The success of antitumor and antiviral vaccines often requires the use of an adjuvant, a substance that is itself not necessarily immunogenic but significantly enhances the immune response of a patient to a coadministered antigen.[1] The adjuvant of choice in many recent immunotherapeutic advances against cancer,[2] human immunodeficiency virus,[3, 4] and malaria[5, 6] is the natural product saponin QS-21.[7] Extensive studies in the development of conjugate cancer vaccines demonstrated the superiority of QS-21 over 18 competing adjuvants in preclinical models.[8]
Isolated in minute quantities from the cortex of the Quillaja saponaria (QS) tree, QS-21 is not a homogeneous substance; rather, it is the 21st of 22 fractions in an early reversed-phase (RP) HPLC trace of semipurified QS-bark extracts.[7, 9] This adjuvant-active fraction comprises at least two principal constituents (Scheme 1), QS-21-Xyl (1) and QS-21-Api (2), which differ in the terminal sugar residue within the linear tetrasaccharide segment.[10, 11] Although the multi-component QS-21 fraction has fueled numerous previous and ongoing vaccine clinical trials, the challenge to procure samples of consistent composition from natural sources is formidable. Indeed, high variability in saponin composition is seen even among QS trees of similar age and local environment (e.g., soil, season).[12] Furthermore, recent metabolomic analyses of semipurified QS-bark extracts revealed a complex mixture of over 100 distinct saponins,[13] considerably more than what the original 22-fraction RP HPLC profile might imply.[7, 9] Because of the variability and heterogeneity of QS extracts, it is difficult to establish the adjuvant activity and toxicity of the component saponins. The possibility that trace quantities of additional saponins may be present in the QS-21 fraction impacts not only on efficacy and formulation aspects, but also on regulatory hurdles in clinical vaccine development in humans.
Scheme 1.
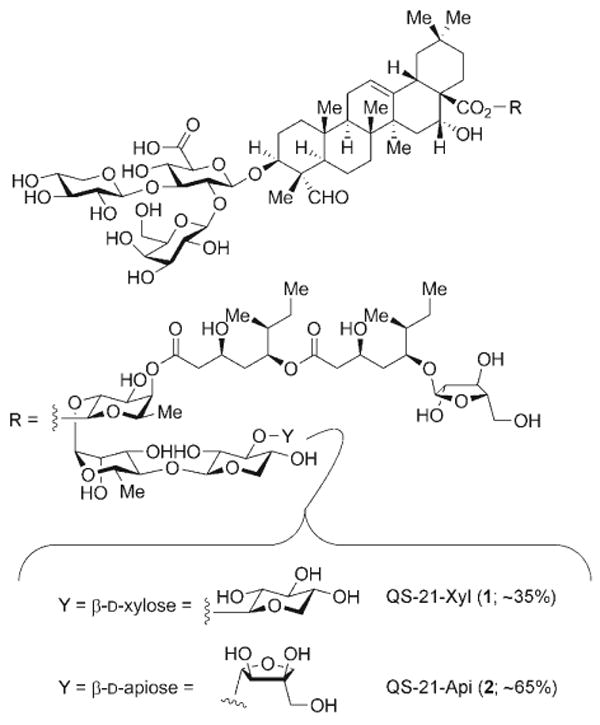
Structures of the principal components of QS-21.
Efforts to further advance QS-21 in the clinic, as well as to illuminate its unknown mechanism of action, require access to adjuvant-active samples of known composition. The recent chemical synthesis of the isomer QS-21-Api (2) enabled independent access to homogenous samples of this particular saponin.[14] We now report the chemical synthesis of the second principal isomer, QS-21-Xyl (1), and thereby verify its structural constitution unambiguously. The synthesis of 1 has enabled preclinical evaluation of the two synthetically derived adjuvant isomers 1 and 2 (administered as a 35:65 mixture to mimic the composition of natural QS-21 isolated from QS bark) by using the GD3-KLH conjugate melanoma vaccine in mice.
The chemical synthesis of QS-21-Xyl (1) builds on the foundation established in the synthesis of QS-21-Api (2).[14] Given the many common structural elements of the two saponins, several of the advanced synthetic intermediates in the synthesis of 2 could be applied directly to the synthesis of 1. These intermediates include the selectively protected trisaccharide–triterpene conjugate 3, the glycosylated pseudodimeric normonoterpene acyl chain 4, and two selectively protected monosaccharides, the xylopyranose 5 and the fucopyranoside 6 (Scheme 2).
Scheme 2.
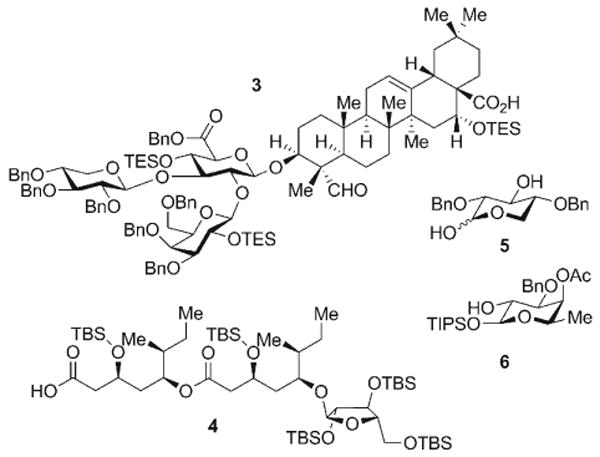
Advanced intermediates prepared during the synthesis of QS-21-Api (2) and employed in the current synthesis of QS-21-Xyl (1). Bn = benzyl, TBS = tert-butyldimethylsilyl, TES = triethylsilyl, TIPS = triisopropylsilyl.
Construction of the linear tetrasaccharide fragment of QS-21-Xyl (1) commenced with 4-O-benzyl-2,3-isopropylidene-l-rhamnopyranose (7; Scheme 3). The silylation of 7 with TIPSOTf afforded the corresponding α-silyl rhamnoside (96%), which was subjected to hydrogenolysis of the 4-O-benzyl ether to provide the rhamnoalcohol 8 (98%). This alcohol served as a competent glycosyl acceptor in a chemo- and stereoselective dehydrative glycosylation (Ph2SO·Tf2O)[15] with the xylopyranose diol 5 to afford the β-disaccharide 9 (75%) as a single anomer. The remaining hydroxy group in disaccharide 9 was then glycosylated with the trichloroacetimidate 11[16] under BF3·OEt2 catalysis to afford the β-trisaccharide 12 (77%). The final sugar residue of the tetrasaccharide was introduced by conversion of the anomeric silylacetal in trisaccharide 12 into its α-trichloroacetimidate 13 in a two-step procedure through exposure first to TBAF and then to trichloroacetonitrile and DBU (89%, 2 steps). This trisaccharide donor was then activated by TMSOTf catalysis to effect α glycosylation of the selectively protected fucopyranoside acceptor 6 and yield the linear tetrasaccharide 14 (75%). Deacetylation of the oxygen atom at C4 with DIBAL-H furnished the fully elaborated tetrasaccharide fragment 15 (83%) for the late-stage convergent assembly of the saponin target 1.
Scheme 3.
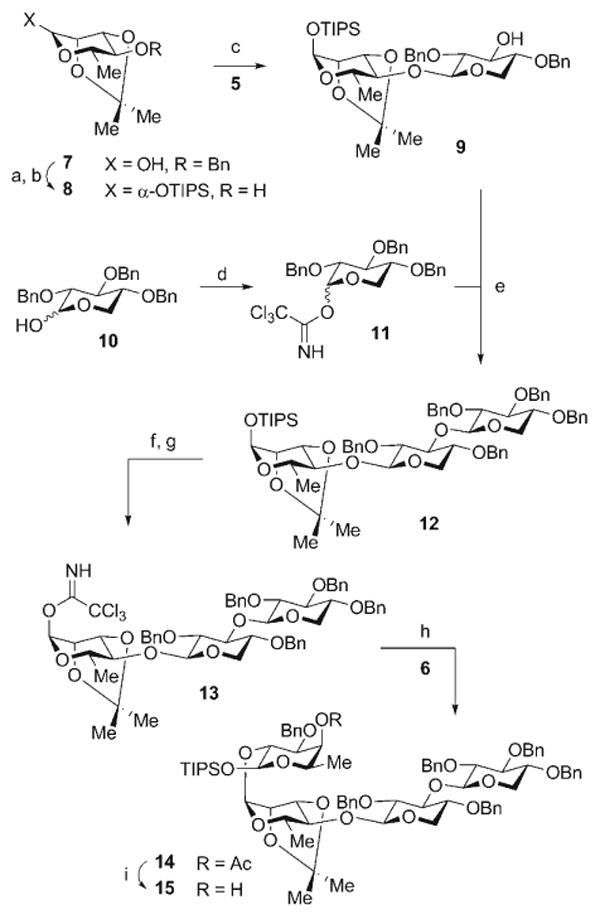
Reagents and conditions: a) TIPSOTf, 2,6-lutidine, CH2Cl2, 0→23 °C, 96%; b) Pd-C, H2, MeOH, 23 °C, 98%; c) 5, Ph2SO, Tf2O, TBP, CH2Cl2, −78°C; then 8, −78→35 °C, 75%; d) CCl3CN, NaH, CH2Cl2, 23°C, 82% (α/β 4:1); e) BF3·OEt2, CH2Cl2, −35°C, 77%; f) TBAF, THF, 0°C, >99%; g) CCl3CN, DBU, CH2Cl2, 0°C, 90%; h) 6, TMSOTf, Et2O, 0°C, 75%; i) DIBAL-H, CH2Cl2, −78°C, 83%. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DIBAL-H = diisobutylaluminum hydride, TBAF = tetrabutylammonium fluoride, TBP = tri-tert-butylpyridine, Tf = trifluoromethanesulfonyl, TMS = trimethylsilyl.
The attachment of the normonoterpene-derived acyl chain 4 to the tetrasaccharide 15 proceeded efficiently under Yamaguchi mixed-anhydride conditions[17] to give 16 (89%). Subsequent desilylation (TBAF, 66%) was followed by the formation of the α-anomeric trichloroacetimidate 17 (73%; Scheme 4). This complex intermediate served as an efficient donor for β glycosylation of the triterpene–trisaccharide conjugate 3 to afford the fully protected saponin target 18 (80%). The final stage of the synthesis required global deprotection, which was optimized through extensive studies, by TFA-mediated hydrolysis of the silyl ethers and isopropylidene ketal, followed by mild hydrogenolysis with Pd-C (Degussa) of all benzyl protecting groups. Care was taken in the final step to avoid reduction of the aldehyde functionality within the triterpene moiety. This sequence resulted in the generation of QS-21-Xyl (1, 64% after RP HPLC), which was found to be identical to the principal component of 1 isolated from natural sources.[18]
Scheme 4.
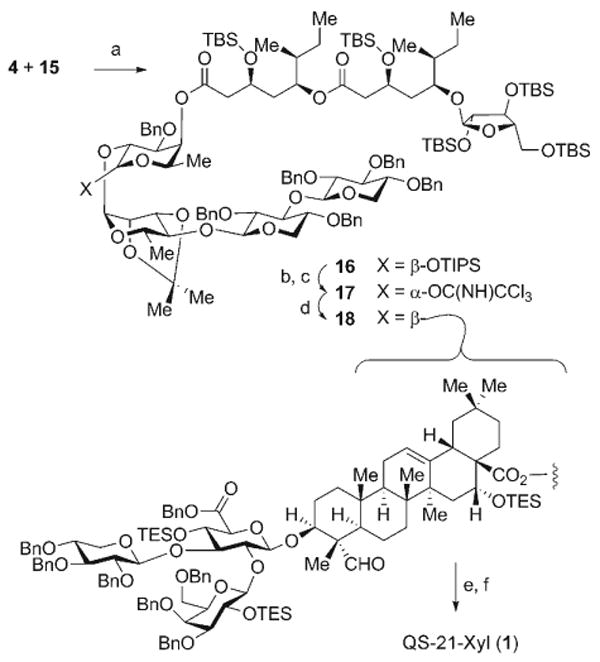
Reagents and conditions: a) 4, 2,4,6-C6H2Cl3COCl, Et3N, PhMe; then 15, DMAP, 23°C, 89%; b) TBAF, THF, 0°C, 66% (16: 12%); c) CCl3CN, DBU, CH2Cl2, 0°C, 73%; d) 3, BF3·OEt2, 4-Å molecular sieves, CH2Cl2, −78→23°C, 80%; e) TFA, H2O, 0°C; f) H2, Pd-C, MeOH, THF, 23°C, 64% (2 steps). DMAP = 4-dimethylaminopyridine, TFA = trifluoroacetic acid.
With QS-21-Xyl (1) and QS-21-Api (2) both accessible by chemical synthesis, it was now possible to assess the adjuvant activity of pure synthetic QS-21 devoid of any potential trace quantities of other natural QS saponins. Adjuvant activity was evaluated with the melanoma vaccine antigen GD3 conjugated to the immunocarrier protein keyhole limpet hemocyanin (KLH; Scheme 5).
Scheme 5.
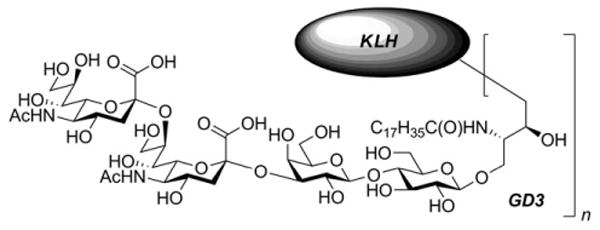
Structure of GD3-KLH conjugate.
Three groups of five C57BL/6J mice (Table 1) received three sequential vaccinations with GD3-KLH conjugate (10 μg equivalent of GD3 in GD3-KLH conjugate). As a negative control, the first group was vaccinated with no adjuvant; the second group received 10 μg of synthetic QS-21 (sQS-21, a 65:35 mixture of QS-21-Api and QS-21-Xyl). As a positive control, the third group was administered 100 μg of GPI-0100, a semisynthetic adjuvant known to be active with this antigen system, albeit with an approximately 10-fold lower potency per dose.[19] Vaccines were administered subcutaneously to each mouse in weeks 1, 2, and 3. Mice were bled 7 days after the third vaccination, and the presence of antibodies was examined by enzyme-linked immunosorbent assays (ELISA) to determine antibody responses against GD3 and KLH.[20] Serially diluted pre- and post-treatment sera were added to GD3- and KLH-coated ELISA plates, which were then washed. Specific antibody detection was carried out by treatment with goat antimouse immunoglobulin M (IgM) or immunoglobulin G (IgG) conjugated with alkaline phosphatase, followed by measurement of the absorbance of the sample at 405 nm. The titer is defined as the highest serum dilution at which an absorbance greater than 0.1 OD405 higher than that of the preserum was observed.
Table 1.
Antibody responses in mice (C57BL/6J; female) after subcutaneous vaccination with GD3-KLH (10 μg) and various adjuvants.[a]
| IgM titer against GD3 | IgG titer against KLH | ||||
|---|---|---|---|---|---|
| No adjuv.[b] | sQS-21 (10 μg)[c,d] | GPI-0100 (100 μg)[e] | No adjuv.[b] | sQS-21 (10 μg)[c,d] | GPI-0100 (100 μg)[e] |
| 0 | 320 | 200 | 3200 | 204800 | 819200 |
| 0 | 80 | 400 | 3200 | 204800 | 204800 |
| 0 | 80 | 100 | 51200 | 819200 | 819200 |
| 0 | 320 | 400 | 12800 | 819200 | 819200 |
| 0 | 640 | 400 | 12800 | 819200 | 819200 |
Serum was collected 7 days after the third injection. Antibody titers (ELISA) for individual mice are reported.
Mouse group 1.
Mouse group 2.
A 35:65 mixture of synthetic 1 and synthetic 2 was used.
Mouse group 3.
No detectable anti-GD3 or anti-KLH antibodies were present in prevaccination sera. As expected, mice immunized with GD3-KLH alone (without the adjuvant) failed to induce any antibody response against GD3 (Table 1, column 1 and Figure 1), and only low titers against KLH were observed (Table 1, column 4). Mice immunized with GD3-KLH conjugate plus either sQS-21 (10 μg) or GPI-0100 (100 μg) produced antibodies against both GD3 and KLH. For mice immunized with GD3-KLH plus sQS-21, the median anti-GD3 IgM and anti-KLH IgG titers were 320 and 819200, respectively (Table 1, columns 2 and 5). These titers were comparable to the respective median values of 400 and 819200 (Table 1, columns 3 and 6) found with the positive control GPI-0100. These data confirm the in vivo adjuvant activity of sQS-21 against GD3-KLH at levels comparable to that previously reported for GD3-KLH with natural QS-21.[21, 22]
Figure 1.
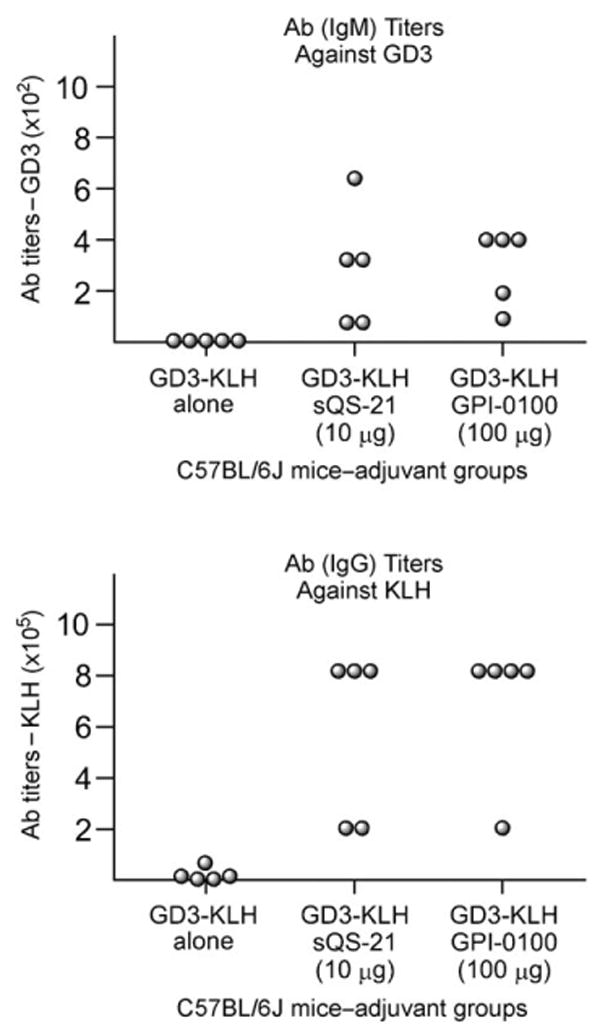
Graphical representation of the results in Table 1. Ab = antibody.
In summary, we have described the chemical synthesis and structural verification of QS-21-Xyl (1), a key isomeric constituent of the clinical adjuvant QS-21. Moreover, the establishment of the preclinical adjuvant activity of synthetic QS-21 marks the necessary first step in the unambiguous establishment of the bioactivity of the two principal constituents of the natural product fraction, QS-21-Xyl (1) and QS-21-Api (2). This study opens the door for the use of synthetic QS saponins of defined molecular composition in imminent clinical trials. Indeed, on the basis of these preclinical data, a protocol for the use of synthetic QS-21 (sQS-21) for a phase-I clinical trial with a GD3 and GD2 bivalent melanoma vaccine has been approved by an institutional review board (IRB).[23]
Supplementary Material
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200801885.
Footnotes
This research was supported by the NIH (GM58833), William H. Goodwin & Alice Goodwin & The Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of MSKCC.
Contributor Information
Govind Ragupathi, Email: ragupatg@mskcc.org.
David Y. Gin, Email: gind@mskcc.org.
References
- 1.Jiang ZH, Koganty RR. Curr Med Chem. 2003;10:1423–1439. doi: 10.2174/0929867033457340. [DOI] [PubMed] [Google Scholar]
- 2.Livingston PO, Ragupathi G. Hum Vaccines. 2006;2:137–143. doi: 10.4161/hv.2941. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki S, Sumino K, Hamajima K, Fukushima J, Ishii N, Kawamoto S, Mohri H, Kensil CR, Okuda K. J Virol. 1998;72:4931–4939. doi: 10.1128/jvi.72.6.4931-4939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans TG, McElrath MJ, Matthews T, Montefiori D, Weinhold K, Wolff M, Keefer MC, Kallas EG, Corey L, Gorse GJ, Belshe R, Graham BS, Spearman PW, Schwartz D, Mulligan MJ, Goepfert P, Fast P, Berman P, Powell M, Francis D. Vaccine. 2001;19:2080–2091. doi: 10.1016/s0264-410x(00)00415-1. [DOI] [PubMed] [Google Scholar]
- 5.Kashala O, Amador R, Valero MV, Moreno A, Barbosa A, Nickel B, Daubenberger CA, Guzman F, Pluschke G, Patarroyo ME. Vaccine. 2002;20:2263–2277. doi: 10.1016/s0264-410x(02)00115-9. [DOI] [PubMed] [Google Scholar]
- 6.Carcaboso AM, Hernandez RM, Igartua M, Rosas JE, Patarroyo ME, Pedraz JL. Vaccine. 2004;22:1423–1432. doi: 10.1016/j.vaccine.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Kensil CR, Patel U, Lennick M, Marciani D. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 8.Kim SK, Ragupathi G, Musselli C, Choi SJ, Park YS, Livingston PO. Vaccine. 1999;18:597–603. doi: 10.1016/s0264-410x(99)00316-3. [DOI] [PubMed] [Google Scholar]
- 9.Kensil CR. Crit Rev Ther Drug Carrier Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 10.Cleland JL, Kensil CR, Lim A, Jacobsen NE, Basa L, Spellman M, Wheeler DA, Wu JY, Powell MF. J Pharm Sci. 1996;85:22–28. doi: 10.1021/js9503136. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen NE, Fairbrother WJ, Kensil CR, Lim A, Wheeler DA, Powell MF. Carbohydr Res. 1996;280:1–14. doi: 10.1016/0008-6215(95)00278-2. [DOI] [PubMed] [Google Scholar]
- 12.Kamstrup S, San Martin R, Doberti A, Grande H, Dalsgaard K. Vaccine. 2000;18:2244–2249. doi: 10.1016/s0264-410x(99)00560-5. [DOI] [PubMed] [Google Scholar]
- 13.Kite GC, Howes MJR, Simmonds MSJ. Rapid Commun Mass Spectrom. 2004;18:2859–2870. doi: 10.1002/rcm.1698. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Wang P, Navarro-Villalobos M, Rohde BD, Derryberry J, Gin DY. J Am Chem Soc. 2006;128:11906–11915. doi: 10.1021/ja062364i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia BA, Gin DY. J Am Chem Soc. 2000;122:4269–4279. [Google Scholar]
- 16.Schmidt RR, Kinzy W. Adv Carbohydr Chem Biochem. 1994;50:21–123. doi: 10.1016/s0065-2318(08)60150-x. [DOI] [PubMed] [Google Scholar]
- 17.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull Chem Soc Jpn. 1979;52:1989–1993. [Google Scholar]
- 18.Natural QS-21-Xyl (1) was purified from commercial Quil A by RP HPLC to approximately 70% purity.
- 19.Marciani DJ, Press JB, Reynolds RC, Pathak AK, Pathak V, Gundy LE, Farmer JT, Koratich MS, May RD. Vaccine. 2000;18:3141–3151. doi: 10.1016/s0264-410x(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 20.Ragupathi G, Meyers M, Adluri S, Howard L, Musselli C, Livingston PO. Int J Cancer. 2000;85:659–666. doi: 10.1002/(sici)1097-0215(20000301)85:5<659::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Helling F, Shang A, Calves M, Zhang SL, Ren SL, Yu RK, Oettgen HF, Livingston PO. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- 22.Direct comparison of clinical-grade natural QS-21 (Antigenics) with sQS-21 was not possible because of licensing restrictions imposed on its use.
- 23.Phase-I clinical trials are scheduled to commence in July 2008. The results will be reported in due course.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200801885.


