Abstract
The influence of estradiol (E2) treatment on temporomandibular joint (TMJ) nociceptive processing in the caudal trigeminal sensory brain stem complex was assessed in ovariectomized female rats by quantitative Fos-immunoreactivity (Fos-LI). After 2 days of daily injections of high (HE2) or low (LE2) dose E2 rats were anesthetized and the small fiber excitant, mustard oil (MO, 0–20%), was injected into the TMJ and after two hours later brains were processed for Fos-LI. TMJ-evoked Fos-LI in laminae I-II at the trigeminal subnucleus caudalis/upper cervical cord (Vc/C1-2) junction and the dorsal paratrigeminal region (dPa5) was significantly greater in HE2 than LE2 rats, while Fos-LI produced at the ventral trigeminal interpolaris/caudalis transition region (Vi/Vcvl) was similar. E2 treatment also modified the influence of N-methyl-D-aspartate (NMDA) and amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptor antagonists on TMJ-evoked Fos-LI. The NMDA antagonist, MK-801, dose-dependently reduced the Fos-LI response at the Vc/C1-2 junction in HE2 rats, while only high dose MK-801 was effective in LE2 rats. MK801 reduced equally the Fos-LI response at the Vi/Vc transition in both groups, while only minor effects were seen at the dPa5 region. The AMPA receptor antagonist, NBQX, reduced Fos-LI at the Vc/C1-2 and Vi/Vcvl regions in HE2 rats, while only high dose NBQX was effective in LE2 rats. NBQX did not reduce Fos-LI at the dPa5 region in either group. These results suggest that estrogen status plays a significant role in TMJ nociceptive processing at the Vc/C1-2 junction mediated, in part, through ionotropic glutamate receptor-dependent mechanisms.
Keywords: pain, trigeminal subnucleus caudalis, NMDA receptor, AMPA receptor, mustard oil
Temporomandibular muscle/joint disorders (TMJD) include a heterogeneous group of conditions that present with pain in the temporomandibular joint (TMJ) and muscles of mastication. TMJD is more prevalent in women than men (Bush, 1993; Lipton, 1993; LeResche, 1997; Huang et al., 2002) and although the basis for this sex difference is not certain, systematic variation in pain severity over the menstrual cycle suggests a role for ovarian hormones (Suenaga et al., 2001; Isselee et al., 2002; LeResche et al., 2003; Landi et al., 2005).
Several aspects of TMJD pain such as poor correlation with peripheral pathology (Ohrbach and Dworkin, 1998) and widespread changes in sensory thresholds outside the jaw joint region (Hollins et al. 1996; Maixner et al., 1998; Sarlani and Greenspan, 2005) suggest a significant contribution by central neural mechanisms. The TMJ region is innervated by small diameter sensory fibers (Ioi et al., 2006; Kido et al., 1995; Takeuchi and Toda, 2003) that project mainly to the trigeminal subnucleus caudalis/upper cervical cord (Vc/C1-2) junction (Shigenaga et al., 1986; Shigenaga et al., 1988). The Vc/C1-2 junction is a laminated region that shares many features with the spinal dorsal horn such as a high density of nuclear estrogen receptors in neurons in the superficial laminae (Amandusson et al., 1996; Bereiter et al., 2005). Laminae I-II of the Vc/C1-2 junction also express moderate to high levels of NMDA (Tallaksen-Greene et al., 1992; Petralia et al., 1994; Bereiter and Bereiter, 2000) and AMPA glutamate receptor subtypes (Petralia and Weinthold, 1992; Florenzano and De Luca, 1999). NMDA and AMPA receptors mediate many aspects of nociceptive processing in the spinal dorsal horn leading to central sensitization (Woolf and Salter, 2000; Ji et al., 2003) including after articular injury (Neugebauer et al., 1993; Schaible and Grubb, 1993; Schaible et al., 2004). In the trigeminal system, preemptive treatment in male rats with MK-801, an open channel NMDA antagonist, greatly reduced the Fos-LI response to TMJ (Bereiter and Bereiter, 2000) or masseter muscle inflammation (Ro et al., 2004) and inhibited jaw muscle responses to TMJ stimulation (Yu et al., 1996); however, similar studies have not assessed the influence of glutamate receptor antagonists on TMJ-evoked Fos-LI in females and the influence of different levels of sex steroids on glutamate receptor mediated modulation on medullary dorsal horn neural activity. In forebrain and limbic regions, estrogen status significantly modifies synaptic plasticity, largely through NMDA-dependent mechanisms (see McEwen, 2002). Although the magnitude of TMJ-evoked Fos-LI at the Vc/C1-2 junction was shown to depend on the stage of the estrous cycle in intact female rats (Bereiter, 2001), it has not been determined if E2 replacement alone was sufficient to mimic this effect in OvX females. The aims of this study were two-fold: to determine if high and low doses of E2 replacement in OvX female rats differentially affected the TMJ-evoked Fos-LI responses in the caudal trigeminal brainstem nuclear complex (TBNC), including the Vc/C1-2 junction, and second, to determine if NMDA and AMPA receptor-mediated modulation of the Fos-LI responses evoked by TMJ stimulation were influenced by E2 replacement.
Experimental procedures
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota and conformed to the established guidelines set by The National Institute of Health guide for the care and use of laboratory animals (Publications No. 99-158, revised 2002).
Animal preparation
Adult ovariectomized (OvX) female rats (225–300 g, Sprague-Dawley, Harlan, Indianapolis, IN) were used within 1–3 weeks after surgery. OvX rats were given daily injections of low (LE2, 2 μg) or high (HE2, 20 μg, sc) dose 17β-estradiol-3-benzoate (E2, Sigma, St. Louis, MO) dissolved in 200 μl sesame oil for two days (2d) prior to the experiment. The timing and dosage of the low and high dose E2 replacement regimens were chosen to mimic the cyclic nature and magnitude of E2 plasma levels in diestrus and proestrus, respectively (Butcher et al., 1974). Data were collected from OvX rats without prior knowledge of the hormone treatment. Plasma E2 levels were not routinely measured in this study; however, in a previous study using a similar dosing regimen we found that low E2 and high E2 treated OvX females had 20–30 and 250–300 pg/ml, respectively (Tashiro et al., 2007). The estrogen status of E2-treated OvX rats was confirmed on the day of the experiment by the vaginal smear cytology taken by gentle lavage. Vaginal smears from LE2 females had (> 80%) small nucleated leukocytes, while smears from HE2 rats had mainly large nucleated epithelial cells plus variable numbers of cornified epithelial cells.
Experimental Design
On the day of the experiment rats were anesthetized with pentobarbital sodium (65 mg/kg, i.p.) and, after tracheotomy, respired artificially throughout the 2h post-stimulus period. Expiratory CO2 was monitored and maintained at 4–5%. Body temperature was maintained at 38°C with a heating blanket. In the first series of experiments the effects of different levels of TMJ stimulation were assessed by the injection of two concentrations of selective small fiber excitant, mustard oil (MO, 2 or 20% allyl isothiocyanate in mineral oil, 25 μl) into the left joint. Sudan black dye was added to the MO solution to aid in localizing the site of injection. Controls received HE2 or LE2 treatment followed by no TMJ stimulation (NS group) or vehicle injection into the TMJ (0% MO group). Sample sizes for the HE2 groups were: NS = 7; 0% MO = 4; 2% MO = 4 and 20% MO = 6. Sample sizes for the LE2 groups were: NS = 6; 0% MO = 4; 2% MO = 4 and 20% MO = 5.
In a second series of experiments, the effects of systemic administration of NMDA and AMPA receptor antagonism on Fos-LI evoked by TMJ stimulation were assessed. The open channel NMDA receptor antagonist, MK-801 (0.02 or 2 mg/kg, ip), or the selective AMPA receptor antagonist, NBQX (0.02 or 2 mg/kg, ip), or vehicle (saline, 0.1ml, ip) was administered intraperitoneally (ip) 10 min prior to TMJ stimulation with 20% MO. Sample sizes for HE2 rats were: vehicle = 6; 0.02 mg/kg MK-801 = 7; 2 mg/kg MK-801 = 8; 0.02 mg/kg NBQX = 7; 2 mg/kg NBQX = 6. Sample sizes for LE2 rats were: vehicle = 5; 0.02 mg/kg MK-801 = 8; 2 mg/kg MK-801 = 8; 0.02 mg/kg NBQX = 6; 2 mg/kg NBQX = 6. Additional controls HE2 and LE2 rats received 2 mg/kg MK-801 or 2 mg/kg NBQX and no TMJ stimulus (n = 4 per group).
Fos Immunocytochemistry
Animals survived two hours after TMJ stimulation and then were given a bolus injection of pentobarbital (60 mg/kg, i.v.) and perfused through the heart with heparinized saline, followed by 250 ml cold fixative (4 % paraformaldehyde, 0.1 M phosphate, pH 7.4). Rats that received no TMJ stimulus (NS groups) also were anesthetized for two hours before perfusion. The lower brainstem and upper cervical spinal cord segment was removed and postfixed for 1–3 h. Transverse sections (50 μm) were cut on a vibratome and collected in cold 0.01 M phosphate buffered saline (PBS). Sections were incubated in 5% normal donkey serum and 0.3% Triton X-100 (60 min), followed by affinity-purified rabbit polyclonal anti-Fos antibody (Ab-5, Millipore; 1: 15000, 40 h at 4°C), biotinylated donkey anti-rabbit antibody (Chemicon; 1: 300, 115 min) and avidin-biotin-peroxidase complex (Vector; 60 min). Fos-positive neuronal nuclei were visualized by incubation in a nickel-cobalt diaminobenzidine solution activated by 0.01 % hydrogen peroxide. After rinsing in PBS, sections were mounted on slides, air-dried and coverslipped with mounting medium. Under bright-field illumination, Fos-positive neuronal nuclei appeared as homogenous gray-black elements with a well-defined border. Specific staining was abolished by omission of primary antiserum.
Data Analysis
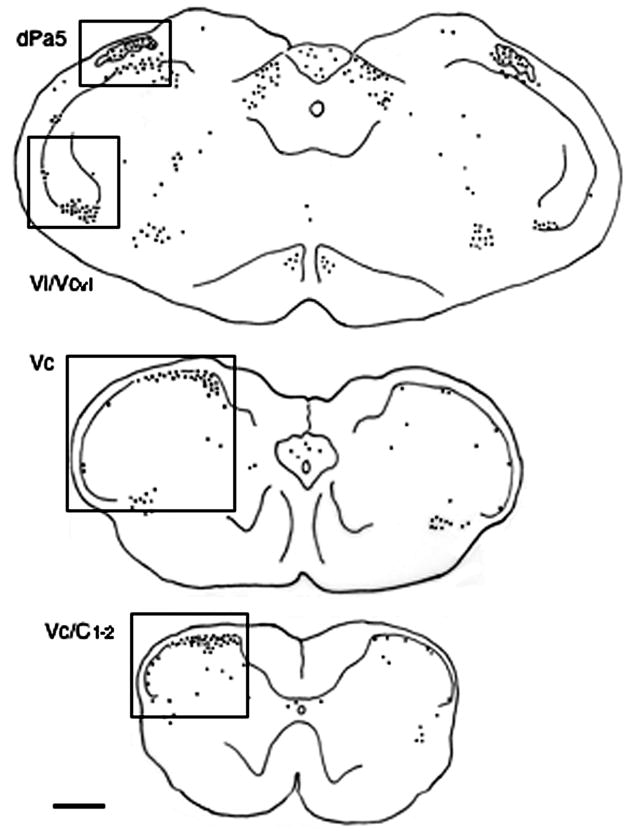
Brainstem sections were categorized according to rostrocaudal level at 500 μm intervals from 2 mm rostral to 7 mm caudal to the obex. The obex is a surface landmark defined by the caudal end of the fourth ventricle approximately 500 μm rostral to the most caudal tip of trigeminal subnucleus interpolaris (see Yoshida et al., 1991). Sixty to 70 sections per animal were counted at 100X magnification without prior knowledge of treatment. To facilitate statistical comparisons across multiple treatments, the average number of Fos-positive neurons per section was compared for the following brainstem regions as shown by the boxed areas in Fig. 1: dorsal paratrigeminal region (dPa5, +0.5 to −0.5 mm relative to the obex), located along the dorsolateral edge of the main body of the trigeminal spinal nucleus and within the spinal trigeminal tract; ventrolateral portion of the trigeminal subnucleus interpolaris/caudalis transition (Vi/Vcvl, +0.5 to −1.0 mm; middle portion of Vc (−2 to −4 mm); and the junction region between Vc and the upper cervical spinal cord (Vc/C1-2, −4.5 to −6.5 mm). At the mid Vc and Vc/C1-2 junction separate cells counts were made for superficial laminae (I-II), deeper laminae (III-V) using established cytological landmarks (see Molander et al. 1989; Bereiter et al. 2005, Fig. 1). Cell counts were compared across treatment groups and ipsilateral versus contralateral to the TMJ injection of MO by two-way analysis of variance. Individual comparisons were made by the Newman-Keuls test after analysis of variance (ANOVA). The TMJ region was inspected after blunt dissection at the end of the experiment to confirm the location of the MO stimulus.
Figure 1.
Camera lucida drawings indicating the pattern of Fos-LI produced in the lower brainstem after acute TMJ stimulation by 20% mustard oil (MO) in a HE2-treated female rat. Left side is ipsilateral to the TMJ stimulus and boxes indicate regions that were quantified bilaterally for Fos-LI. Approximate rostrocaudal levels caudal to obex: upper panel, 0.5 mm; middle panel, 3.0 mm; lower panel, 5 mm. Abbreviations: dPa5, dorsal paratrigeminal region; Vc, trigeminal subnucleus caudalis; Vi/Vcvl, ventrolateral portion of trigeminal subnucleus interpolaris/caudalis transition region; Vc/C1-2, spinomedullary junction region. Each dot = 1 Fos-positive neuron. Calibration = 0.5 mm.
Results
Effect of E2 treatment on Fos-LI produced by TMJ stimulation
The general pattern of Fos-LI produced in caudal portions of the TBNC after TMJ stimulation was similar in LE2 and HE2 rats and agreed well with that seen in cycling female rats (Bereiter 2001). After unilateral intra-TMJ injection of 20% MO, Fos-LI appeared as a rostral cell group at peri-obex levels and a caudal group at the spinomedullary junction ipsilateral to the stimulus, whereas on the contralateral side Fos-LI was increased above controls only at peri-obex levels (Fig 2). Only sparse Fos-LI was found in the TBNC rostral to the levels shown in Figs 1 and 2. Additional brainstem regions, mainly associated with control of autonomic function, such as the nucleus tractus solitarii and the caudal ventrolateral medulla, displayed significant levels of Fos-LI bilaterally (see Fig 1, upper panel); however, these areas were not quantified.
Figure 2.
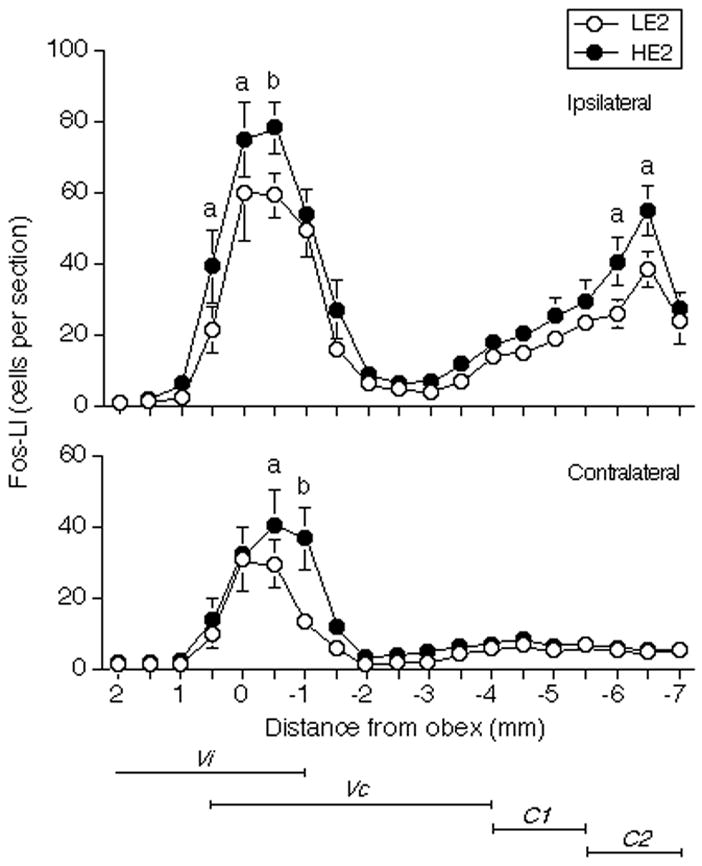
Mean number of Fos-positive neurons produced within the caudal TBNC and upper cervical dorsal horn after injection of mustard oil (20% solution, 25 μl) into the TMJ region expressed as a function of the distance from the obex in HE2 and LE2 rats. a = P < 0.05, b = P < 0.01 HE2 versus LE2. Abbreviations: C1, first cervical segment; C2, second cervical segment; Vc, trigeminal subnucleus caudalis; Vi, trigeminal subnucleus interpolaris.
The Fos-LI produced at periobex levels included separate groups of cells in the Vi/Vcvl transition and dPa5 regions. At the ipsilateral Vi/Vcvl transition, Fos-LI was increased after vehicle injection alone (F = 8.26, df = 7,32, P < 0.001) in both LE2 and HE2 rats (Fig 3, upper panel). Increasing concentrations of MO had only a modest effect on Fos-LI in HE2 rats and caused no further increase in LE2 rats above vehicle injections. Fos-LI produced at the contralateral Vi/Vcvl transition was similar after vehicle (HE2 = 3 ± 1; LE2 = 10 ± 2 cells per section) and 20% MO injections (HE2 = 12 ± 4; LE2 = 12 ± 3 cells per section). At the ipsilateral dPa5 region Fos-LI increased above vehicle-injected controls only in HE2 rats after injection of 20% MO (F = 7.23, df = 7,32, P < 0.001, Fig 3, middle panel). Fos-LI produced in the contralateral dPa5 was similar after vehicle (HE2 = 6 ± 3; LE2 = 8 ± 1 cells per section) and 20% MO injections (HE2 = 13 ± 3; LE2 = 10 ± 2 cells per section) in both groups.
Figure 3.
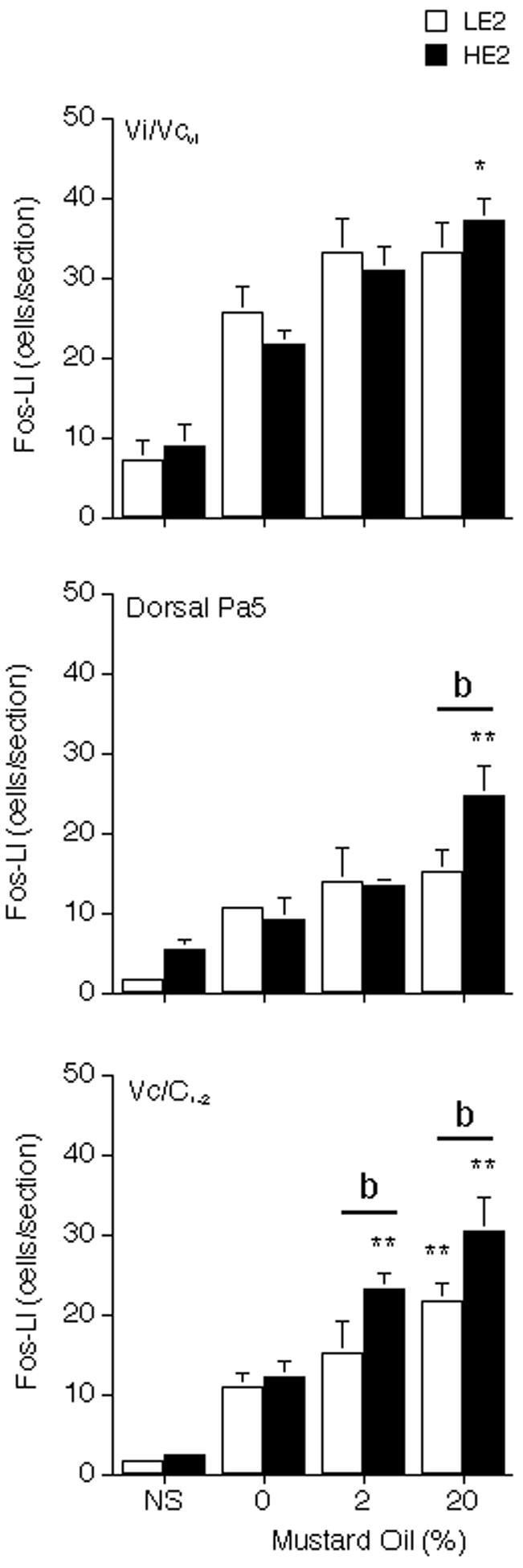
Effect of different concentrations of MO on the average number of Fos-positive neurons produced in the ipsilateral TBNC in HE2 and LE2 rats after TMJ stimulation. Upper panel, Vi/Vcvl transition region; middle panel, dorsal Pa5 region; lower panel, Vc/C1-2, junction region. Abbreviations and symbols: NS = no stimulus controls; *P < 0.05, **P < 0.01, versus 0% MO group; b = P < 0.01 HE2 versus LE2.
The laminated portion of the TBNC, subnucleus Vc, extends from the periobex level caudally to the cervical spinal cord. In general, Fos-LI was seen only in the superficial laminae at the mid Vc and at the Vc/C1-2 junction regions. At the mid Vc region Fos-LI found in laminae I-II averaged 5–7 cells per section ipsilateral to 20% MO injection and 2–4 cells per section contralateral in HE2 and LE2 rats. Fos-LI found in deep laminae at the mid Vc region averaged less than 3 cells per section in HE2 and LE2 rats and was not different from vehicle-injected controls (data not shown). TMJ-evoked Fos-LI in the mid Vc region was not different across treatment groups (F = 1.54, df = 7,32, P > 0.05). By contrast, Fos-LI produced in laminae I-II at the ipsilateral Vc/C1-2 junction increased in proportion to the MO concentration and was significantly different in HE2 than LE2 rats (F = 14.02, df = 7,32, P < 0.001). TMJ stimulation did not affect Fos-LI in laminae I-II contralateral to the injection (2–5 cells per section) or in deeper laminae (2–4 cells per section) in HE2 or LE2 rats.
Effect of MK-801 and NBQX on Fos-LI evoked by TMJ stimulation
Pretreatment with the NMDA receptor antagonist, MK-801, caused a dose-dependent decrease in the number of Fos-LI neurons in animals that received MO injections into the TMJ at the ipsilateral Vi/Vcvl transition region in HE2 and LE2 rats (F = 8.28, df = 5,37, P < 0.001, Fig 4, upper panel). At the ipsilateral dPa5 region, high dose MK-801 reduced TMJ-evoked Fos-LI response in HE2, but not in LE2 rats (F = 3.30, df = 5, 37, P < 0.025, Fig 4, middle panel). Low dose MK-801 was sufficient to cause maximum reduction of MO-evoked Fos-LI in laminae I-II at the Vc/C1-2 junction in HE2 rats, whereas LE2 rats displayed a significant increase after low dose MK-801 and only minor reductions even after high dose compared to naïve LE2 rat (F = 8.10, df = 5, 37, P < 0.001, Fig 4, lower panel). HE2 and LE2 rats given high dose MK-801 alone and no TMJ stimulus displayed a pattern of Fos-LI in the TBNC similar to that of NS controls (data not shown). MK-801 did not affect the Fos-LI response in these areas contralateral to MO injection significantly compared to vehicle group (data not shown).
Figure 4.
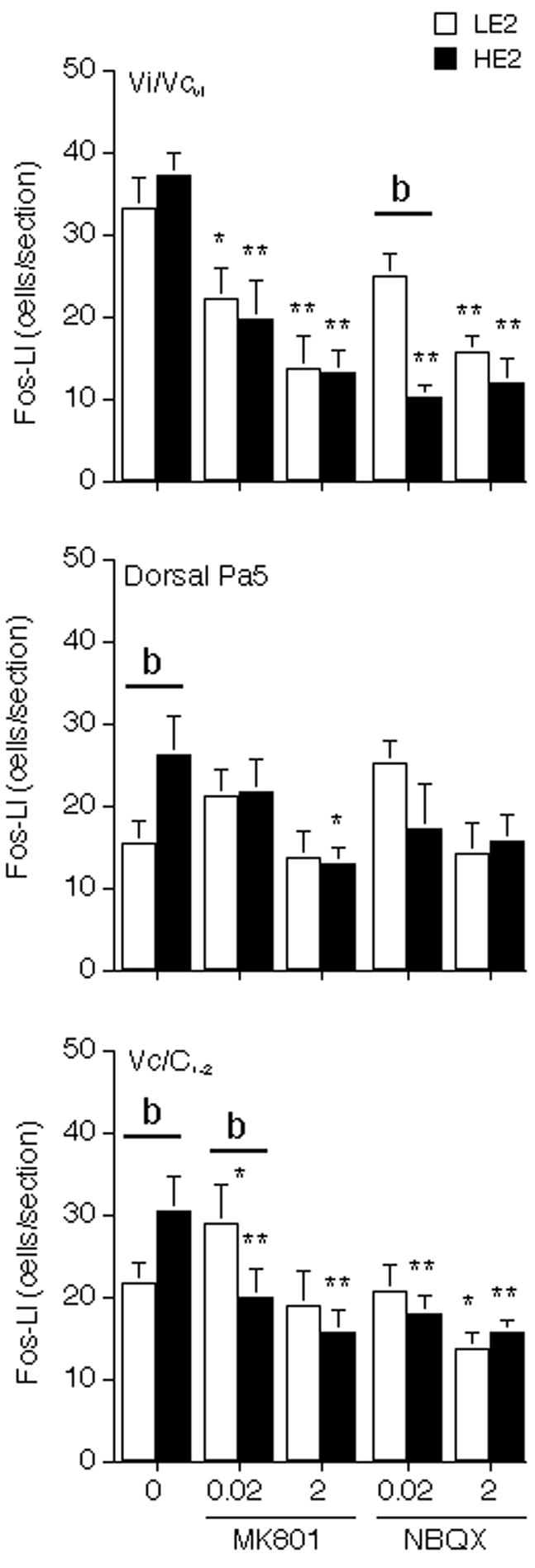
Effect of pretreatment with MK-801 and NBQX on the average number of Fos-positive neurons produced Vi/Vcvl transition region (upper panel), dPa5 region (middle panel) and laminae I-II at the Vc/C1-2, junction region (lower panel) after 20% MO stimulation. Abbreviations and symbols: 0 = vehicle+20% MO; *P < 0.05, **P < 0.01, versus vehicle+20% MO; b = P < 0.01 HE2 versus LE2.
As seen in Fig 4, pretreatment with low dose AMPA receptor antagonist, NBQX, caused maximum decreases in MO injection into TMJ-evoked Fos-LI at the ipsilateral Vi/Vcvl transition (F = 12.19, df = 5,30, P < 0.001) and in laminae I-II at the Vc/C1-2 junction region in HE2 rats, while only high dose NBQX was effective in LE2 rats (F = 5.27, df = 5,30, P < 0.005). NBQX caused a small overall reduction in Fos-LI produced at the dPa5 region in HE2 and LE2 rats (F = 3.14, df = 5,30, P < 0.05); however, there were no significant group differences. In LE2 rats, low dose of NBQX showed a small, but not significant increase in Fos-LI in the dPa5 region. HE2 and LE2 rats given high dose NBQX alone and no TMJ stimulus displayed a pattern of Fos-LI in the TBNC similar to that of NS controls (data not shown). NBQX did not affect the Fos-LI response in these areas contralateral to MO injection significantly compared to vehicle group (data not shown).
Discussion
The main findings of this study revealed that the TMJ-evoked Fos-LI responses in the superficial laminae at the Vc/C1-2 junction and dPa5 regions were significantly enhanced in an E2-dependent manner, while Fos-LI produced in other regions of the caudal TBNC was not affected by estrogen status. Secondly, the effectiveness of both NMDA and AMPA receptor antagonists to reduce TMJ-evoked Fos-LI at the Vc/C1-2 junction was greater under high E2 than low E2 conditions. These results supported the hypothesis that a change in estrogen status alone was sufficient to modify the processing of noxious sensory input from the TMJ by neurons in superficial laminae at the Vc/C1-2 junction by acting, in part, through ionotropic glutamatergic mechanisms.
Female sex hormone status is a recognized risk factor in TMJD (Huang et al., 2002; Slade et al., 2007); however, the basis for this relationship remains uncertain. The actions of estrogens on neural tissues are complex and likely involve multiple mechanisms (McEwen, 2001; Ronnekleiv and Kelly, 2005). The classical estrogen receptor exists as two subtypes, ERα and ERβ, and is expressed in sensory ganglia (Bereiter et al., 2005; Sohrajbi et al., 1994), dorsal horn (Amandusson et al., 1996; Bereiter et al., 2005; Papka et al., 2001) and supraspinal brain regions associated with pain modulation (Merchenthaler et al., 2004; Shughrue et al., 2001). In the TBNC, ERα-positive neurons were found in abundance in laminae I-II of Vc with only sparse staining in other laminae and subnuclei, while ERβ-positive neurons were more evenly distributed throughout the TBNC (Amandusson et al., 1996; Bereiter et al., 2005). Previously, we reported that the Fos-LI response in the laminae I-II at the Vc/C1-2 junction was proportional to the intensity of the TMJ stimulus and varied systematically over different phases of the estrous cycle in female rats (Bereiter, 2001). This suggested that the cyclic variation in E2, as occurs during the estrous cycle, was a significant factor in TMJ nociceptive processing at the Vc/C1-2 junction region. This hypothesis was further supported by neurophysiological studies in which the encoding properties of TMJ-responsive neurons in laminae I-II at the Vc/C1-2 junction were shown to vary over the estrous cycle in intact females (Okamoto et al., 2003) and to differ under HE2 and LE2 conditions in OvX female rats, whereas TMJ units in deep laminae were not affected by E2 treatment (Tashiro et al., 2007). This suggested that regions of the TBNC that displayed differential responses under high and low E2 conditions were more likely to occur in regions that also displayed a high density of ERα-positive neurons. Thus, the greatest effect of E2 treatment on TMJ-evoked Fos-LI was seen in laminae I-II at the Vc/C1-2 junction, whereas the Fos-LI responses at the Vi/Vcvl transition in HE2 and LE2 rats were similar, a region where ER-positive neurons were sparse (Bereiter et al., 2005). An exception to this relationship was the dPa5 region, where few ERα-positive neurons were seen, yet TMJ-evoked Fos-LI was significantly greater in HE2 than LE2 rats. The dPa5 region receives significant direct afferent input from visceral afferents as well as trigeminal nerves and is thought to be important for sensory-autonomic integration (Saxon and Hopkins, 1998; Caous et al., 2001). These data do not, however, exclude the possibility that E2 acted on peripheral tissues or modified the properties of trigeminal ganglion (TG) cells. ER-LI is expressed in several non-neuronal cell types within the TMJ region (Yamada et al., 2003) and in a significant percentage of TG neurons (Bereiter et al., 2005), including those that innervate the TMJ region (Flake et al., 2005). Several studies have reported that chronic E2 treatment enhanced the responsiveness of TG neurons in vitro (Flake et al., 2005; Diogenes et al., 2006) and in vivo (Dong et al., 2007) in female rodents.
It is well established that NMDA and non-NMDA glutamate receptors are critically involved in nociceptive processing (see Woolf and Salter, 2000; Bleakman et al., 2006). Although considerable evidence indicates that E2 treatment has both short- and long-term effects on neural function elsewhere in the brain involving NMDA and AMPA receptor pathways (see Smith, 1994; Cyr et al., 2001; McEwen, 2002; Liu et al., 2008), the relationship between estrogen status and ionotropic glutamate receptors in nociception has been less well defined. The present study found that NMDA and AMPA receptor antagonists markedly inhibited TMJ-evoked Fos-LI in laminae I-II at the Vc/C1-2 junction in HE2 rats after only low doses of MK-801 or NBQX. By contrast, even high dose MK-801 did not reduce Fos-LI in LE2 rats and high dose NBQX caused only small reductions. At the Vi/Vcvl transition HE2 and LE2 rats displayed similar reductions in TMJ-evoked Fos-LI after MK-801, whereas NBQX was more effective in HE2 than LE2 rats. The superficial laminae of the medullary and spinal dorsal horns express high densities of NMDA (Petralia et al., 1994; Bereiter and Bereiter, 2000; Nagy et al., 2004b) and AMPA receptors (Florenzano and De Luca, 1999; Tang et al., 2001; Nagy et al., 2004a). Since a high percentage of glutamatergic synapses in spinal dorsal horn (Nagy et al., 2004b), other brainstem regions (Tse et al., 2008) and limbic brain areas co-express NMDA and AMPA receptors (Takumi et al., 1999), it was predicted that Fos-LI evoked within a given TBNC region should be similarly responsive to NMDA and AMPA receptor antagonism. This appeared to be the case in the present study when considering data within each E2 treatment group. For example, TMJ-evoked Fos-LI was markedly inhibited by low dose MK-801 or NBQX at the Vc/C1-2 junction and Vi/Vcvl transition regions in HE2 rats, whereas neither drug was effective in reducing Fos-LI at the dPa5 region. The finding that NMDA and AMPA receptor blockade had similar effects on TMJ-evoked Fos-LI for a given TBNC region within each E2 treatment group agreed well with previous studies in male rats in which Fos-LI evoked by ocular surface (Bereiter and Bereiter, 1996) or intracisternal chemical stimulation (Mitsikostas et al., 1998; Mitsikostas et al., 1999) was reduced similarly by NMDA or non-NMDA antagonists. However, in spinal dorsal horn it has been reported that NMDA and non-NMDA antagonists differentially reduced Fos-LI after cutaneous or muscle inflammation, respectively, in male rats (Hu and Zhao, 2001). The basis for this discrepancy is not certain but may be due to differences in the neurochemical organization of trigeminal versus spinal nociceptive systems or methodological aspects such as drug choice or dose or animal preparation. The present results also revealed possible sex differences in the response to NMDA antagonism. Pretreatment with MK-801 caused a marked reduction in TMJ-evoked Fos-LI at the Vi/Vcvl transition in HE2 and LE2 females, whereas similar doses of MK-801 in males failed to reduce Fos-LI evoked by TMJ (Bereiter and Bereiter, 2000) or masseter muscle stimulation with 20% MO (Ro et al., 2004). The function of the Vi/Vcvl transition in TMJ nociceptive processing remains uncertain since TMJ-evoked Fos-LI was not proportional to stimulus intensity and most anatomical studies report no direct primary afferent input to this region from deep cranial facial tissues supplied by the mandibular branch of the trigeminal nerve (Shigenaga et al., 1988; Dessem et al., 2007; however, see Wang et al., 2006). TMJ-evoked Fos-LI at the Vi/Vcvl transition may originate from intersubnuclear relays in the Vc/C1-2 junction, since blockade of the Vc/C1-2 junction completely prevented increases in glutamate release at the Vi/Vcvl transition as seen by microdialysis (Bereiter et al., 2002). As discussed previously (Meng et al., 1997; Bereiter, 2001), the Vi/Vcvl transition may be involved in the recruitment of endogenous pain controls from supraspinal brain regions. The Vi/Vcvl transition is unique among other TBNC regions and has a dense projection to thalamic nucleus submedius (Yoshida et al., 1991). It will be important to determine if sensory encoding of deep craniofacial inputs by neurons at the Vi/Vcvl transition is similar in males and females, since one theory to explain the higher prevalence of TMJD in women proposes a deficiency in the ability to recruit endogenous pain controls (Kashima et al., 1999; Bragdon et al., 2002).
The mechanisms for E2 modulation of glutamate receptor involvement in TMJ nociception are not certain and could involve peripheral as well as central neural mechanisms. In the periphery, TG neurons express multiple subtypes for NMDA and non-NMDA receptors (Dong et al., 2007; Lee and Ro, 2007). E2 treatment may have affected glutamate binding in the TG or TBNC by increasing receptor protein levels or binding affinity as occurs in other brain areas (see Cyr et al., 2001; McEwen et al., 2001). E2 may have affected the availability of glutamate by increasing glutamate transporter activity in neurons or glia (Mitrovic et al., 1999; Pawlak et al., 2005). Recently, we reported that glutamate transporter activity at the Vc/C1-2 junction was significantly greater in HE2 compared to LE2 females or males (Bereiter and Benetti, 2006). E2 treatment has been reported to increase the expression of several proteins such as PSD95 that are critical for glutamatergic synaptic function in other brain regions (d’Anglemont de Tassigny et al., 2007; Liu et al., 2008); however, it is not known if similar effects occur in the dorsal horn. Since many articular afferents can be classified as “silent nociceptors” (Schaible and Grubb, 1993; Michaelis et al., 1996), while in the dorsal horn so-called “silent synapses” exhibit mainly NMDA receptor-mediated current (Kerchner et al., 1999), the net effect of high E2 conditions may be to unmask silent TMJ afferents by lowering sensory thresholds or disinhibition of primary afferent synaptic contacts in the TBNC. Direct action of E2 at the first synapse may explain why the greatest differences in TMJ-evoked Fos-LI between HE2 and LE2 groups were seen at Vc/C1-2 junction.
Acknowledgments
This study was supported by a grant from the NIDCR (DE12758).
Abbreviations
- AMPA
amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid
- dPa5
dorsal paratrigeminal region
- E2
estradiol
- Fos-LI
Fos-immunoreactivity
- MO
allyl isothiocyanate
- NMDA
N-methyl-D-aspartate
- TBNC
trigeminal brainstem nuclear complex
- TMJ
temporomandibular joint
- TMJD
temporomandibular joint disorder
- Vc/C1-2
trigeminal subnucleus caudalis/upper cervical cord
- Vi/Vcvl
ventral trigeminal interpolaris/caudalis transition region
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amandusson A, Hermanson O, Blomqvist A. Colocalization of oestrogen receptor immunoreactivity and preproenkephalin mRNA expression to neurons in the superficial laminae of the spinal and medullary dorsal horn of rats. Eur J Neurosci. 1996;8:2440–2445. doi: 10.1111/j.1460-9568.1996.tb01207.x. [DOI] [PubMed] [Google Scholar]
- Bereiter DA. Sex differences in brainstem neural activation after injury to the TMJ region. Cells Tissues Organs. 2001;169:226–237. doi: 10.1159/000047886. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Benetti AP. Amino acid release at the spinomedullary junction after inflammation of the TMJ region in male and female rats. Pain. 2006;126:175–183. doi: 10.1016/j.pain.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Bereiter DF. N-methyl-D-aspartate and non-N-methyl-D-aspartate receptor antagonism reduces Fos-like immunoreactivity in central trigeminal neurons after corneal stimulation in the rat. Neuroscience. 1996;73:249–258. doi: 10.1016/0306-4522(96)00038-3. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Bereiter DF. Morphine and NMDA receptor antagonism reduce c-fos expression in spinal trigeminal nucleus produced by acute injury to the TMJ region. Pain. 2000;85:65–77. doi: 10.1016/s0304-3959(99)00246-8. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Shen S, Benetti AP. Sex differences in amino acid release from rostral trigeminal subnucleus caudalis after acute injury to the TMJ region. Pain. 2002;98:89–99. doi: 10.1016/s0304-3959(01)00476-6. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bragdon EE, Light KC, Costello NL, Sigurdsson A, Bunting S, Bhalang K, Maixner W. Group differences in pain modulation: pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96:227–237. doi: 10.1016/S0304-3959(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Bush FM, Harkins SW, Harrington WG, Price DD. Analysis of gender effects on pain perception and symptom presentation in temporomandibular pain. Pain. 1993;53:73–80. doi: 10.1016/0304-3959(93)90058-W. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Caous CA, de Sousa Buck H, Lindsey CJ. Neuronal connections of the paratrigeminal nucleus: a topographic analysis of neurons projecting to bulbar, pontine and thalamic nuclei related to cardiovascular, respiratory and sensory functions. Auton Neurosci. 2001;94:14–24. doi: 10.1016/s1566-0702(01)00338-1. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Brain Res Rev. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- D’Anglemont de Tassigny X, Campagne C, Dehouck B, Leroy D, Holstein GR, Beauvillain JC, Buee-Scherrer V, Prevot V. Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. J Neurosci. 2007;27:6103–6114. doi: 10.1523/JNEUROSCI.5595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessem D, Moritani M, Ambalavanar R. Nociceptive craniofacial muscle primary afferent neurons synapse in both the rostral and caudal brain stem. J Neurophysiol. 2007;98:214–223. doi: 10.1152/jn.00990.2006. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26:8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience. 2007;146:822–832. doi: 10.1016/j.neuroscience.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol. 2005;93:1585–1597. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florenzano F, Luca BD. Nociceptive stimulation induces glutamate receptor down-regulation in the trigeminal nulceus. Neuroscience. 1999;90:201–207. doi: 10.1016/s0306-4522(98)00388-1. [DOI] [PubMed] [Google Scholar]
- Hollins M, Sigurdsson A, Fillingim L, Goble AK. Vibrotactile threshold is elevated in temporomandibular disorders. Pain. 1996;67:89–96. doi: 10.1016/0304-3959(96)03083-7. [DOI] [PubMed] [Google Scholar]
- Hu JY, Zhao ZQ. Differential contributions of NMDA and non-NMDA receptors to spinal Fos expression evoked by superficial tissue and muscle inflammation in the rat. Neuroscience. 2001;106:823–831. doi: 10.1016/s0306-4522(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Huang GJ, LeResche L, Critchlow CW, Martin MD, Drangsholt MT. Risk factors for diagnostic subgroups of painful temporomandibular disorders (TMD) J Dent Res. 2002;8:284–288. doi: 10.1177/154405910208100412. [DOI] [PubMed] [Google Scholar]
- Ioi H, Kido MA, Zhang JQ, Yamaza T, Nakata S, Nakasima A, Tanaka T. Capsaicin receptor expression in the rat temporomandibular joint. Cell Tissue Res. 2006;325:47–54. doi: 10.1007/s00441-006-0183-7. [DOI] [PubMed] [Google Scholar]
- Isselee H, Laat AD, Mot BD, Lysens R. Pressure-pain threshold variation in temporomandibular disorder myalgia over the course of the menstrual cycle. J Orofac Pain. 2002;16:105–117. [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kashima K, Rahman OI, Sakoda S, Shiba R. Increased pain sensitivity of the upper extremities of TMD patients with myalgia to experimentally-evoked noxious stimulation: possibility of worsened endogenous opioid systems. Cranio. 1999;17:241–246. doi: 10.1080/08869634.1999.11746100. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Li P, Zhuo M. Speaking out of turn: a role for silent synapses in pain. IUBMB Life. 1999;48:251–256. doi: 10.1080/713803505. [DOI] [PubMed] [Google Scholar]
- Kido MA, Kiyoshima T, Ibuki T, Shimizu S, Kondo T, Terada Y, Tanaka T. A topographical and ultrastructural study of sensory trigeminal nerve endings in the rat temporomandibular joint as demonstrated by anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) J Dent Res. 1995;74:1353–1359. doi: 10.1177/00220345950740070601. [DOI] [PubMed] [Google Scholar]
- Landi N, Lombardi I, Manfredini D, Casarosa E, Biondi K, Gabbanini M, Bosco M. Sexual hormone serum levels and temporomandibular disorders. A preliminary study. Gynecol Endocrinol. 2005;20:99–103. doi: 10.1080/09513590400021136. [DOI] [PubMed] [Google Scholar]
- Lee J, Ro JY. Differential regulation of glutamate receptors in trigeminal ganglia following masseter inflammation. Neurosci Lett. 2007;421:91–95. doi: 10.1016/j.neulet.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiological factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Nat Acad Sci. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Benetti AP, Bereiter DA. Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. J Neurophysiol. 1997;77:43–56. doi: 10.1152/jn.1997.77.1.43. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor a and b in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Habler HJ, Jaenig W. Silent afferents: a separate class of primary afferents? Clin Exp Pharmacol Physiol. 1996;23:99–105. doi: 10.1111/j.1440-1681.1996.tb02579.x. [DOI] [PubMed] [Google Scholar]
- Mitrovic AD, Maddison JE, Johnston GAR. Influence of the oestrous cycle on L-glutamate and L-aspartate transport in rat brain synaptosomes. Neurochem Int. 1999;34:101–108. doi: 10.1016/s0197-0186(98)00066-7. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Rio MSD, Waeber C, Moskowitz M, Cutrer FM. The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain. 1998;76:239–248. doi: 10.1016/s0304-3959(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M, Waeber C, Huang Z, Cutrer FM, Moskowitz MA. Non-NMDA glutamate receptors modulate capsaicin induced c-fos expression within trigeminal nucleus caudalis. Br J Pharmacol. 1999;127:623–630. doi: 10.1038/sj.bjp.0702584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander C, Xu Q, Rivero-Melian C, Grant G. Cytoarchitectonic organization of the spinal cord in the rat: II. The cervical and upper thoracic cord. J Comp Neurol. 1989;289:375–385. doi: 10.1002/cne.902890303. [DOI] [PubMed] [Google Scholar]
- Nagy GG, Al-Ayyan M, Andrew D, Fukaya M, Watanabe M, Todd AJ. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J Neurosci. 2004a;24:5766–5777. doi: 10.1523/JNEUROSCI.1237-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004b;20:3301–3312. doi: 10.1111/j.1460-9568.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Lucke T, Schaible HG. N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists block the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat’s knee joint. J Neurophysiol. 1993;70:1365–1377. doi: 10.1152/jn.1993.70.4.1365. [DOI] [PubMed] [Google Scholar]
- Ohrbach R, Dworkin SF. Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain. 1998;74:315–326. doi: 10.1016/s0304-3959(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Hirata H, Takeshita S, Bereiter DA. Response properties of TMJ neurons in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J Neurophysiol. 2003;89:1467–1477. doi: 10.1152/jn.00795.2002. [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-a and -b immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro JY, Capra NF, Masri R. Contribution of peripheral N-methyl-D-aspartate receptors to c-fos expression in the trigeminal spinal nucleus following acute masseteric inflammation. Neuroscience. 2004;123:213–219. doi: 10.1016/s0306-4522(03)00465-2. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Greenspan JD. Why look in the brain for answers to temporomandibular disorder pain? Cells Tissues Organs. 2005;180:69–75. doi: 10.1159/000086200. [DOI] [PubMed] [Google Scholar]
- Saxon DW, Hopkins DA. Efferent and collateral organization of paratrigeminal nucleus projections: an anterograde and retrograde fluorescent tracer study in the rat. J Comp Neurol. 1998;402:93–110. [PubMed] [Google Scholar]
- Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- Schaible HG. Spinal mechanisms contributing to joint pain. Novartis Found Symp. 2004;260:4–22. [PubMed] [Google Scholar]
- Shigenaga Y, Chen IC, Suemune S, Nishimori T, Nasution ID, Yoshida A, Sato H, Okamoto T, Sera M, Hosoi M. Oral and facial representation within the medullary and upper cervical dorsal horns in the cat. J Comp Neurol. 1986;243:388–408. doi: 10.1002/cne.902430309. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol. 1988;268:489–507. doi: 10.1002/cne.902680403. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor b immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- Slade GD, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim RB, Belfer I, Max MB, Goldman D, Maixner W. Influence of psychological factors on risk of temporomandibular disorders. J Dent Res. 2007;86:1120–1125. doi: 10.1177/154405910708601119. [DOI] [PubMed] [Google Scholar]
- Smith SS. Female sex steroid hormones: From receptors to networks to performance-actions on the sensorimotor system. Progr Neurobiol. 1994;44:55–86. doi: 10.1016/0301-0082(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga S, Abeyama K, Indo H, Shigeta K, Noikura T. Temporomandibular disorders: MR assessment of inflammatory changes in the posterior disk attachment during the menstrual cycle. J Comput Assist Tomogr. 2001;25:476–481. doi: 10.1097/00004728-200105000-00023. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Toda K. Subtypes of nociceptive units in the rat temporomandibular joint. Brain Res Bull. 2003;61:603–608. doi: 10.1016/s0361-9230(03)00219-3. [DOI] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Tallaksen-Greene SJ, Young AB, Penny JB, Beitz AJ. Excitatory amino acid binding sites in the trigeminal principal sensory and spinal trigeminal nuclei of the rat. Neurosci Lett. 1992;141:79–83. doi: 10.1016/0304-3940(92)90339-9. [DOI] [PubMed] [Google Scholar]
- Tang FR, Yeo JF, Leong SK. Qualitative light and electron microscope study of glutamate receptors in the caudal spinal trigeminal nucleus of the rat. J Dent Res. 2001;80:1736–1741. doi: 10.1177/00220345010800081101. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Milam SB, Bereiter DA. Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in female rats. J Neurophysiol. 2007;98:3242–3253. doi: 10.1152/jn.00677.2007. [DOI] [PubMed] [Google Scholar]
- Tse YC, Lai CH, Lai SK, Liu JX, Yung KK, Shum DK, Chan YS. Developmental expression of NMDA and AMPA receptor subunits in vestibular nuclear neurons that encode gravity-related horizontal orientations. J Comp Neurol. 2008;508:343–364. doi: 10.1002/cne.21688. [DOI] [PubMed] [Google Scholar]
- Wang H, Wei F, Dubner R, Ren K. Selective distribution and function of primary afferent nociceptive inputs from deep muscle tissue to the brainstem trigeminal transition zone. J Comp Neurol. 2006;498:390–402. doi: 10.1002/cne.21062. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nozawa-Inoue K, Kawano Y, Kohno S, Amizuka N, Iwanaga T, Maeda T. Expression of estrogen receptor alpha (ERa) in the rat temporomandibular joint. Anat Rec A. 2003;274A:934–941. doi: 10.1002/ar.a.10107. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Dostrovsky JO, Sessle BJ, Chiang CY. Trigeminal projections to the nucleus submedius of the thalamus in the rat. J Comp Neurol. 1991;307:609–625. doi: 10.1002/cne.903070408. [DOI] [PubMed] [Google Scholar]
- Yu X-M, Sessle BJ, Haas DA, Izzo A, Vernon H, Hu JW. Involvement of NMDA receptor mechanisms in jaw electromyographic activity and plasma extravasation induced by inflammatory irritant application to temporomandibular joint region of rats. Pain. 1996;68:169–178. doi: 10.1016/S0304-3959(96)03181-8. [DOI] [PubMed] [Google Scholar]