free heme can be a major source of iron, which participates in the generation of more detrimental hydroxyl radicals from hydrogen peroxide via Fenton reaction.40, 41 We have shown that 5% CO-UW could prevent degradation of cytochrome P450 and prevent heme/iron release during IRI in kidney28. Our observations in this study may support the role of CO in binding heme protein and inhibiting heme/iron-derived graft injuries. Although we do not have clear explanation why 100% CO did not prevent IRI, we postulate that high concentrations of CO might be harmful by completely inhibiting enzymatic function of remained cytochrome P450, altering other critical heme proteins required to maintain cell viability, or releasing CO into the recipient circulation after transplantation. In addition, Taille et al. showed that CO modulates redox signaling by interacting with the heme moiety of NAD(P)H oxidase and/or the respiratory chain.42
In summary, our results demonstrate that the addition of CO to the preservation solution utilized for flush and cold storage reduces IRI of lung grafts resulting in improved graft function and reduced inflammation.. This approach is straightforward and could be easily adapted in the clinic and could potentially have a positive impact on patient care in the early post-transplant period and improve long-term outcomes.
Acknowledgments
Supported by the NIH Grants HL076265 (McCurry), GEMI fund (Nakao).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ultramini-Abstract: Carbon monoxide at low concentration is known to have potent cytoprotective effects. Our study using rat lung transplantation model demonstrated that 5% CO as an additive to the cold flush/preservation solution can ameliorate cold ischemia/reperfusion injury following prolonged cold preservation.
references
- 1.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–6. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 3.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171(7):786–91. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 4.Boehler A, Estenne M. Obliterative bronchiolitis after lung transplantation. Curr Opin Pulm Med. 2000;6(2):133–9. doi: 10.1097/00063198-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome Ann Thorac Surg 20027341041–7.; discussion 1047-8. [DOI] [PubMed] [Google Scholar]
- 6.Hicks M, Hing A, Gao L, et al. Organ preservation. Methods Mol Biol. 2006;333:331–74. doi: 10.1385/1-59745-049-9:331. [DOI] [PubMed] [Google Scholar]
- 7.de Antonio DG, Marcos R, Laporta R, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant. 2007;26(5):529–34. doi: 10.1016/j.healun.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Ardehali A, Laks H, Russell H, et al. Modified reperfusion and ischemia-reperfusion injury in human lung transplantation. J Thorac Cardiovasc Surg. 2003;126(6):1929–34. doi: 10.1016/s0022-5223(03)00976-0. [DOI] [PubMed] [Google Scholar]
- 9.Botha P, Jeyakanthan M, Rao JN, et al. Inhaled nitric oxide for modulation of ischemia-reperfusion injury in lung transplantation. J Heart Lung Transplant. 2007;26(11):1199–205. doi: 10.1016/j.healun.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006;10(3):650–71. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohmoto J, Nakao A, Kaizu T, et al. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery. 2006;140(2):179–85. doi: 10.1016/j.surg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Kohmoto J, Nakao A, Stolz DB, et al. Carbon Monoxide Protects Rat Lung Transplants From Ischemia-Reperfusion Injury via a Mechanism Involving p38 MAPK Pathway. Am J Transplant. 2007;7(10):2279–90. doi: 10.1111/j.1600-6143.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakao A, Toyokawa H, Tsung A, et al. Ex vivo application of carbon monoxide in university of wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant. 2006;6(10):2243–55. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakao A, Schmidt J, Harada T, et al. A single intraperitoneal dose of carbon monoxide-saturated Ringer’s lactate solution ameliorates post-operative ileus in mice. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.106.108654. [DOI] [PubMed] [Google Scholar]
- 15.Mizuta T, Kawaguchi A, Nakahara K, Kawashima Y. Simplified rat lung transplantation using a cuff technique. J Thorac Cardiovasc Surg. 1989;97(4):578–81. [PubMed] [Google Scholar]
- 16.Nakao A, Kimizuka K, Stolz DB, et al. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol. 2003;163(4):1587–98. doi: 10.1016/S0002-9440(10)63515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eppinger MJ, Jones ML, Deeb GM, et al. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. J Surg Res. 1995;58(6):713–8. doi: 10.1006/jsre.1995.1112. [DOI] [PubMed] [Google Scholar]
- 18.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 19.Fiser SM, Tribble CG, Long SM, et al. Pulmonary macrophages are involved in reperfusion injury after lung transplantation Ann Thorac Surg 20017141134–8.; discussion 1138-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhao M, Fernandez LG, Doctor A, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L1018–26. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 21.Eppinger MJ, Deeb GM, Bolling SF, Ward PA. Mediators of ischemia-reperfusion injury of rat lung. Am J Pathol. 1997;150(5):1773–84. [PMC free article] [PubMed] [Google Scholar]
- 22.Serrick C, Adoumie R, Giaid A, Shennib H. The early release of interleukin-2, tumor necrosis factor-alpha and interferon-gamma after ischemia reperfusion injury in the lung allograft. Transplantation. 1994;58(11):1158–62. [PubMed] [Google Scholar]
- 23.Amersi F, Buelow R, Kato H, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104(11):1631–9. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coito AJ, Buelow R, Shen XD, et al. Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury. Transplantation. 2002;74(1):96–102. doi: 10.1097/00007890-200207150-00017. [DOI] [PubMed] [Google Scholar]
- 25.Akamatsu Y, Haga M, Tyagi S, et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. Faseb J. 2004;18(6):771–2. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 26.Clark JE, Naughton P, Shurey S, et al. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93(2):e2–8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 27.Bagul A, Hosgood SA, Kaushik M, Nicholson ML. Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation. 2008;85(4):576–81. doi: 10.1097/TP.0b013e318160516a. [DOI] [PubMed] [Google Scholar]
- 28.Nakao A, Faleo G, Shimizu H, et al. Ex vivo delivered carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts Kidney Int 2008; in press. [DOI] [PubMed] [Google Scholar]
- 29.Sandouka A, Fuller BJ, Mann BE, et al. Treatment with CO-RMs during cold storage improves renal function at reperfusion. Kidney Int. 2006;69(2):239–47. doi: 10.1038/sj.ki.5000016. [DOI] [PubMed] [Google Scholar]
- 30.Estabrook RW, Franklin MR, Hildebrandt AG. Factors influencing the inhibitory effect of carbon monoxide on cytochrome P-450-catalyzed mixed function oxidation reactions. Ann N Y Acad Sci. 1970;174(1):218–32. doi: 10.1111/j.1749-6632.1970.tb49788.x. [DOI] [PubMed] [Google Scholar]
- 31.Uchida T, Sato E, Sato A, et al. CO-dependent activity-controlling mechanism of heme-containing CO-sensor protein, neuronal PAS domain protein 2. J Biol Chem. 2005;280(22):21358–68. doi: 10.1074/jbc.M412350200. [DOI] [PubMed] [Google Scholar]
- 32.Maines MD, Mayer RD, Ewing JF, McCoubrey WK., Jr Induction of kidney heme oxygenase-1 (HSP32) mRNA and protein by ischemia/reperfusion: possible role of heme as both promotor of tissue damage and regulator of HSP32. J Pharmacol Exp Ther. 1993;264(1):457–62. [PubMed] [Google Scholar]
- 33.Baliga R, Zhang Z, Baliga M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int. 1996;49(2):362–9. doi: 10.1038/ki.1996.53. [DOI] [PubMed] [Google Scholar]
- 34.Bysani GK, Kennedy TP, Ky N, et al. Role of cytochrome P-450 in reperfusion injury of the rabbit lung. J Clin Invest. 1990;86(5):1434–41. doi: 10.1172/JCI114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nath KA, Balla J, Croatt AJ, Vercellotti GM. Heme protein-mediated renal injury: a protective role for 21-aminosteroids in vitro and in vivo. Kidney Int. 1995;47(2):592–602. doi: 10.1038/ki.1995.75. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157(3):175–88. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Nath KA, Vercellotti GM, Grande JP, et al. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59(1):106–17. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- 38.Wagener FA, Eggert A, Boerman OC, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98(6):1802–11. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 39.Wagener FA, Feldman E, de Witte T, Abraham NG. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216(3):456–63. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 40.Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988;34(4):474–80. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- 41.Baliga R, Zhang Z, Baliga M, et al. Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;54(5):1562–9. doi: 10.1046/j.1523-1755.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 42.Taille C, El-Benna J, Lanone S, et al. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280(27):25350–60. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]