Abstract
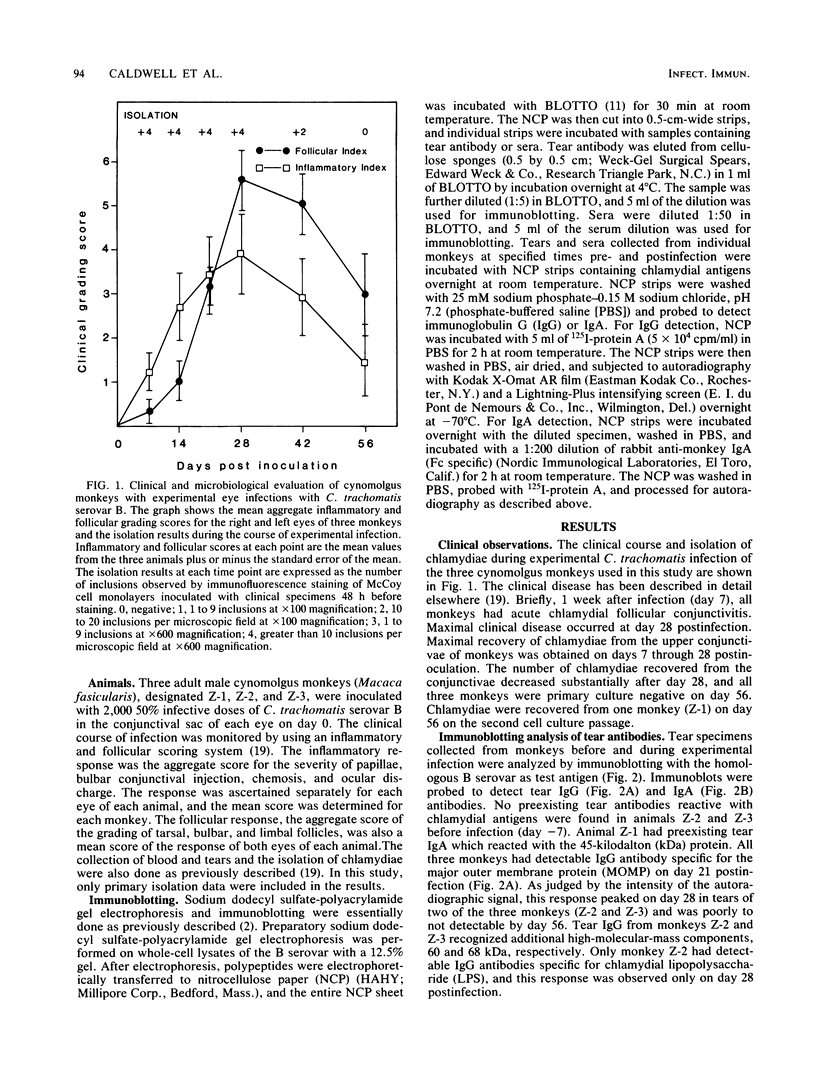
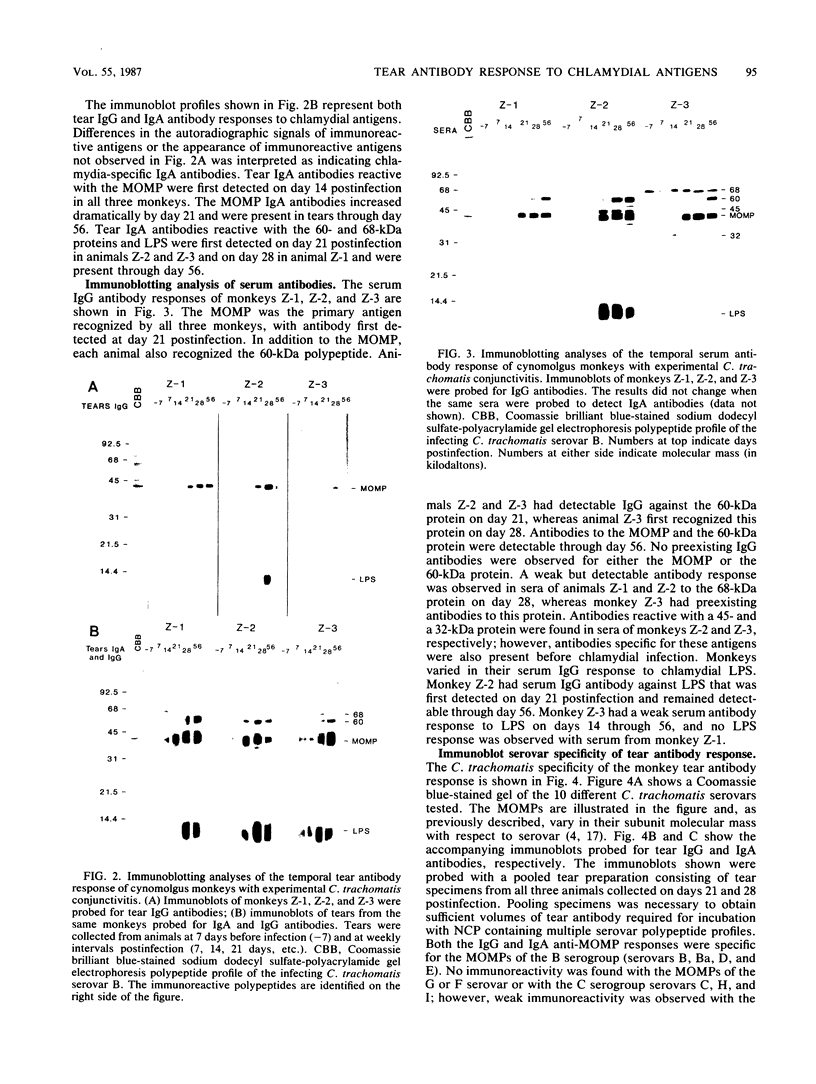
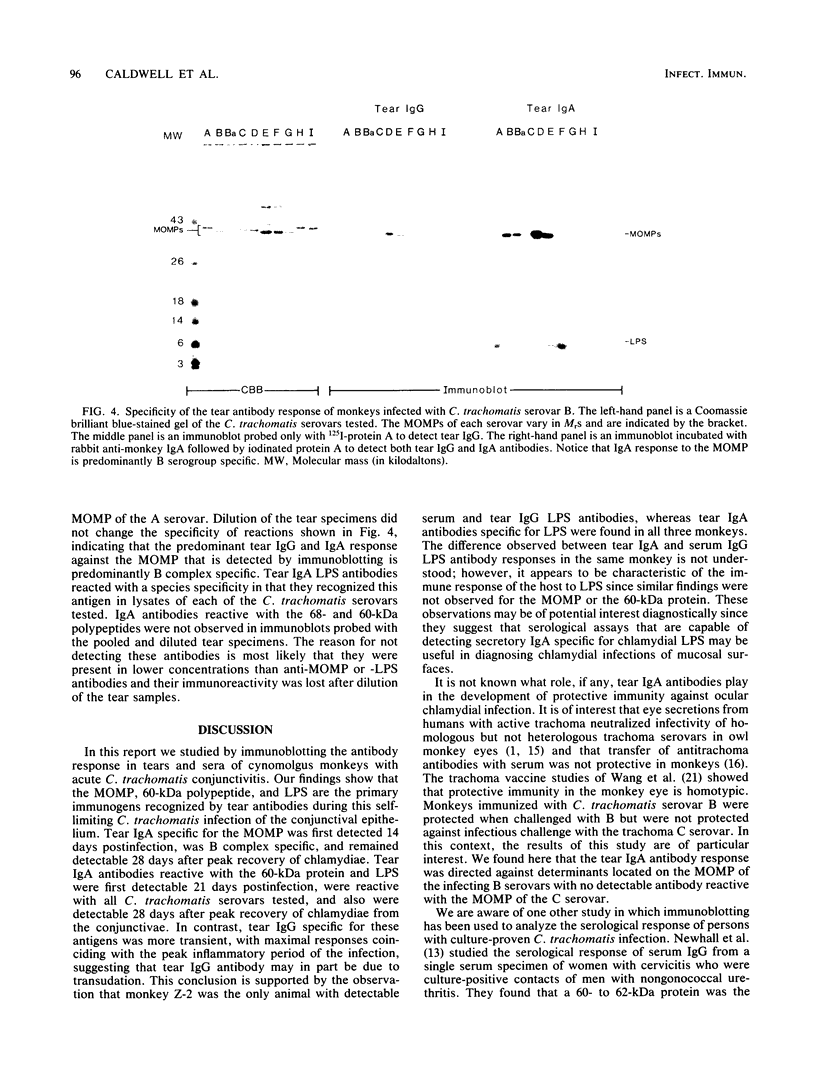
In this study, we examined the temporal antibody response by immunoblotting analysis in tears and sera of three cynomolgus monkeys (Macaca fasicularis) with primary acute Chlamydia trachomatis serovar B conjunctivitis. The objective was to identify chlamydial antigens stimulating antibody during the host responses in the course of this self-limiting infection with the rationale that they may be protective antigens. The major outer membrane protein (MOMP), lipopolysaccharide (LPS), and polypeptides of 60 and 68 kilodaltons (kDa) were the predominant antigens recognized by immunoglobulin A (IgA) in monkey tears. Tear IgA antibody specific for the MOMP was first detected 14 days postinfection, whereas tear IgA reactive with LPS or the 68- and 60-kDa polypeptides was first detectable on day 21. Tear IgA antibodies specific for each of these antigens persisted in tears through day 56, 4 weeks after both peak clinical disease and recovery of the organism from the conjunctivae. In contrast, tear IgG antibodies peaked at approximately 28 days postinfection, the time of maximal inflammatory response. The IgG response in monkey sera was similar to that observed for tear antibodies, in that the MOMP, 60-, and 68-kDa polypeptides were the primary immunogens. The exception was that IgG antibody against these antigens was detected 1 week later than that observed for tear IgA antibodies. Of three monkeys that responded with tear IgA antibody against LPS, one did not have detectable serum IgG LPS antibody. The specificity of the tear IgA antibody response of monkeys was determined by immunoblotting nine other C. trachomatis serovars in addition to the homologous B serovar. The tear IgA response to the MOMP was predominantly B complex subspecies-specific (serovars B, Ba, D, and E), whereas the response to chlamydial LPS was found to be species-specific. The significance of these observations in relation to previous vaccine studies in nonhuman primates is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenfanger J., MacDonald A. B. The role of immunoglobulin in the neutralization of trachoma infectivity. J Immunol. 1974 Nov;113(5):1607–1617. [PubMed] [Google Scholar]
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson C. R., Schachter J. Strategies for treatment and control of blinding trachoma: cost-effectiveness of topical or systemic antibiotics. Rev Infect Dis. 1985 Nov-Dec;7(6):768–773. doi: 10.1093/clinids/7.6.768. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Yeh L. J., Kuo C. C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov-Dec;7(6):717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- Matikainen M. T., Terho P. Immunochemical analysis of antigenic determinants of Chlamydia trachomatis by monoclonal antibodies. J Gen Microbiol. 1983 Aug;129(8):2343–2350. doi: 10.1099/00221287-129-8-2343. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., 5th, Terho P., Wilde C. E., 3rd, Batteiger B. E., Jones R. B. Serovar determination of Chlamydia trachomatis isolates by using type-specific monoclonal antibodies. J Clin Microbiol. 1986 Feb;23(2):333–338. doi: 10.1128/jcm.23.2.333-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R. L., Oertley R. E., Fraser E. C., MacDonald A. B., McComb D. E. Immunity to chlamydial infections of the eye. VI. Homologous neutralization of trachoma infectivity for the owl monkey conjuctivae by eye secretions from humans with trachoma. J Infect Dis. 1973 Apr;127(4):429–432. doi: 10.1093/infdis/127.4.429. [DOI] [PubMed] [Google Scholar]
- Salari S. H., Ward M. E. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981 Apr;123(2):197–207. doi: 10.1099/00221287-123-2-197. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Taylor H. R., Prendergast R. A., Dawson C. R., Schachter J., Silverstein A. M. An animal model for cicatrizing trachoma. Invest Ophthalmol Vis Sci. 1981 Sep;21(3):422–433. [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Alexander E. R. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967 May;63(5 Suppl):1615–1630. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]