Abstract
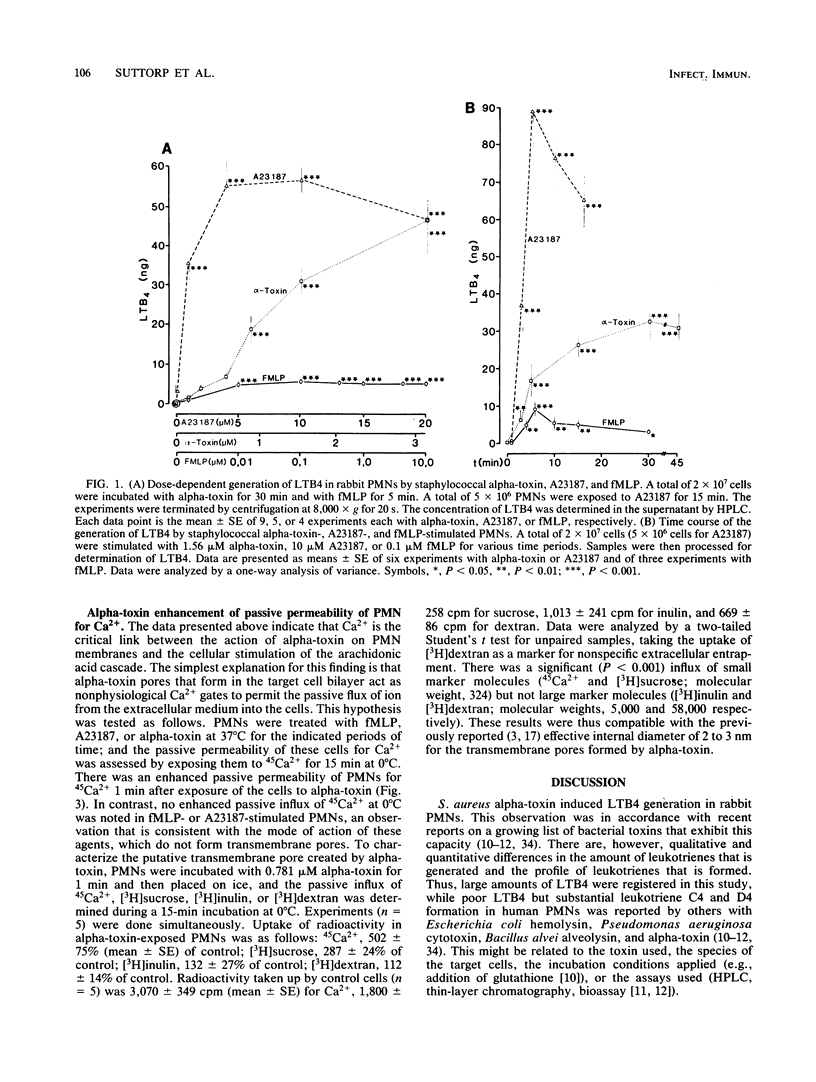
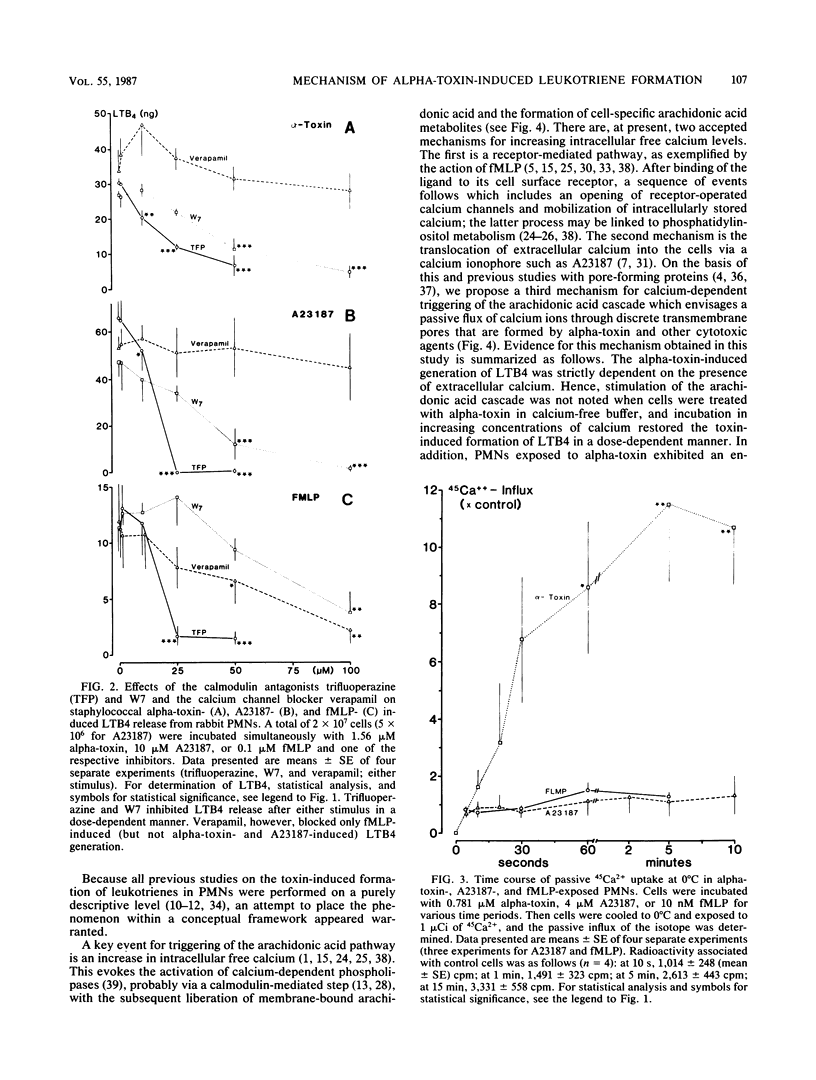
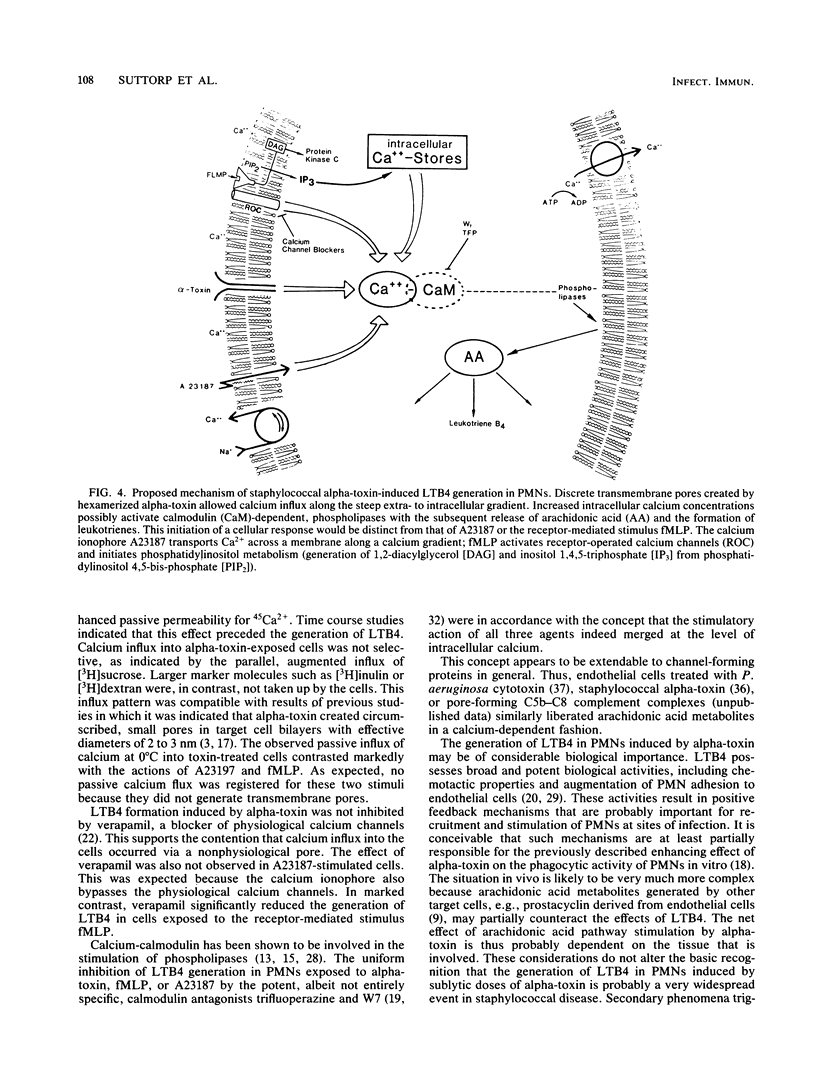
The effects of staphylococcal alpha-toxin on arachidonic acid metabolism in rabbit polymorphonuclear leukocytes (PMNs) were investigated and compared with those of the ionophore A23187 and the chemotactic tripeptide formylmethionyl-leucyl-phenylalanine (fMLP). Sublytic amounts of alpha-toxin stimulated the release of leukotriene B4 (LTB4) in PMNs in a dose-dependent manner. The toxin was several times more potent than fMLP but was not as effective as the ionophore. Preincubation of the toxin with neutralizing antibodies abolished the effect. Extracellular calcium was strictly required for eliciting LTB4 generation. Verapamil, a calcium channel blocker, inhibited fMLP-mediated LTB4 generation but had no effect on alpha-toxin- or A23187-exposed PMNs. Agents such as trifluoperazine and N-6(aminohexyl)-5-chloro-1-naphthalene sulfonamid that interfered with calmodulin activity, however, inhibited LTB4 generation in all cases. One minute after the addition of alpha-toxin, PMNs exhibited a severalfold enhancement in passive permeability to 45Ca2+. In addition, these cells became permeable to sucrose but not to inulin or dextran. The influx pattern was consistent with the previous observation that alpha-toxin creates discrete transmembrane channels in erythrocytes with an effective internal diameter of 2 to 3 nm. The results suggest that alpha-toxin triggers the arachidonic acid pathway in PMNs by facilitating calcium influx into the cells, possibly via transmembrane toxin pores that serve as calcium gates. Generation of arachidonic acid metabolites in PMNs by sublytic amounts of alpha-toxin may represent an important cellular reaction that generally occurs during infections with Staphylococcus aureus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Füssle R., Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Mechanism of complement cytolysis and the concept of channel-forming proteins. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):311–324. doi: 10.1098/rstb.1984.0092. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Stimulation of arachidonic acid metabolism in the polymorphonuclear leukocyte by an N-formylated peptide. Comparison with ionophore A23187. J Biol Chem. 1980 Nov 10;255(21):10223–10226. [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Schmidt M., Yoder M., Baehner R. L. Inhibition of polymorphonuclear leukocyte adherence by prostacyclin. J Lab Clin Med. 1980 May;95(5):672–678. [PubMed] [Google Scholar]
- Bremm K. D., Brom H. J., Alouf J. E., König W., Spur B., Crea A., Peters W. Generation of leukotrienes from human granulocytes by alveolysin from Bacillus alvei. Infect Immun. 1984 Apr;44(1):188–193. doi: 10.1128/iai.44.1.188-193.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., Brom J., König W., Spur B., Crea A., Bhakdi S., Lutz F., Fehrenbach F. J. Generation of leukotrienes and lipoxygenase factors from human polymorphonuclear granulocytes during bacterial phagocytosis and interaction with bacterial exotoxins. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Jul;254(4):500–514. [PubMed] [Google Scholar]
- Bremm K. D., König W., Pfeiffer P., Rauschen I., Theobald K., Thelestam M., Alouf J. E. Effect of thiol-activated toxins (streptolysin O, alveolysin, and theta toxin) on the generation of leukotrienes and leukotriene-inducing and -metabolizing enzymes from human polymorphonuclear granulocytes. Infect Immun. 1985 Dec;50(3):844–851. doi: 10.1128/iai.50.3.844-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Ca2+.Calmodulin-dependent release of arachidonic acid for renal medullary prostaglandin synthesis. Evidence for involvement of phospholipases A2 and C. J Biol Chem. 1983 Apr 25;258(8):4814–4823. [PubMed] [Google Scholar]
- Ford-Hutchinson A. W. Leukotrienes: their formation and role as inflammatory mediators. Fed Proc. 1985 Jan;44(1 Pt 1):25–29. [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell C. G., Peterson P. K., Schmeling D. J., Quie P. G. Effect of staphylococcal alpha-toxin on phagocytosis of staphylococci by human polymorphonuclear leukocytes. Infect Immun. 1982 Dec;38(3):975–980. doi: 10.1128/iai.38.3.975-980.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Asano M., Tanaka T. Activity-structure relationship of calmodulin antagonists, Naphthalenesulfonamide derivatives. Mol Pharmacol. 1981 Nov;20(3):571–578. [PubMed] [Google Scholar]
- Hoover R. L., Karnovsky M. J., Austen K. F., Corey E. J., Lewis R. A. Leukotriene B4 action on endothelium mediates augmented neutrophil/endothelial adhesion. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2191–2193. doi: 10.1073/pnas.81.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa D. K., Osifchin N. E., Paznekas W. A., Shin M. L., Mayer M. M. Consequences of cell membrane attack by complement: release of arachidonate and formation of inflammatory derivatives. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6647–6651. doi: 10.1073/pnas.80.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis R. A., Scriabine A. Sites of action of Ca2+ channel inhibitors. Biochem Pharmacol. 1983 Dec 1;32(23):3499–3507. doi: 10.1016/0006-2952(83)90295-2. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Rutherford L. E., Weissmann G. Stimulus response coupling in the human neutrophil. I. Kinetic analysis of changes in calcium permeability. J Biol Chem. 1984 Apr 10;259(7):4070–4075. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Weissmann G. Neutrophil stimulation: receptor, membrane, and metabolic events. Fed Proc. 1984 Sep;43(12):2749–2754. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Moskowitz N., Shapiro L., Schook W., Puszkin S. Phospholipase A2 modulation by calmodulin, prostaglandins and cyclic nucleotides. Biochem Biophys Res Commun. 1983 Aug 30;115(1):94–99. doi: 10.1016/0006-291x(83)90973-7. [DOI] [PubMed] [Google Scholar]
- Palmblad J., Malmsten C. L., Udén A. M., Rådmark O., Engstedt L., Samuelsson B. Leukotriene B4 is a potent and stereospecific stimulator of neutrophil chemotaxis and adherence. Blood. 1981 Sep;58(3):658–661. [PubMed] [Google Scholar]
- Palmer R. M., Salmon J. A. Release of leukotriene B4 from human neutrophils and its relationship to degranulation induced by N-formyl-methionyl-leucyl-phenylalanine, serum-treated zymosan and the ionophore A23187. Immunology. 1983 Sep;50(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Powell W. S. Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes. J Biol Chem. 1984 Mar 10;259(5):3082–3089. [PubMed] [Google Scholar]
- Salari H., Braquet P., Naccache P., Borgeat P. Characterization of effect of N-formyl-methionyl-leucyl-phenylalanine on leukotriene synthesis in human polymorphonuclear leukocytes. Inflammation. 1985 Jun;9(2):127–138. doi: 10.1007/BF00917585. [DOI] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Dewein E., Bhakdi S., Roka L. Staphylococcal alpha-toxin-induced PGI2 production in endothelial cells: role of calcium. Am J Physiol. 1985 Jan;248(1 Pt 1):C127–C134. doi: 10.1152/ajpcell.1985.248.1.C127. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Uhl J., Lutz F., Roka L. Pseudomonas aeruginosa cytotoxin stimulates prostacyclin production in cultured pulmonary artery endothelial cells: membrane attack and calcium influx. J Cell Physiol. 1985 Apr;123(1):64–72. doi: 10.1002/jcp.1041230111. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Homma Y., Nagai Y. Role of Ca2+ in phosphatidylinositol response and arachidonic acid release in formylated tripeptide- or Ca2+ ionophore A23187-stimulated guinea pig neutrophils. J Immunol. 1983 Jun;130(6):2849–2855. [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]



