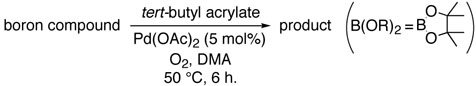
Abstract
We report herein the development of a general and mild protocol of oxygen promoted Pd(II) catalysis resulting in the selective cross-couplings of alkenyl- and arylboron compounds with various olefins. Unlike most cross-coupling reactions, this new methodology works well even in the absence of bases, consequently averting undesired homo-couplings. Nitrogen-based ligands including dimethyl-phenanathroline enhance reactivities and offer a highly efficient and stereoselective methodology to overcome challenging substrate limitations. For instance, oxidative palladium(II) catalysis is effective with highly substituted alkenes and cyclic alkenes, which are known to be incompatible with other known catalytic conditions. Most examined reactions progressed smoothly to completion at low temperatures and in short times. These interesting results provide mechanistic insights and utilities for a new paradigm of palladium catalytic cycles without bases.
Introduction
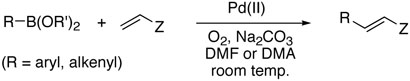
Transition metal catalyzed coupling reactions are extremely powerful tools for carbon-carbon bond formation, and have been widely utilized in a great number of syntheses. In particular, palladium catalyzed arylation and vinylation of alkenes known as Mizoroki-Heck (Y = H),1 Suzuki-Miyaura (Y = B(OR')2),2 and Stille (Y = SnR″3)3 reactions have become classical vehicles to various carbon-carbon bonds (Scheme 1). Among these methods, Heck reaction has been considered the best choice presumably because Suzuki-Miyaura and Stille couplings often require stereoselective syntheses of both substrates prior to couplings. However, this methodology has been limited primarily to monosubstituted olefins due to inefficiency, low selectivity, and harsh reaction conditions when used for di- and trisubstituted olefins.
Scheme 1.
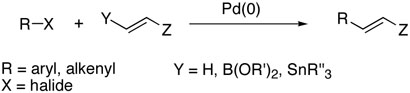
To overcome these limitations, two distinct approaches have been pursued by a number of research groups. In one approach, mild conditions have been sought by changing reaction parameters as seen in recent reports by Fu4(a) and Hartwig.4(b) On the contrary, other groups investigated alternative halide surrogates such as diazonium halide salt,5 triflate,6 acid chloride,7 telluronium salt,8 and etc. These efforts to find new conditions have provided high efficiency under mild conditions, yet these methods are not widely used. Our group has pursued a combined strategy of aforementioned approaches by changing halides to boronic acids9 and modifying the reaction parameters such as atmosphere.10 This approach presents a novel paradigm for these types of coupling, which stems from a change in reaction mechanism, namely Pd(0) to Pd(II) catalysis under an oxygen atmosphere.
Recently, we reported early developments of an oxygen-promoted palladium(II) catalysis for the cross-coupling of organoboron compounds and alkenes to afford arylalkene and alkene-alkene compounds (Scheme 2).11 In this carbon-carbon bond formation protocol, the most characteristic feature is utilization of molecular oxygen as a reoxidant of palladium(0) species.13 Coupling reactions proceed smoothly with various substrates and the reactions are highly efficient and stereoselective presumably due to mildness of the reaction conditions employed. In this definitive report, we offer general conditions for wide applications.
Scheme 2.
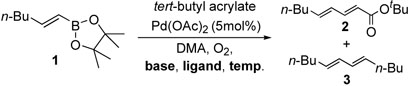
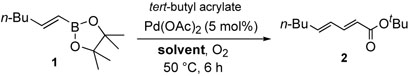
As representatively shown in table 1, we have discovered benign conditions to enhance the desired cross-coupling process. Our early studies on oxidative Pd(II) catalysis exhibited superior activities and selectivities, however these conditions accompanied homocouplings to a small extent (entry 1).14 Although these undesired homo-coupled products can be easily separated out by column chromatography, it would be ideal to avoid them for wide application, especially in library generation. In this context, we embarked on efforts to eliminate homo-coupling products by scrutinizing possible mechanisms and control elements.
Table 1.
Coupling of 1-Hexenyl Boronic Ester 1 with tert-Butyl Acrylate under Various Conditions
 | ||||||
|---|---|---|---|---|---|---|
| entry | base | ligand | temp.(°C) | time (h) | yield (%) |
|
| 2 | 3 | |||||
| 1 | Na2CO3 | - | 23 | 3 | 85 | 10 |
| 2 | - | - | 50 | 6 | 95 | - |
| 3 | - | phena | 50 | 2 | 98 | - |
phen = 1,10-phenanthroline
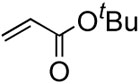
First, we considered value of using bases. Reactions run in the absence of base consequently yielded cross-coupled compound 2 without any concomitant formation of 3 (entry 2). Despite the success, the base-free conditions needed a longer reaction time. In the meanwhile, the palladium catalyst started to precipitate out. Therefore, we next pursued stabilizing the palladium catalysts and maximizing their catalytic efficiency. This goal was successfully achieved by adopting amine ligands such as 1,10-phenanthroline resulting in the exclusive formation of the desired cross-coupling product in just 2 hours (entry 3).15
We believe that these newly developed methods can serve as the optimal conditions for these types of cross-coupling reactions. The current account addresses these control elements, the scope of each methodology, and mechanistic insights. Based upon the observed results, a new mechanism is proposed herein to account for the desired catalytic cycle, avoidance of by-products, base-free and ligand-present conditions, and most importantly the mildness and efficiency relative to known methodologies.
Results and Discussion
1. Cross-Coupling Reaction of Alkenylboron Compounds and Olefins
1-1. Base Effect on the Cross-Coupling Reaction
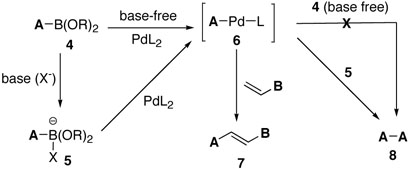
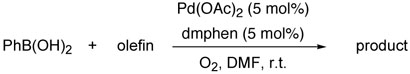
Generally, bases are well known to be a pivotal component in palladium catalyzed coupling reactions such as the Miyaura-Suzuki reaction.2 The role of the bases in these reactions is to facilitate transmetallation of organoboron compounds via formation of organoborate salts resulting in the enhancement of coupling reactions. Likewise, in our protocol, the reactive borate salts 5 should accelerate the coupling processes as shown in Scheme 3. However, homo-coupled compounds 8 were concomitantly formed albeit in low yields due to the reactive borate salts 5. Therefore, we anticipated exclusive cross-coupling by avoiding generation of reactive borate salts. Based upon this assumption, we conducted oxidative Pd(II) cross coupling reaction in the absence of bases and the results are summarized in Table 2.
Scheme 3.
Table 2.
a The Effect of Bases and Temperature on the Cross-Coupling Reactions of Alkenyl Boronic Ester with Alkene
 | |||||
|---|---|---|---|---|---|
| entry | base | temperature | time (h) | yield (%) |
|
| 2 | 3 | ||||
| 1b | Na2CO3 | 23 | 3 | 85 | 10 |
| 2b | Na2CO3 | 50 | 1 | 75 | 23 |
| 3 | - | 23 | 6 | 17 | - |
| 4 | - | 50 | 6 | 95 | - |
All reactions were carried out with 1 (0.5 mmol), tert-butyl acrylate (1.5 mmol),and Pd(OAc)2 (5 mol%) in DMA (dimethylacetamide, 2.5 mL).
Na2CO3 (1.0 mmol)
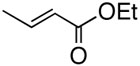
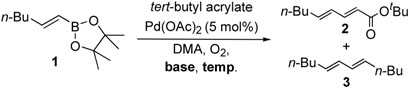
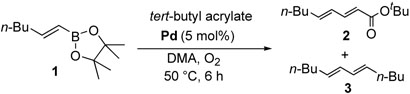
We examined the base effect on the cross-coupling of trans-1-hexenyl pinacolboron ester (1) and tert-butyl acrylate. The cross-coupling reaction in the presence of Na2CO3 as a base at room temperature provided a 85% yield of conjugated diene compound 2 and 10% of homo-coupled compound 3 (entry 1). At a higher temperature, 50 °C, although reaction rate was accelerated, the yield of homo-coupled compound was also increased to 23% (entry 2). In the absence of a base, the reactivity was attenuated significantly to effect low conversion, however, the desired cross-coupling was observed exclusively (entry 3). Hence, an elevated temperature was required to facilitate the catalysis. At 50 °C, the cross-coupling reaction proceeded smoothly to give 95% yield of the desired diene compound 2, and no homo-coupled compound 3 was detected (entry 4). These results implied that the homocoupled reaction should be prohibited under the base free conditions due to the avoidance of the reactive borate salts as we hypothesized.
Based on these results, we screened the reaction conditions by varying solvents and palladium catalysts to determine optimal base-free conditions (Table 3). In the cases of THF and toluene, the reactions were incomplete even after 36 hours, and the yields were very low (entries 4, 5). However, in polar solvents such as DMA, acetonitrile and DMF, the desired (E,E)-conjugated diene 2 was obtained in excellent yields (entries 1, 2 and 3). The homo-coupled product was not detected at all in any of those cases.
Table 3.
The Effect of Solvents on the Cross-Coupling Reactions of Alkenyl Boronic Ester with Alkene under Base-Free Conditions
 | ||
|---|---|---|
| entry | solvent | yield (%) |
| 1 | DMA | 95 |
| 2 | CH3CN | 89 |
| 3 | DMF | 92 |
| 4 | THF | 12 |
| 5 | Toluene | 17 |
The effect of the palladium catalyst was investigated as demonstrated in table 4. In the case of PdCl2, the conversions were unsatisfactorily low (entry 2). With Pd2(dba)3, the reaction did not proceed at all. These results were contrary to our expectation because the cross coupling reaction took place well in the presence of bases independent of palladium species described in our previous communications.
Table 4.
The Effect of Palladium Catalysts on the Cross-Coupling Reactions of Alkenyl Boronic Ester with Alkene under Base-Free Conditions
 | |||
|---|---|---|---|
| entry | catalyst | yield (%) |
|
| 2 | 3 | ||
| 1 | Pd(OAc)2 | 95 | - |
| 2 | PdCl2 | 21 | - |
| 3 | Pd2(dba)3 | No reaction | |
| 4 | Pd(PPh3)4 | 46 | 51 |
These phenomena can be rationalized by considering the reactivity of the oxopalladium complex in situ generated by reaction of PdL2 (L = Cl, dba) with bases (OH−, R'O−, and RCO2−).2(a) The ligand (RO) on the oxo-palladium complex will coordinate to the boron atom, which can facilitate the transfer of the organic group on the boron atom to the palladium atom resulting in the enhancement of transmetallation. In the absence of bases, there would be no such oxo-palladium complex in the cases of PdCl2 and Pd2dba3, which may explain the low yield and no reaction. Interestingly, coupling reaction with Pd(PPh3)4 afforded homo-coupled compound 3 as the major product along with 46% of the desired compound 2.16 These results indicate that palladium ligands can play a key role in the coupling reactions under base-free conditions, which prompted us to investigate studies on the use of various ligands (vide infra).
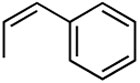
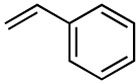
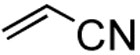
Having established optimized conditions, various alkenes were examined for the feasibility of the methodology as shown in table 5. Styrene reacted with hexenyl boronic acid efficiently to generate diene compound 9 in a high yield (entry 2). Furthermore, the unreactive allyl ether was subjected to coupling reaction to deliver product 10 in a good yield of 87% (entry 3). These couplings were highly stereoselective to furnish E,E-isomers only. However, acrylonitrile furnished a mixture of E- and Z-isomers as expected, mainly due to the lack of steric presence (entry 4).17 We examined a number of cases in addition to the reported ones here, and came to a conclusion that the E-selectivity was prominent.
Table 5.
Cross-Coupling Reactions of Hexenyl Boronic Ester with Various Alkenes under Base-Free Conditions
Isolated yields.
E : Z = 3 : 2
1,4- and 1,3-diene ratio is 2.8 to 1.
EE and EZ ratio is 6 to 1.
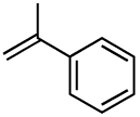
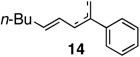
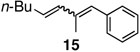
The reaction with disubstituted alkenes such as tert-butyl methacrylate and ethyl crotonate afforded the corresponding methyl substituted diene products 12 and 13 in 90% and 89% yields, respectively, while exhibiting excellent E-selectivities (entries 5 and 6). Interestingly, in the case of α-methyl styrene, the coupling reactions provided the isomeric mixtures of diene compounds 14 due to the different pathways of β-hydride elimination during the reaction cycle (entry 7). The reaction with cis-β-methyl styrene also provided an isomeric mixture of coupling compound 15 in moderate yield (entry 8).
Regardless of the steric and electronic nature of the alkenes, the reaction under the base free conditions delivered the corresponding cross-coupled products exclusively without homo-coupled products. To our delight, yields were generally desirably high due to excellent chemoselectivities, and stereoselective outcomes were outstandingly in favor of the E-isomer. In particular, E-selective preparation of trisubstituted alkenes should be of great value because of known difficulty in their stereoselective synthesis.
To further examine the method versatility, we carried out cross-coupling reactions of various alkenylboronic esters with tert-butyl acrylate (Table 6). The coupling reaction with boronic acids furnished considerable amounts of homo-coupled products because alkenylboronic acids are known to be more reactive than the corresponding boronic esters. However, the second transmetallation for the alkenylboronic esters may be slower than that for the corresponding acids due to the repulsion between the alkenylpalladium complex and the sterically hindered ketal group of alkenylboronic esters, averting the unwanted homocouplings. For example, the cross-coupling reaction with hexenyl boronic acid (16) and tert-butyl acrylate afforded 64% of diene compound 2 along with 35% of homo-coupled product 3 even under base free conditions (entry 1). For these reasons, we conducted the coupling reactions with boron ester compounds, which were easily prepared form the corresponding boronic acids in nearly quantitative yields.
Table 6.
Cross-Coupling Reactions of tert-Butyl Acrylate with Various Alkenyl Boron Compounds under Base-Free Conditions
Isolated yields.
TMS group was deprotected during column chromatography
The reaction time is 8 hours.
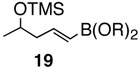
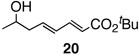
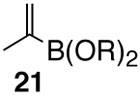
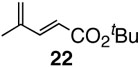
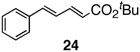
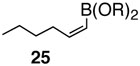
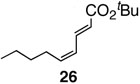
Pinacol boron esters 17 and 19 containing the alcohol functional moieties were subjected to base-free oxidative Pd(II) catalysis, giving rise to the exclusive formation of (E,E)-dienes 18 and 20 in high yields, respectively. Highly substituted alkenylboron ester compounds were also compatible with this protocol. Isopropenyl pinacol boron ester (21) prepared from the corresponding bromide via Grignard reaction with the alkyl borate18 ,25 reacted smoothly to offer diene compound 22 in 86% yield and with high selectivity. Aryl substituted boronic ester 23 gave the corresponding (E,E)-dienoate 24 efficiently. Furthermore, palladium catalyzed cross-coupling with cis-hexenyl pinacolboronic ester 2519 yielded (E,Z)-diene 26 with full retention of the olefin stereochemistry. Advantageously, highly functionalized structural groups can be incorporated for further manipulation utilizing this protocol.
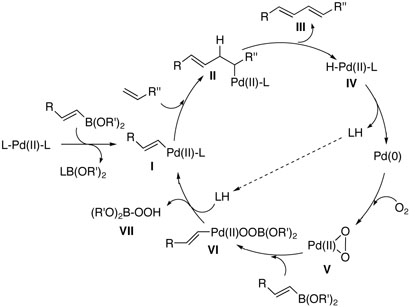
Based upon these results, we would like to suggest a plausible catalytic mechanism for base-free oxygen promoted Pd(II) catalysis as follows (Figure 1): initial transmetallation should be feasible with an alkene boron compound even without activating it to an “ate” complex, giving the palladium (II) intermediate I; the second incorporation of an alkenyl group can be carried out by migratory insertion, which would be followed by β-hydride elimination to produce cross-coupling product III and Pd(0) species without the aid of bases; molecular oxygen would then oxidize the resulting Pd (0) to a peroxopalladium complex V,12 which can react with another alkenylboron compound to regenerate alkene- Pd(II)-L complex I.13 As we confirmed in the previous work,11 oxygen is crucial for the catalytic cycle, acting as an efficient Pd(0) reoxidant. The most notable feature of the proposed mechanism is that transmetallation takes place in the first alkenylation process, facilitating the whole catalysis under mild conditions. We believe that this transmetallation operation is facile due to lack of steric repulsion, which is prevalent in a number of organometallic reactions. Other known cross-coupling methods require transmetallation in the second alkenylation step after already adsorbing the first alkenyl group through Pd(0) catalyzed oxidative addition. This is not the case with our newly developed methodology.
Figure 1.
Proposed Mechanism for the Cross-Coupling Reactions under Base-Free Conditions
1-2. Ligand Effect on Cross-Coupling Reaction of Alkenylboron Compounds and Olefins
The Heck type reaction is usually carried out in the presence of phosphine ligands, which stabilize the active Pd(0) species and accelerate the reaction rate.1(c),(d) In our basefree reaction protocol, we found that palladium catalyst was quite easily precipitated and the coupling reaction was retarded. To stabilize and minimize the precipitation of palladium catalysts, we decided to employ organic ligands in our base-free conditions. The most suitable ligands for cross-couplings we found during this study were bidentated nitrogen ligands,20 which have been known to facilitate the efficient redoxidation of Pd(0) by molecular oxygen and remain stable upon exposure to air and moisture unlike their phosphine counterparts. Many phosphine ligands are sensitive to air and moisture, and subsequently convert to phosphine oxide species upon exposure.
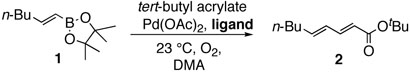
First, we examined the cross-coupling reaction of trans-1-hexenyl pinacolboron ester (1) and t-butyl acrylate by screening with various bidentated aliphatic and aryl amines, pyridine, and phosphine ligands as shown in table 7. The reactions with alkyl amines provided the cross-coupled compound 2 in low yields (entries 1, 2, and 3) whereas tetra-N-methyl- propyldiamine resulted in an excellent yield (entry 4). However, in the case of the phosphine analogue of tetra-N-methyl-propyldiamine, the yield was dramatically reduced (entry 5). In general, phosphine ligands were observed to be incompatible with our coupling system. Aniline derivatives were similarly inefficient for the coupling reaction (entries 6, 7). However, reactions with 2,2′-bipyridine and 1,10-phenanthroline afforded the desired compound in excellent yields (entries 8, 9).
Table 7.
The Effect of Ligands on the Cross-Coupling Reactions under Base-Free Conditions
 | |||||
|---|---|---|---|---|---|
| entry | ligand | yield (%)a,b | entry | ligand | yield (%)a,b |
| 1 |  |
38 | 6 | 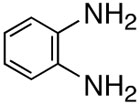 |
0 |
| 2 |  |
35 | 7 | 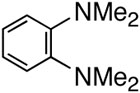 |
23 |
| 3 | 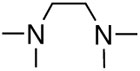 |
32 | 8 | 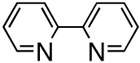 |
95 |
| 4 | 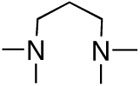 |
91 | 9 | 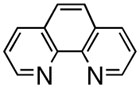 |
95 |
| 5 |  |
15 | 10 | 0 | |
All reactions were carried out with 1 (0.5 mmol), tert-butyl acrylate (1 mmol), and Pd(OAc)2 (5 mol%) in DMA (dimethylacetamide, 2.5 mL).
Isolated yields.
In none of these cases, was the precipitation of palladium catalysts detected, which indicated stabilization of the Pd species by the bidentate ligands. However, all ligands did not enhance the coupling reaction. We envisioned the bidentate amine ligands should be located at two adjacent sites among the square chelating sites of palladium catalysts leaving the other two sites open for the coupling reaction. Notably, cis-configuration of two ligand groups in the square planar structure would be more favorable for the migratory insertion steps during the catalytic cycle, resulting in the enhancement of the coupling reaction.21 This idea was supported by the reaction with 4,4'-bipyridine as a ligand (entry 10). Coupling reaction with 4,4'-bipyridine did not proceed and the palladium catalyst precipitated early in the reaction presumably because 4,4'-bipyridine did not effectively form a Pd-nitrogen complex due to its linear structure.
These results indicate the coupling reactions are sensitive to the structure of the Pdligand complex: Pd-nitrogen chelating length, coordination angle, and steric environment. Based on these factors, phenanthroline was selected as the best choice for the coupling reaction.
Subsequently, we sought optimal conditions by screening various temperatures and Pd/ligand amounts. The results are summarized in table 8. At room temperature, reduction of catalyst loading provided diminished yields (entry 2). However, at a higher temperature, the reaction proceeded well in shorter reaction time to afford the coupling compound in an excellent yield. Further reduction of catalyst loading at 50 °C provided slightly diminished yields. In conclusion, phenanthroline is the ligand of choice under the optimal coupling reaction conditions. These conditions are general and efficient to afford (E,E)-diene as the exclusive product in high yields, when the undesirable homo-coupled product is not observed. In particular, there is no need for base, and a minimal loading of catalyst is sufficient for high turnover up to 108.
Table 8.
The Effect of Pd(II) Catalyst Amount Employed on the Cross-Coupling Reactions in the Presence of Amine Ligands
 | ||||
|---|---|---|---|---|
| entry | Pd / ligand | T (°C) | t (hr) | yield (%)a |
| 1 | 5 mol% | 23 | 12 | 95 |
| 2 | 2 mol% | 23 | 12 | 32 |
| 3 | 2 mol% | 50 | 2 | 98 |
| 4 | 1 mol% | 50 | 2 | 81 |
| 5 | 0.5 mol% | 50 | 2 | 54 |
Isolated yields.
By utilizing the optimized reaction conditions, we examined the cross-coupling reaction of alkenylboronic ester 1 with various olefins as shown in table 9. All the examined mono-substituted alkenes reacted smoothly, furnishing exclusively the corresponding conjugated dienes in excellent yields (entries 1, 2, and 3). Regardless of substituents, cross-couplings were successful, and this protocol was generally applicable to a number of alkene systems.
Table 9.
Cross-Coupling Reactions of Hexenyl Boronic Ester with Various Alkenes in the Presence of Ligand
Isolated yields.
The ratio of 1,4- and 1,3-diene was 6:1 by 1H-NMR analysis.
The .ratio of E to Z isomer was 9:1 by 1H-NMR analysis.
Due to difficulty and low stereoselectivity, highly substituted olefins are poor substrates for Heck reaction as pointed out earlier. Therefore, a coupling reaction compatible with di- and trisubstituted olefins can be highly valuable, prompting us to probe the feasibility of Pd-nitrogen ligand catalysis. The reaction with α-methyl styrene (entry 4) furnished an inseparable mixture of 1,4- and 1,3-diene compound 14 in 87% yield via two possible β-elimination pathways during the reaction cycle. With cis β-methyl styrene (entry 5), the 9:1 mixture of (E,E)- and (E,Z)-dienes was obtained in 91% overall yield. Compared to the previous results in the base-free and ligandless conditions (Table 5), the yields were dramatically increased and the reactions went to completion faster. We believe these reactivities and selectivities illustrate the efficiency and feasibility of our carbon-carbon bond forming protocol with palladium(II)-nitrogenous ligand complexes under oxidative and base-free conditions.
2. Cross-Coupling Reaction of Aryl Compounds and Olefins
2.1 Base Effect on the Cross-Coupling Reaction of Aryl Compounds and Olefins
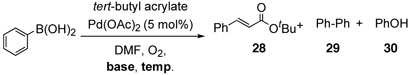
The base-free oxidative catalysis was next examined for the cross-coupling of arylboron compounds and alkenes. In the previous communication, we reported the development of an oxygen promoted Pd(II) catalyzed coupling of arylboronic acids and alkenes in the presence of bases. As with the alkenylboron compounds, the homo-coupled side product was also generated under the reaction conditions. In addition, phenol was unfortunately formed by oxidation of the corresponding arylboron compounds by boron peroxide, which was generated during the catalytic cycle.22 To prevent homo-couplings, we attempted the coupling reaction of phenylboronic acid and tert-butyl acrylate in the absence of bases, adopting the same methodology.
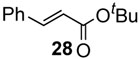
In the presence of Na2CO3 as a base, the reactions proceeded smoothly to afford the coupling product 28 in good yields along with homo-coupled compound 29 and phenol 30 at room temperature (entry 1, Table 10). At an elevated temperature, the reaction furnished the same products in similar yields, albeit in shorter reaction time (entry 2). Under base free conditions, however, the coupling reaction did not proceed well in spite of longer reaction time and higher temperature. Interestingly, the homo-coupled products were not detected (entries 3 and 4).
Table 10.
aThe Effect of Bases and Temperature on the Cross-Coupling Reactions of Aryl Boronic Acid with Alkene under Base-Free Conditions
 | ||||||
|---|---|---|---|---|---|---|
| entry | base | temp.(°C) | t (h) | yield (%)b |
||
| 28 | 29 | 30 | ||||
| 1c | Na2CO3 | 23 | 8 | 78 | 2 | 15 |
| 2c | Na2CO3 | 50 | 1 | 81 | 7 | 5 |
| 3c | - | 23 | >24d | 28 | - | 10 |
| 4 | - | 50 | >24d | 35 | - | 11 |
All reactions were carried out with phenylboronic acid (1 mmol), tert-butyl acrylate (1.5 mmol), and Pd(OAc)2 (5 mol%) in DMF (2.5 mL).
isolated yield.
Na2CO3 (2 mmol).
The reaction was incomplet after 24 hours.
We also examined coupling reactions with p-methoxy and m-acetyl phenyl boronic acids. The yields for these coupling reactions were 37% and 41%, respectively, indicating the electronic variation in arylboron compounds does not affect the coupling reaction under the base free condtions.
Based on these results, the bases seem to be a critical element in the coupling reaction between arylboronic compounds and alkenes contrary to the case of alkenylboron compounds. In consideration of reaction time and temperature under the same conditions, the rate of transmetallation for the aryl boron compounds was slower than that for alkenyl boron compounds. These results imply that transmetallation for aryl boron compounds does not occur effectively and requires other elements under base-free condtions.
2-2. Ligand Effect on Cross-Coupling Reaction of Arylboron Compounds and Olefins
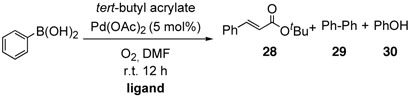
Shifting our attetintion to ligand effect on the cross-coupling reaction of arylboron compounds and olefins, we conducted the coupling reaction with phenyl boronic acid and tert-butyl acrylate using various ligands in the absence of bases at room temperature (Table 12).
Table 12.
a The Effect of Ligands on the Cross-Coupling Reactions of Aryl Boronic Acid with Alkene under Base-Free Conditions
 | ||||
|---|---|---|---|---|
| entry | ligand | yield (%)b |
||
| 28 | 29 | 30 | ||
| 1 |  |
13 | 44 | 17 |
| 2 | 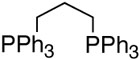 |
10 | 60 | 5 |
| 3 | 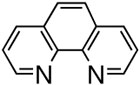 |
76 | - | 23 |
| 4 | 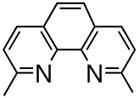 |
84 | - | 13 |
All reactions were carried out with phenylboronic acid (1 mmol), tert-butyl acrylate (1.5 mmol), and Pd(OAc)2 (5 mol%) in DMF (2.5 mL).
isolated yield.
Reaction with N,N,N′,N′-tetramethylpropylamine afforded only 13% of the desired product, 17% of phenol, and 44% of homo-coupled compound as the major product, which was contrary to the previous results for the coupling with alkenylboron compounds (entry 1). Using diphenylphosphonopropane as a ligand, homo-coupling was also predominant (entry 2). With 1,10-phenanthroline, the coupling reaction proceeded smoothly to give 28 in a good yield without homo-coupled compound 29, resembling the case of alkeylboron compounds (entry 3). Base-free conditions with 2,9-dimethyl-1,10-phenanthroline afforded better yields than 1,10-phenanthroline as representatively illustrated in entry 4, thus offering a general method of aryl-alkenyl couplings devoid of homo-coupling side pathways. In practice, phenolic side products are easily removed by washing away during the aqueous work-up.
Using 2,9-dimethyl-1,10-phenanthroline as a palladium ligand, we screened various solvents in the absence of bases. Reactions performed in DMF, DMA, and NMP gave the similarly satisfactory results. However, the reaction in CH3CN and THF afforded the desired coupling compound in low yields. Therefore, using 2,9-dimethyl-1,10- phenanthroline as a ligand and DMF as a solvent proved to be the best choice for the coupling reaction with arylboronic compounds.
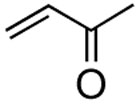
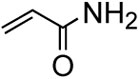
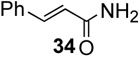
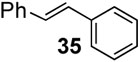
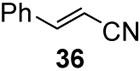
With the optimized condtions in hand, we carried out the coupling reaction of various alkenes with phenylboronic acid. As shown in table 14, tert-butyl acrylate was converted efficiently to (E)-t-butyl cinnamate 28 in 87% yield (entry 1). The reaction with methyl vinyl ketone and acryl amine afforded (E)-4-phenyl-3-butene-2-one 33 and (E)-3- phenyl-2-propenamide 34 in 71% and 64% yields, respectively (entries 2, 3). Furthermore, styrene and acrylonitrile reacted with phenylboronic acid to give 85% and 84% yields of (E)-stilbene 35 and (E)-3-phenyl-2-propenenitrile 36, respectively (entries 4, 5). The coupling with unreactive allyl benzyl ether proceeded successfully, affording benzyl (E)- cinnamyl ether 37 in 78% yield (entry 6).
Table 14.
Cross-Coupling Reactions of Phenyl Boronic Acid with Various Alkenes in the Presence of Ligand
Isolated yields.
The yield was calculated based on phenylboronic acid
The yield was calculated based on olefin.
The ratio of Heck and migrated products was 1:1.4 by 1H-NMR analysis
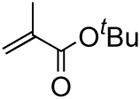
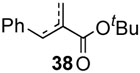
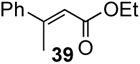
In the case of highly substitued alkenes, coupling reaction of tert-butyl methyl methacrylate with phenylbronic acid afforded a mixture of migrated product 38 along with α-methyl cinnamte. Ethyl crotonate delivered ethyl β-methyl cinnamte 39 with exclusive (E)-configuration in 82% yield. Since phenol was not factored in, the conversion yields based on alkenes were generally higher up to 96% (yield B) than those based on arylboron compounds (yield A), exhibiting excellent efficiency and selectivity.
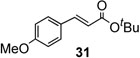
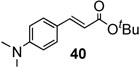
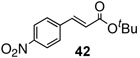
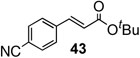
To further investigate the scope and limitation of this methodology, we carried out cross-couplings of various arylboronic acids and tert-butyl acrylate as summarized in table 15. Reactions of 4-methoxy phenyl boronic acid and N,N-dimethyl phenyl boronic acid, substituted with electron-donating groups, took place smoothly to provide the desired compound, tert-butyl-(E)-4-methoxycinnamate 31 and tert-butyl-(E)-3-(4-dimethylamino) phenyl) acrylate 40 and in 61% and 94% yields, respectively (entries 1, 2). Also, the reactions with meta- and para-acetyl phenyl boronic acids, substituted with an electron withdrawing group, afforded 32 and 41 in 72% and 71% yields, respectively (entries 3, 4). 4-Nitro phenyl boronic acid and 4-cyano phenyl boronic acid possessing highly electron withdrawing groups reacted with tert-butyl acrylate to give arylated products 42 and 43 in 57% and 69% yields, respectively (entries 5, 6). The coupled reaction of sterically hindered 2-methyl phenyl boronic acid with tert-butyl acrylate afforded 84% yield of the desired product 44 (entry 7).
Table 15.
Cross-Coupling Reactions of tert-Butyl Acrylate with Various Aryl Boron Compounds in the Presence of Ligand
 | |||
|---|---|---|---|
| entry | arylboron compound | product | yield (%)a |
| 1 | 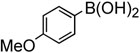 |
 |
61 |
| 2 | 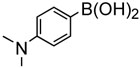 |
 |
94 |
| 3 | 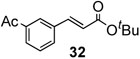 |
72 | |
| 4 | 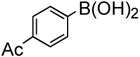 |
 |
71 |
| 5 | 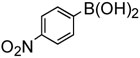 |
 |
57 |
| 6 | 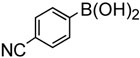 |
 |
69 |
| 7 | 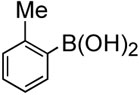 |
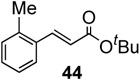 |
84 |
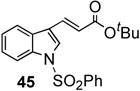
| 8 | 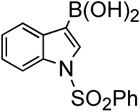 |
 |
63 |
| 9 | 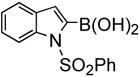 |
 |
49 |
Isolated yields.
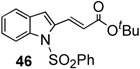
In addition, we examined the reaction of heterocyclic boronic acids. The coupling reaction involving 1-(phenylsulfonyl)-indol-3-boronic acid proceeded to afforded the corresponding cross-coupling compound 45 in 63% yield (entry 8). Reactions with 1- (phenylsulfonyl)-indol-2-boronic acid gave the cross-coupling compound 46 in only moderate yields due to protodeboronation (entry 9). It is well known that heteroarylboronic acids bearing the boron atom ortho to the heteroatom are prone to protodeboronation.23 Nontheless, this synthetic method appears to be applicable to the synthesis of heterocyclic templates used in medicinal chemistry. Overall, this oxidative palladium(II) catalysis offers an efficient method for aryl-alkenyl C-C bond formation under mild conditions.
3. Oxidative Pd(II) Catalysis for Unusual Cycloalkenyl Substrates
During the catalytic cycle, β-elimination has been known to occur through concerted syn-elimination of Pd-H without aid of bases, which explained the high E-stereoselectivity of the Heck type reaction. Nonetheless, the Pd-H was scavenged quickly by bases after syn-elimination step, and bases seem to play a key role in the β-elimination step. Contrary to acyclic substrates, the base is found to be an important factor for the coupling reaction with cyclic substrates in our protocol as described below.
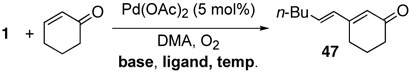
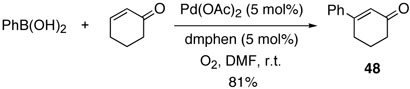
Although cyclohexenone has been known to be a difficult substrate for direct Hecktype conversion, we obtained the desired cross-coupling product 47 in a high yield along with the homo-coupled product in the presence of bases (table 16, entry 1).24 However, the same base-free reaction conditions for acyclic alkenes yielded diene compound 47 in a poor yield, implying that β-elimination was inefficient in the absence of a base (entry 2). However, when 1,10-phenanthroline was used as a palladium ligand, the catalysis was rapid, and the yield was increased to 82% without the homo-coupled product. Likewise, the cross-coupling reaction of cyclohexeneone with phenylboronic acid afforded the desired product 48 in a good yield (Scheme 4).
Table 16.
The Effect of Bases and Ligands on the Cross-Coupling Reaction of Cyclohexenone with Vinyl Boronic Compounds
amount of palladium was 2 mol%.
phen = 1,10-phenanthroline
Scheme 4.
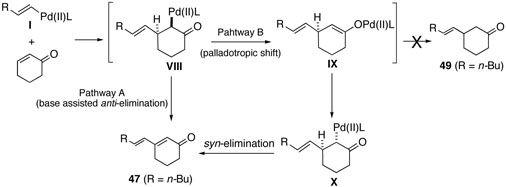
As shown in scheme 5, we assumed two pathways for the coupling reaction with cyclohexenone: i) base catalyzed β-elimination via E2 like mechanism (pathway A); ii) palladotropic shift followed by normal syn-elimination (pathway B).1(c) We thought that β-elimination in the presence of bases would take place in an anti-fashion from VIII in pathway A, because the syn-elimination in pathway B would not be affected to a great extent by the presence of bases. In addition, Michael addition product 49 was not observed at all, suggesting that pathway B can be ruled out. Therefore, in the absence of bases, the pathway A was very slow resulting in low yields of the coupled product. These findings suggested base assisted β-elimination process should be considered in the mechanism for the Heck type reaction with cyclic systems. Based on this assumption, phenanthroline would work as not only a palladium ligand but also a base participated in the β-elimination step.
Scheme 5.
4. Competition Experiments
In order to understand kinetic and mechanistic differences, we studied intermolecular competition reaction using p-methoxyiodobenzene, N,N-dimethylaminophenyl boronic acid, and tert-butyl acrylate. As shown in scheme 6, coupling reaction with an equimolar amount of each compound under the oxidative conditions afforded only boron Heck-type compound 40 without any other coupling compounds such as Heck and Suzuki coupling products.
Scheme 6.
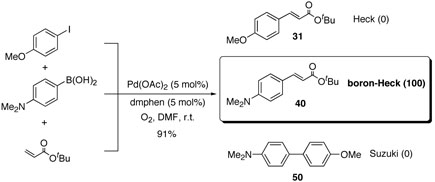
This result implied that Heck reaction was suppressed under these coupling conditions due to lack of bases, relatively low temperature, or oxygen atmosphere. To confirm these assumptions, we conducted Heck coupling reaction of pmethoxyiodobenzene with tert-butyl acrylate under the same conditions except nitrogen atomosphere (table 17).
Table 17.
The Bases and Temperature Effect on the Suzuki-Miyaura Reaction in the Presence of Ligand
 | |||
|---|---|---|---|
| entry | base | temp | yield |
| 1 | - | r.t. | no reaction |
| 2 | Na2CO3 | r.t. | < 10% |
| 3 | Na2CO3 | 50 °C | 95% |
Independent of bases, Heck coupling reaction was not efficient at room temperature (entries 1 and 2). Only at high temperature, the coupling reaction proceeded well to afford the coupling compound 31 with good yield in the presence of bases (entry 3). These results showed that oxidative boron Heck was more feasible than Heck reaction.
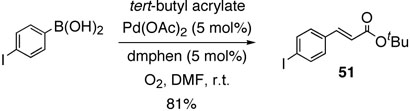
With these results, we undertook intramolecular competition experiment with 4-iodophenylboronic acid and tert-butyl acrylate (Scheme 7). As expected, boron Heck reaction product 51 was exclusively produced, and no other coupling products were obtained. This established high chemoselectivity would open the door to wide application.
Scheme 7.
Conclusion
We have demonstrated the first example of oxygen promoted palladium catalyzed cross-coupling of alkenyl and aryl boron compounds with various alkenes in the absence of bases, which afford the corresponding conjugated diene compounds without homo-coupled products. While Heck and Suzuki type reactions require bases to facilitate coupling reactions, bases are not a requisite element for the oxidative palladium(II) catalysis, but useful in transmetallation and β-hydride elimination steps resulting in the enhancement of reaction rates. Additionally, base-free and bidentated nitrogen ligands stabilized palladium(II) species successfully catalyzed cross-coupling reactions to prevent precipiation of Pd(0) species and enhanced reactivity of palladium catalysts, resulting in the enhancement of yields under milder reaction conditions. Therefore, our new synthetic protocol is advantageous over the conventional and modified Heck conditions and allows a wide array of substrates otherwise difficult or impossible to utilize. Due to its easy and convenient nature, this methodology for carbon-carbon bond formation is highly practical, holding great promise for wide use in organic and medicinal chemistry fields. Studies on asymmetric catalysis are currently in progress by using chiral amine ligands, and their successful results will be reported in due coure.
Experimental Section
General procedure A for the coupling reaction with alkenyl pinacolboronic ester and olefin in the absence of base (Base Free Conditions):
To a solution of olefin (1.5 mmol) in N,N-dimethylacetamide (2.5 mL, C = 0.2 M), was added pinacolboronic ester (0.5 mmol) followed by a single addition of Pd(OAc)2 (0.025 mmol). The reaction flask was fitted with an oxygen balloon. The resulting reaction was stirred for 6 hours at 50 °C, diluted with ethyl acetate (20 mL), and washed with water (2 X 10 mL). The separated organic layer was dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated in vacuo, and subjected to flash chromatography affording a cross-coupling compound.
General procedure B for the couling reaction with alkenyl pinacolboronic ester and olefin in the presence 1,10-phenanthroline as a ligand:
To a premixed solution of palladium acetate (0.025 mole) and 1,10-phenanthroline as a ligand (0.028 mmol) in DMF (2.5 mL) for 30 minutes, was added olefin (1.5 mmol) and pinacolboronic ester (0.5 mmol). The reaction flask was fitted with an oxygen balloon and the reaction mixture was stirred at room temperature for 12 hour, then diluted with ethyl acetate (20 mL), and washed with water and brine (2 X 10 mL). The separated organic layer was dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated in a vacuo and the residue was chromatographed on silica gel to give a cross-coupled product.
General procedure C for the coupling reaction with arylboronic acid and olefin in the presence of 2,9-dimethyl-phenanthroline as a ligand:
To a premixed solution of palladium acetate (0.025 mole) and 2,9-dimethyl-phenanthroline as a ligand (0.028 mmol) in DMF (2.5 mL) for 30 minutes, were added olefin (1.5 mmol) and arylboronic acid (0.5 mmol). The reaction flask was fitted with an oxygen balloon and the reaction mixture was stirred at room temperature for 12 hours, then diluted with ethyl acetate (20 mL), and washed with saturated aqueous NaHCO3, water and brine (2 X 10 mL). The separated organic layer was dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated in vacuo and the residue was chromatographed on silica gel to give a cross-coupled product.
Procedures for the coupling of cyclohexenone:
(1E)-3-Hex-1-enyl-cyclohex-2-enone (47):
Following the general procedure A, cross coupling reaction of boronic ester 1 with cyclohexenone afforded diene 47 (37%). Following the general procedure B, cross coupling reaction of boronic ester 1 with cyclohexenone afforded diene 47 for 2 hours (82%): 1H NMR (250 MHz, CDCl3) δ = 6.19 (m, 2 H), 5.85 (s, 1 H), 2.41 (m, 4 H), 2.16 (m, 2 H), 2.00 (m, 2 H), 1.35 (m, 4 H), 0.89 (t, J = 7.0 Hz, 3 H); 13C NMR (63 MHz, CDCl3) δ = 200.5, 157.7, 139.2, 131.3, 126.4, 37.7, 32.9, 31.0, 25.0, 22.3, 22.2, 13.9; Anal. calcd for C12H18O: C 80.85, H 10.18, found: C 80.82, H 10.19
(1E)-3-Phenyl-cyclohex-2-enone (48):
Following the general procedure C, cross coupling reaction of phenylboronic acid with cyclohexenone afforded 48 (81%): 1H NMR (300 MHz, CDCl3) δ = 7.52 (m, 2H), 7.39 (m, 3H), 6.40 (s, 1H), 2.76 (t, J = 5.6 Hz, 2H), 2.47 (t, J = 6.8 Hz, 2 H), 2.14 (q, J = 6.8 Hz, 2 H); 13C NMR (75.5 MHz, CDCl3) δ = 199.9, 159.8, 138.6, 130.0, 128.7, 126.0, 125.3, 115.4, 37.1, 28.0, 22.7; Anal. calcd for C12H12O: C 83.69, H 7.02, found: C 83.69, H 7.05
Supplementary Material
Table 11.
The Effect of Substituent on the Arylboron Compounds on the Cross-Coupling Reactions under Base-Free Conditions
 | |||
|---|---|---|---|
| entry | arylboron compound | product | yield (%)a |
| 1 |  |
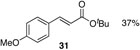 |
37% |
| 2 | 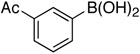 |
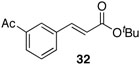 |
41% |
isolated yield
Table 13.
a The Effect of Solvents on the Cross-Coupling Reactions of Aryl Boronic Acid with Alkene in the Presence of Ligand
 | ||||
|---|---|---|---|---|
| entry | solvent | yield (%)b |
||
| 28 | 29 | 30 | ||
| 1 | DMF | 84 | - | 13 |
| 2 | DMA | 77 | - | 22 |
| 3 | NMP | 77 | - | 23 |
| 4 | MeCN | 63 | - | 37 |
| 5 | THF | 35 | 23 | 42 |
All reactions were carried out with phenylboronic acid (1 mmol), tert-butyl acrylate (1.5 mmol), 1,10-dimethylphenanthroline (5mol%), and Pd(OAc)2 (5 mol%) in solvent (2.5 mL).
isolated yield.
Acknowledgments
We acknowledge generous financial support from the National Institute of General Medical Sciences of the National Institutes of Health (RO1 GM 71495). We thank professor Chulbom Lee for helpful discussion. This paper is dedicated to Professor William Weber for his retirement from the 37 years of service at USC.
Footnotes
Supporting Information Available
Full experimental procedures and spectral data (1H and 13C NMR) for all products (pdf). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Heck RF. Org. React. 1982;27:345. For reviews of the Heck reaction, see: [Google Scholar]; (b) Gürtler C, Buchwald SL. Chem. Eur. J. 1999;5:3107. [Google Scholar]; (c) Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]; (d) Whitcombe NJ, Hii KK, Gibson SE. Tetrahedron. 2001;57:7449. [Google Scholar]; (e) Kondolff I, Doucet H, Santelli M. Tetrahedron Lett. 2003;44:8487. [Google Scholar]
- 2.(a) Miyaura N. J. Organomet. Chem. 2002;653:54. For reviews of the Suzuki-Miyaura reaction, see: [Google Scholar]; (b) Miyaura N. Top. Curr. Chem. 2002;219:11. [Google Scholar]; (c) Suzuki A. J. Organomet. Chem. 1999;576:147. [Google Scholar]; (d) Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]
- 3.Farina V, Krishnamurthy V, Scott W. J. Org. React. 1997;50:1–652. For a review of the Stille reaction, see: [Google Scholar]
- 4.(a) Littke AF, Fu GC. J. Am. Chem. Soc. 2001;123:6989. doi: 10.1021/ja010988c. [DOI] [PubMed] [Google Scholar]; (b) Stambuli JP, Stauffer SR, Shaughnessy KH, Hartwig JF. J. Am. Chem. Soc. 2001;123:2677. doi: 10.1021/ja0058435. [DOI] [PubMed] [Google Scholar]
- 5.(a) Akiyama F, Miyazaki H, Kaneda K, Teranishi S, Fujiwara Y. J. Org. Chem. 1980;45:2359. [Google Scholar]; (b) Kikukara K, Nagira K, Wada F, Matsuda T. Tetrahedron. 1981;37:31. [Google Scholar]; (c) Sengupta S, Bhattacharyya S. J. Chem. Soc., Perkin Trans. 1993;1:1943. [Google Scholar]
- 6.(a) Hamann BC, Hartwig JF. J. Am. Chem. Soc. 1998;120:7369. [Google Scholar]; (b) Fu X, Thiruvengadam TK, Zhang F. Tetrahedron Lett. 2002;43:573. [Google Scholar]; (c) Fu X, Zhang S, Yin J, Schumacher DP. Tetrahedron Lett. 2002;43:6673. [Google Scholar]; (d) Roy AH, Hartwig JF. J. Am. Chem. Soc. 2003;125:8704. doi: 10.1021/ja035835z. [DOI] [PubMed] [Google Scholar]; (e) Roy AH, Hartwig JF. Organometallics. 2004;23:194. [Google Scholar]; (f) Klapars A, Campos KR, Chen C-Y, Volante RP. Org. Lett. 2005;7:1185. doi: 10.1021/ol050117y. [DOI] [PubMed] [Google Scholar]; (g) Hanse AL, Skrydstrup T. Org. Lett. 2005;7:5585. doi: 10.1021/ol052136d. [DOI] [PubMed] [Google Scholar]
- 7.(a) Blaser H-U, Spencer A. J. Organomet. Chem. 1982;233:267. [Google Scholar]; (b) Spencer A. J. Organomet. Chem. 1983;247:117. [Google Scholar]; (c) Spencer A. J. Organomet. Chem. 1984;270:115. [Google Scholar]
- 8.Zeni G, Lu DS, Panatieri RB, Braga AL. Chem. Rev. 2006;106:1032. doi: 10.1021/cr0505730. [DOI] [PubMed] [Google Scholar]
- 9. Compared to the time when Heck discovered the original conditions, there are currently an astronomical number of commercially available boronic acids and esters.
- 10.(a) Andappan MMS, Nilsson P, Larhed M. Chem. Commun. 2004. p. 218. For oxygen promoted Pd catalyzed cross-coupling reactions, see: [DOI] [PubMed]; (b) Zou G, Zhu J, Tang J. Tetrahedron Lett. 2003;44:8709. [Google Scholar]; (c) Dams M, De Vos DE, Celen S, Jacobs PA. Angew. Chem. Int. Ed. 2003;42:3512. doi: 10.1002/anie.200351524. [DOI] [PubMed] [Google Scholar]; (d) Matoba K, Motofusa S-I, Cho CS, Ohe K, Uemura S. J. Organomet. Chem. 1999;574:3. [Google Scholar]; (e) Du X, Suguro M, Hirabayashi K, Mori A. Org. Lett. 2001;3:3313. doi: 10.1021/ol016529y. For Pd catalyzed cross-coupling reactions with Cu(II) as an oxidant: [DOI] [PubMed] [Google Scholar]
- 11.(a) Yoon CH, Yoo KS, Yi SW, Mishra RK, Jung KW. Org. Lett. 2004;6:4037. doi: 10.1021/ol0483192. [DOI] [PubMed] [Google Scholar]; (b) Jung YC, Mishra RK, Yoon CH, Jung KW. Org. Lett. 2003;5:2231. doi: 10.1021/ol034458s. [DOI] [PubMed] [Google Scholar]; (c) Parrish JP, Jung YC, Shin SI, Jung KW. J. Org. Chem. 2002;67:7127. doi: 10.1021/jo020159p. [DOI] [PubMed] [Google Scholar]
- 12.(a) Stahl SS. Angew. Chem. Int. Ed. 2004;43:3400. doi: 10.1002/anie.200300630. [DOI] [PubMed] [Google Scholar]; (b) Steinhoff BA, Fix SR, Stahl SS. J. Am. Chem. Soc. 2002;124:766. doi: 10.1021/ja016806w. [DOI] [PubMed] [Google Scholar]; (c) Stahl SS, Thorman JL, Nelson RC, Kozee MA. J. Am. Chem. Soc. 2001;123:7188. doi: 10.1021/ja015683c. [DOI] [PubMed] [Google Scholar]; (d) Ferreira EM, Stoltz BM. J. Am. Chem. Soc. 2001;123:7725. doi: 10.1021/ja015791z. [DOI] [PubMed] [Google Scholar]; (e) Jensen DR, Pugsley JS, Sigman MS. J. Am. Chem. Soc. 2001;123:7475. doi: 10.1021/ja015827n. [DOI] [PubMed] [Google Scholar]; (f) Brink GT, Arends IWCE, Sheldon RA. Science. 2000;287:1636. doi: 10.1126/science.287.5458.1636. [DOI] [PubMed] [Google Scholar]; (g) Peterson KP, Larock RC. J. Org. Chem. 1998;63:3185. [Google Scholar]
- 13.Adamo C, Amatore C, Ciofini L, Jutand A, Lakmini H. J. Am. Chem. Soc. 2006;128:6829. doi: 10.1021/ja0569959. [DOI] [PubMed] [Google Scholar]
- 14.(a) Yoshida H, Yamaryo Y, Ohshita J, Kunai A. Tetrahedron Lett. 2003;44:1541. For oxygen promoted Pd catalyzed homocoupling reactions, see: [Google Scholar]; (b) Mukhopadhyay S, Rothenberg G, Lando G, Agbaria K, Kazanci M, Sasson Y. Adv. Synth. Catal. 2001;343:455. [Google Scholar]; (c) Hossain KM, Kameyama T, Shibata T, Tagaki K. Bull. Chem. Soc. Jpn. 2001;74:2415. [Google Scholar]; (d) Wong MS, Zhang XL. Tetrahedron Lett. 2001;42:4087. [Google Scholar]; (e) Ohe T, Tanaka T, Kuroda M, Cho CS, Uemura S. Bull. Chem. Soc. Jpn. 1999;72:1851. [Google Scholar]; (f) Smith KA, Campi EM, Jackson WR, Marcuccio S, Maeslund CGM, Deacon GB. Synlett. 1997. p. 131.
- 15. Without amine ligands, the reaction mixture turned to black while the solution stayed clear in the presence of amine ligands.
- 16.Yoshida H, Yamaryo Y, Ohshita J, Kunai A. Tetrahedron Lett. 2003;44:1541. For an example of homocoupling with Pd(OAc)2 in the presence of phosphine ligands, see; [Google Scholar]
- 17.(a) Beletskaya IP, Cheprakov AV. Chem. Rev. 2002;100:3009. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]; (b) Crisp GT, Glink PT. Tetrahedron. 1994;50:2623. [Google Scholar]; (c) Kim J-I, Patel BA, Heck RF. J. Org. Chem. 1981;46:1067. [Google Scholar]
- 18.(a) Molander GA, Ribagorda M. J. Am. Chem. Soc. 2003;125:11148. doi: 10.1021/ja0351140. [DOI] [PubMed] [Google Scholar]; (b) Ueda M, Saitoh A, Miyaura N. J. Organomet. Chem. 2002;642:145. [Google Scholar]
- 19.(a) Costa A, Najera C, Sansano JM. J. Org. Chem. 2002;67:5216. doi: 10.1021/jo025620s. [DOI] [PubMed] [Google Scholar]; (b) Gelpke AES, Veerman JJN, Goedheijt MS, Kamer PCJ, van Leeuwen PWMN, Hiemstra H. Tetrahedron. 1999;55:6657. [Google Scholar]; (c) Genet JP, Blart E, Savignac M. Synlett. 1992. p. 715.
- 20.(a) Enquist P-A, Lindh J, Nilsson P, Larhed M. Green Chem. 2006;8:338. [Google Scholar]; (b) Andappan MMS, Nilsson P, Larhed M. Chem. Commun. 2004. p. 218. [DOI] [PubMed]; (c) von Schenck H, Akermark B, Svensson M. J. Am. Chem. Soc. 2003;125:3503. doi: 10.1021/ja028755o. [DOI] [PubMed] [Google Scholar]; (d) von Schenck H, Akermark B, Svensson M. Organomet. 2002;21:2248. [Google Scholar]; (e) Olofsson K, Larhed M, Hallberg A. J. Org. Chem. 2000;65:7235. doi: 10.1021/jo000824z. [DOI] [PubMed] [Google Scholar]; (f) Cabri W, Candiani I, Bedeschi A. J. Org. Chem. 1993;58:7421. [Google Scholar]
- 21.Portnoy M, Ben-David. Y, Roussa I, Milstein D. Organometallics. 1994;13:3465. [Google Scholar]
- 22.Moreno-Manas M, Perez M, Pleixats R. J. Org. Chem. 1996;61:2346. doi: 10.1021/jo9514331. [DOI] [PubMed] [Google Scholar]
- 23.(a) Roques BP, Florentin D, Callanquin M. J. Heterocycl. Chem. 1975;12:195. [Google Scholar]; (b) Florentin D, Fournie-Zaluski MC, Callanquin M, Roques BP. J. Heterocycl. Chem. 1976;13:1265. [Google Scholar]
- 24.(a) Krishna TR, Jayaraman N. Tetraheron Lett. 2004;60:10325. For the Heck reaction with cyclohexenone, see: [Google Scholar]; (b) Gupta AK, Song CH, Oh CH. Tetrahedron Lett. 2004;45:4113. [Google Scholar]; (c) Gelpke AES, Veerman JJN, Goedheijt MS, Kamer PCJ, van Leeuwen PWNM, Hiemstra H. Tetrahedron. 1999;55:6657. [Google Scholar]
- 25.Laurent D, Morris S. J. Org. Chem. 1994;59:6871. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.