Abstract
The intestinal epithelial cell-surface molecule, CD98 is a type II membrane glycoprotein. Molecular orientation studies have demonstrated that the C-terminal tail of human CD98 (hCD98), which contains a PDZ-binding domain, is extracellular. In intestinal epithelial cells, CD98 is covalently linked to an amino-acid transporter with which it forms a heterodimer. This heterodimer associates with β1-integrin and intercellular adhesion molecular 1 (ICAM-1) to form a macromolecular complex in the basolateral membranes of polarized intestinal epithelial cells. This review focuses on the many functional roles of CD98, including involvement in extracellular signaling, adhesion/polarity, and amino-acid transporter expression in intestinal epithelia. A role for CD98 in intestinal inflammation, such as Intestinal Bowel Disease (IBD), is also proposed.
Keywords: Intestinal Epithelial CD98, Extracellular PDZ binding domain, Adhesion, Cell polarity, Amino acid transporters, Intestinal inflammation
1) Introduction
Polarized intestinal epithelial cells contain distinct apical and basolateral membranes with unique protein and lipid compositions. This asymmetric distribution of plasma membrane components is a fundamental characteristic of epithelial cells (1–5). For example, the ability of epithelium to secrete and absorb fluid is closely linked to the asymmetric distribution of ion-transport processes in the membranes (6–11). Accumulating experimental and clinical evidence indicates that surface adhesion molecules, such as integrins, are required for normal epithelial development; mutation or absence of these molecular adhesins can disrupt growth control and/or alter epithelial cell function and cell polarity (12–13). Because epithelial cells are in direct contact with the extracellular matrix (ECM), it is reasonable to expect that specific interactions exist between basolateral "receptors", such as integrins, and their cognate extracellular “ligands” in the ECM. Indeed, on binding to ECM ligands, integrins deliver signals that control cell proliferation, gene expression, differentiation, and polarization (14–16).
The CD98 complex, a cell-surface amino-acid transporter formed by covalent linkage of the CD98 heavy chain (CD98hc) with several different light chains, has recently been shown to function as a β1-integrin regulator (17–25). Because most of the reported studies have been performed in non-polarized cells, the physiological relevance of this regulatory function is unclear. Here, we focus on CD98 in polarized intestinal epithelia, exploring its role as a potential regulator of multiple functions, including adhesion, epithelial cell polarity, and amino-acid transport.
2) Expression of CD98 in intestinal polarized epithelia
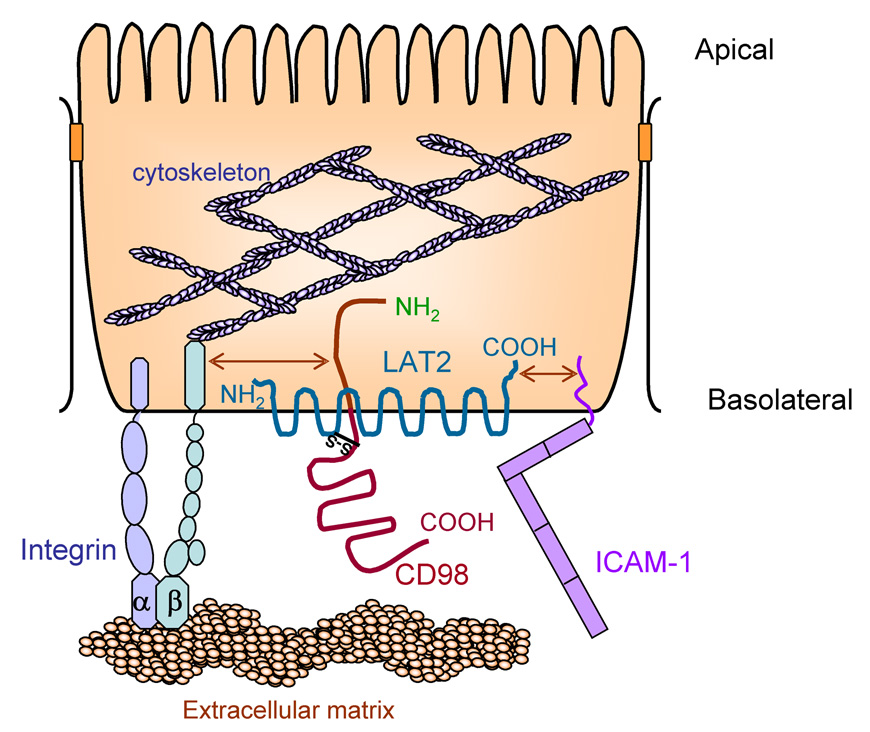
CD98 is expressed in all cell types with the exception of platelets, and is expressed at highest levels in the tubules of the kidney and the gastrointestinal tract (26, 27). In polarized epithelial cells, CD98 is targeted to the basolateral domain of the epithelium (26, 27), and is found exclusively on the basolateral surface of intestinal epithelia in culture (24) and mouse small intestine (27). The intestinal epithelial CD98 cell-surface molecule is a 125-kDa type II membrane glycoprotein heterodimer composed of a 40-kDa non-glycosylated light chain (amino-acid transporter) and an 85-kDa glycosylated heavy chain (CD98) (24; and Figure 1). The CD98 transcript is 1863 base pairs (bp) long with an open reading frame of 1590 bp that encodes a glycoprotein of 529 amino acids with a predicted size of 80 kDa. The hydropathy plot of CD98 predicts the presence of a single membrane-spanning domain (24–31). Amino acids 1–81 form the cytoplasmic portion, amino acids 82–104 represent the single-pass transmembrane region, and amino acids 105–529 comprise the extracellular domain of the CD98 glycoprotein (30). Interestingly, the C-terminus of human CD98 (hCD98) contains a potential class II PDZ-binding domain (amino acids 520 to 529; GLLLRFPYAA), suggesting that hCD98 may associate with extracellular PDZ-domain-containing proteins (32). The primary sequence contains four potentially glycosylated asparagine residues at positions 264, 280, 323, and 405, all of which are located in the C-terminal half of the protein. Cys-109 has been shown to participate in disulfide-bond formation with amino-acid transporters. Five vertebrate “glycoprotein-associated” amino acid transporters, LAT1, LAT-2, y+LAT1, y+LAT-2, and xCT (33–39), have been shown to associate with CD98. However, intestinal CD98 is linked to the L-type amino-acid transporters LAT-2 and y+LAT1, which appear to be expressed only in epithelia (27, 36). LAT-2, and presumably also y+LAT1, is exclusively expressed in the basolateral membrane domains of intestinal epithelial cells and is covalently linked to CD98 to form a functional heterodimer (39–42). Studies have demonstrated that basolaterally expressed CD98/LAT-2 in intestinal epithelial cells represents the minimal functional unit for an Na+-independent transporter of zwitterionic amino acids of any size (36, 38). Hydrophobicity studies indicate that LAT2 is composed of 12 transmembrane domains with intracellular N- and C-terminal segments, a structure that is predicted to be shared by other proteins of this family (33, 43). CD98 is responsible for recognition of LAT-2 and also ensures proper translocation of the transporter complex to the plasma membrane (44). However, CD98 has also been shown to be capable of guiding LAT-2 to the plasma membrane in the absence of a disulfide linkage between the two proteins, implying the existence of important noncovalent, steric interactions (44). A model of the CD98/LAT-2 heterodimer is depicted in Figure 1.
Figure 1. Model of possible functional macro-complex including heterodimer of CD98 and LAT2, β1 integrins and ICAM-1 in intestinal epithelia.
The model shows that CD98 controls β1 integrin activation that and amino acid transport activities via LAT2. In addition, ICAM-1 is also associated to CD98 and modulates amino acid transporter activities via LAT2. Extracellular C-terminal domain that contain PDZ binding domain controls LAT2 transport activity since intracellular/juxta-intramembrane N terminal domain of CD98 controls β1 integrin activation in intestinal epithelia.
3) Potential Role of the Extracellular PDZ Binding Domain of CD98
The C-terminal region of CD98 contains a potential PDZ class II-binding domain (amino acids 520 to 529; GLLLRFPYAA), indicating that the C-terminal tail of hCD98 might bind to PDZ class II proteins in the extracellular space (32). Such interactions might be expected to occur on the basolateral side of intestinal monolayers, given that proteins containing PDZ class II domains are often localized to specific subcellular sites near the plasma membranes of polarized cells, such as intestinal epithelial cells. One protein that may bind to the C-terminus of hCD98 in the extracellular space is human calcium/calmodulin-dependent serine kinase (hCASK), a member of the MAGUK protein family (45, 46). This protein is the human homolog of the Caenorhabditis elegans LIN-2 and Drosophila Camguk proteins and is widely expressed in human tissues (47, 48). The protein has a unique domain structure that includes an N-terminal region with homology to the calcium/calmodulin-dependent protein kinase, followed by the three characteristic MAGUK domains (PDZ class II consensus domain, SH3 domain, and GUK domain) (46). Although hCASK-associated protein complexes in epithelial cells have not yet been fully characterized, hCASK is reportedly basolaterally localized in intestinal epithelial cells (46). In agreement with these reports, we have recently shown that hCASK is basolaterally expressed in the Caco2-BBE intestinal epithelial cell line (32). We have further shown that hCASK may be inserted into the cell membranes of Caco2-BBE cells with an orientation that positions the C-terminus of hCASK and its PDZ domain on the extracellular side of the membrane (32). Although hCASK has previously been shown to access the intracellular side of the membrane (47), this is the first report that hCASK may also access extracellular ligands (32). In this orientation, hCASK would be capable of using extracellular nucleoside triphosphates as co-substrates (48, 49). In addition, we have demonstrated that hCD98 and hCASK co-precipitate and co-localize both in vitro and in vivo, and that the PDZ-binding domain of hCD98 is directly involved in this interaction (32). The most critical residue for PDZ recognition was found to be a phosphorylatable amino acid (tyrosine), further suggesting that tyrosine phosphorylation is a common mechanism for regulating the hCASK/CD98 interaction (47), perhaps via the action of ectokinases (49).
Although most protein phosphorylation studies have focused on intracellular protein kinases, some have reported evidence of ectokinase activities on the surfaces of a variety of cells (49–53). Importantly, a recent study identified ectokinase activity on the surface of human neutrophils (54), which can interact with the basolateral aspect of intestinal epithelial cells, and thus might regulate the hCD98/hCASK interaction. Recent reports demonstrate the importance of the 15 C-terminal residues of CD98 that contain the PDZ binding domain, showing that these residues are required for the transport function of the heterodimer. Mutation of the conserved C-terminal residue, leucine 523, to glutamine, reduced the Vmax values for arginine and leucine uptake (66). Collectively, these observations suggest that the PDZ-binding domain of CD98 might be a functional entity capable of regulating important processes, such as amino acid transport activity via LAT-2, in intestinal epithelial cells.
4) Epithelial CD98/LAT2 as a regulator of intestinal epithelial adhesion and cell polarity
Integrins, including β1-integrins, are expressed in the basolateral domain and along cell–cell junctions (lateral domain), where they have a role in maintaining cell–cell adhesion and organization of the subcortical cytoskeleton (55). It has been suggested that integrin function is readily modulated by various proteins and protein complexes, including oncogenes (56). Several classes of cell-surface glycoproteins have been shown to play a role in integrin-mediated events, including CD98, CD36, CD63, and CD9 (17, 57, 58, 59). β1-integrins co-localize with CD98/LAT-2 to the intercellular contact sites in Caco2-BBE monolayers, which indicates that CD98 may also regulate β1-integrin function (24). Given the importance of integrin cytoplasmic tails in integrin activation, proteins such as CD98 that interact with integrin cytoplasmic domains are excellent candidates as modifiers of integrin activation. Integrins are dynamic molecules, and recent studies have reported that a number of surface transmembrane glycoproteins can associate with integrins and thereby modulate their function (17, 60). Several classes of cell-surface glycoproteins have been shown to play a role in integrin-mediated events, including the integrin-associated protein (CD47) and members of the transmembrane 4 superfamily (TM4SF or tetraspannins). CD47 associates with αvβ3-integrin and seems to affect integrin-mediated signal transduction, phagocytosis, and cell migration (58, 59). Numerous TM4SF members such as CD36, CD63, and CD9 have been shown to be associated with β1-integrins (17, 57). Experiments in non-polarized epithelial CHO cells have shown that CD98 can modify the function of β1-splice variants (19). In addition, a genetic complementation strategy identified an interaction between a transmembrane protein, CD98, and the integrin β1 cytoplasmic domain (18, 19, 23). Together, such observations performed in non polarized cells indicate that CD98, if basolaterally expressed by polarized epithelial cells, could affect attachment, adherence, and integrin-mediated cytoskeletal functions.
Epithelial cell polarity is determined by a combination of events mediated by cell–cell and cell–substratum adhesion. Cell adhesion to the ECM is mediated by the integrin superfamily of adhesion receptors and is thought to play a critical role in subsequent ordering of the cytoskeleton and formation of polarity. However, the interactions between integrins and the ECM, although triggering a crude initial polarity, are unlikely to be sufficient to organize the high level of polarity displayed by polarized columnar epithelia. For development of this level of polarity, it is likely that additional cell–cell interactions are required to restrict the localization of basolateral proteins. We have shown that expression of human heterodimeric (human CD98/endogenous canine light chain) or monomeric CD98 in a CD98-deficient cell line (MDCK) disrupts intercellular adhesion, leading to cytoskeletal disorder (24). This phenotypic conversion, which probably depends on the interaction of human CD98 with respective "ligands", is accompanied by reorganization of the actin cytoskeleton. However, interactions between CD98 and the amino-acid transporter are unlikely to be involved in this process, as overexpression of CD98 modified at a specific residue 109 (C109 was mutated to serine) preventing disulfide linkage between human CD98 and canine amino acid transporter does not inhibit the process. The later observation is in agreement with the finding that CD98/amino acid transporter association is not required for the interaction of CD98 with integrins (19). By contrast, it has recently been demonstrated that heterodimeric CD98, but not monomeric CD98, causes transformation of fibroblasts cells; however, in this case, expression of the amino-acid transporter was thought essential to achieve this phenotype (62). A potential ligand for CD98 is β1-integrin, which is expressed by MDCK cells. The phenotypic conversion observed with CD98 could be related to altered β1 recognition of extracellular ligands (18), and CD98 has been shown to affect β1-integrin function (18, 19, 25, 63). Consistent with this view, overexpression of a CD98 mutant protein lacking the cytoplasmic tail and part of the transmembrane domain did not induce this phenotypic change, whereas a CD98 protein with a partially truncated cytoplasmic tail did show this phenotypic change. These results indicate that the cytoplasmic juxtamembrane domain domain is crucial in this phenotype change and are in agreement with the recent study demonstrating that both the transmembrane domain and the proximal part of the cytoplasmic tail play an important role in modulating integrin-dependent cell adhesion and migration as well as branching morphogenesis of polarized renal epithelial cells. However, another study using non polarized cells has reported that CD98 interaction with the integrin β subunit cytoplasmic domain was necessary to mediate adhesive signaling signaling (64). The reason for this discrepancy may be due to the different model system utilized. The co-localization of CD98/LAT-2 and β1-integrin suggests a possible interaction between these three proteins. We speculate that a specific molecular ratio of CD98/LAT-2 heterodimer and β1-integrin may be required for polarization of epithelial cells. Expression of human CD98 in a human CD98-deficient cell line (MDCK) may change the canine CD98/amino-acid transporter and β1-integrin molecular ratio, which may have a consequent effect on cell adherence and polarity. The cytoplasmic juxtamembrane domain seems to be crucial in this process. Recently it has been proposed that CD98 expression participates in fibronectin matrix assembly by mediating integrin signaling (64). Under this context changes in CD98hc expression, or its association with integrins, may influence a wide range of developmental that include cell polarity processes through the regulation of matrix assembly (64).
5) Epithelial CD98 and ICAM-1 regulate the activity of amino acid transporter, LAT-2
The mechanisms by which CD98 and LAT-2 regulate amino-acid transport, and the possible interactions involved, remain largely unstudied. However, we have demonstrated that cross-linking of CD98 affects the intrinsic activity of the LAT-2 transporter by increasing the affinity and reducing the capacity of LAT-2-mediated leucine uptake in Caco2-BBE monolayers (65). In addition, we have reported that cross-linking of CD98 regulates LAT-2-dependent leucine efflux (65). Other studies have shown that a series of CD98 C-terminal truncates (ranging from 15 to 404 residues) caused a complete loss of light-chain function, although all heterodimers were expressed at the cell surface. This indicates that the 15 C-terminal residues of CD98 are required for the transport function of the heterodimer. Mutation of the conserved residue leucine 523 to glutamine in the C-terminus reduced the Vmax of arginine and leucine uptake (66). Another study have demonstrated that the substitution of the extracellular domain of CD98hc with that of CD69 preserved effects on integrin function but abolished amino acid transport activity (19). Together, these observations suggest that extracellular domain of CD98 regulates the LAT-2 transport activity that contrast to the intracellular domain of CD98 which is crucial for regulating β1 integrin activation.
It has been reported in non polarized cells that CD98 can regulate different types of adhesion molecules such as CD147, LFA-1 through distinct mechanisms, reinforcing the notion that CD98 acts as a ‘molecular facilitator’ in the plasma membrane (19). In the context of intestinal inflammation, ICAM-1, a cell adhesion molecule is expressed in intestinal epithelial cells during intestinal inflammation (67, 68). ICAM-1 plays an important role in cell-cell, cell-extracellular matrix interactions and cellular interactions such as the immune response (69). Furthermore, ICAM-1 is known to be the receptor to the heterodimer of CD11a, and CD18 (β2 integrin) is expressed in leukocytes that interact with the intestinal epithelia during inflammation. ICAM-1 is constrictively expressed to both apical and basolateral domain membranes of the model intestinal epithelial cell line Caco2-BBE (65). Under this observation, it is conceivable that in the intestinal epithelia, ICAM-1 may be part of a multicomponent web that includes CD98/LAT2 and integrin β1. Recently, we have demonstrated that ICAM-1 associates with the heterodimer CD98/LAT2 (65). This result suggests that, in adhesion, ICAM-1 functions not only as an individual receptor but also as a component of supramolecular complexes at the plasma membrane in epithelial cells. In addition, association of the heterodimer CD98/LAT-2 and ICAM-1 indicate that this supramolecular complex may have a significant role in mediation of cellular regulation. We have shown that cross-linking of ICAM-1 reduces the affinity and increases the capacity of LAT-2 mediated leucine uptake (65).
Furthermore, cross-linking of ICAM-1 increases the rate of leucine efflux across the basolateral membranes of Caco2-BBE cells (65). However, it is not known if ICAM-1 interacts directly or indirectly with LAT2. Further investigations will have to be perform to elucidate the ICAM1 interaction(s) with CD98/LAT2 β1 integrin complexe. The supramolecular complex may signal via the amino-acid transporter LAT-2 to regulate multiple aspects of cell physiology. For example, LAT-2 medicated regulation of intracellular amino-acid availability may modulate the activity signaling pathway, leading to phosphorylation of an intracellular target protein. In addition, it has been shown that the intracellular amino-acid supply modulates several important regulatory translation factors through a variety of mechanisms (70). Furthermore, it has been shown that leucine availability regulates the activity of the signaling pathway, which leads to the activation of p70 S6 kinase (which is a 70-kDa protein kinase that acts on the ribosomal protein S6) (71, 72). Indeed, we have demonstrated that cross-linking of CD98 or ICAM-1, which mimics natural ligands for these proteins, modifies LAT-2-mediated leucine transport activity. Interestingly, cross-linked CD98 and cross-linked ICAM-1 have different effects on LAT-2 transport activity. We suggest that the transport activity changes are the result of a direct or indirect phosphorylation of LAT-2. In conclusion, the amino-acid transporter LAT-2 is regulated by adhesion molecules such as ICAM-1 and CD98 in epithelial cells. CD98 and ICAM-1 may have roles in transduction of intracellular signals. Changes in amino-acid-transport activity resulting from CD98 and ICAM interaction may coordinate events such as cell adhesion.
6) Regulation of CD98 Expression in intestinal Inflammation
In the context of intestinal inflammation, CD98 protein expression in human colonic epithelium was shown to be upregulated by pro-inflammatory cytokines such as interferon γ (73), and increased expression levels of lymphocyte-activation antigens for CD98 were found at the cell surface of intestinal B cells, T cells, CD4+ T cells, and CD8+ T cells isolated from patients with Crohn's disease and ulcerative colitis (74). These and other results indicate that markedly increased intestinal lymphocyte activation is an important immunological alteration in inflammatory bowel disease (IBD) (75). In addition, 5-aminosalicylic acid (which is used for the treatment of intestinal inflammation in IBD) dose-dependently inhibited expression of the CD98 cell-surface-activation antigen in mitogen-activated peripheral blood lymphocytes, further suggesting that CD98 plays an important inflammatory role. However, in contrast to the high expression of intestinal epithelial CD98 expression, it has been demonstrated that T-cells have low levels of CD98 transcripts and has been shown to be the result of a block to transcription elongation within the exon 1 intron 1 regions (76). These findings indicated that a removal of the block to mRNA elongation stimulates the induction of CD98 in activated T-cells (76). The latter observation suggests that regulation CD98 expression could be tissue dependent. Dextran sulfate sodium (DSS)-induced colitis is a useful model for examining the role of CD98 in the colonic mucosa (74, 76), and we found that the effect of DSS on epithelial CD98 expression is mediated via interferon-γ (IFN-γ) (77). IFN-γ is present at high levels in tissues affected by IBD, where it helps enterocytes to function in host defense (75). Antibody-based inhibition of endogenous IFN-γ has been shown to ameliorate the chronic stage of colitis, indicating that IFN-γ is likely to be a key mediator of intestinal inflammation (78). Recently, we showed that CD98 transcription is activated in IFN-γ-treated intestinal epithelial cells, and investigated the underlying mechanisms in the colonic epithelial cells (77, 79). We have isolated and functionally characterized the 5’-flanking region of the CD98 gene in the Caco2-BBE epithelial cell line. Sequence analysis revealed four GC/GT boxes potentially capable of binding Sp1 transcription factors, together with a nuclear factor-κB (NF-κB)-transcription-factor-binding site (79). This result is in agreement with results of sequence analysis of the cloned CD98 DNA from the HPB-MLT human T-cell tumor line, which revealed that the 5' flanking region of the CD98 gene contains a hypomethylated CpG island and four potential binding sites for the Spl transcription factors but does not contain TATA or CCAAT boxes (80). Both competition electrophoretic mobility shift assay (EMSA) and antibody supershift experiments revealed that Sp1 and NF-κB interact with the promoter region. We found that different Sp1 binding sites have different DNA–protein interaction profiles, indicating that each of these binding sites has distinct functional properties. Furthermore, chromatin immunoprecipitation studies have shown that DNA interacts with Sp1 and NF-κB in vivo under basal and IFN-γ-stimulated conditions. The 5`-flanking region does not contain TATA or CCAAT boxes, and primer extension and rapid amplification of cDNA ends (RACE) assays revealed that a major transcriptional initiation site is located 129 bases upstream of the first ATG codon. This is in agreement with previous identification of single start sites for TATA-less promoters in other genes, including the genes encoding thymidine kinase (81), dihydrofolate reductase (82), and adenine deaminase (83). Transfection of Caco2-BBE cells with luciferase reporter constructs fused to the CD98 gene promoter region or its serially truncated mutants revealed that specific DNA regulatory elements are located within 0.33 kb of the major transcription start site. IFN-γ increases transcription of CD98 via the Sp1 and NF-kB transcription factors in intestinal epithelial cells (79). These results indicate that, during intestinal inflammation, CD98 expression is upregulated in immune cells and in intestinal epithelial cells. The upregulation of CD98 in intestinal epithelial cells could affect functions such as β1-integrin mediated events (18, 21) that have been implicated in the etiology of various pathologic conditions, including inflammatory disorders such as IBD.
7) Perspectives
Intestinal epithelial CD98 plays an important role in coordinating intestinal epithelia events such as adhesion/polarity, amino-acid transport, and the direct binding of cell-surface molecules. The unique molecular orientation of CD98, with a PDZ-binding domain in the extracellular C-terminal tail, suggests that extracellular signaling may play a role in the multiple functions of CD98. For example, one of the challenges would be to study the role of extracellular phosphorylation of CD98 and its effects in intestinal epithelial functions in the normal and disease states.
Acknowledgements
This work was supported by National Institutes of Health of Diabetes and Digestive and Kidney Diseases under a center grant (R24-DK-064399), RO1-DK061941-02 (to D. Merlin), RO1-DK55850 (S. Sitaraman). Y. Yan is recipient of a research fellowship award from the Crohn’s and Colitis Foundation of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yutao Yan, Department of Medicine, Division of Digestive Diseases, Emory University School of Medicine, Atlanta, GA 30322.
Sona Vasudevan, National Biomedical Research Foundation, Protein Information Resource, Department of Biochemistry and Molecular Biology, Georgetown University Medical Center, Washington DC 20057.
Hang Nguyen, Department of Medicine, Division of Digestive Diseases, Emory University School of Medicine, Atlanta, GA 30322.
Didier Merlin, Department of Medicine, Division of Digestive Diseases, Emory University School of Medicine, Atlanta, GA 30322.
REFERENCES
- 1.Danielsen EM, Hansen GH. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim. Biophys. Acta. 2003;1617:1–9. doi: 10.1016/j.bbamem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Danielsen EM, Hansen GH. Lipid raft organization and function in brush borders of epithelial cells. Mol Membr Biol. 2006;23:71–79. doi: 10.1080/09687860500445604. [DOI] [PubMed] [Google Scholar]
- 3.Izumi Y, Hirose T, Tamai TY, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell. Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20(14):3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 6.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 7.Greger R. Role of CFTR in the colon. Annu Rev Physiol. 2000;62:467–491. doi: 10.1146/annurev.physiol.62.1.467. [DOI] [PubMed] [Google Scholar]
- 8.Frizzell RA, Field M, Schultz SG. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979;236:F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman EK, Schettino T, Marshall WS. The role of volume-sensitive ion transport systems in regulation of epithelial transport. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:29–43. doi: 10.1016/j.cbpa.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Haas M, Forbush. B B. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2006;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 11.Isenring P, Forbush B. Ion and bumetanide binding by the Na-K-Cl cotransporter, Importance of transmembrane domains. J Biol Chem. 1997;272:24556–24562. doi: 10.1074/jbc.272.39.24556. [DOI] [PubMed] [Google Scholar]
- 12.Gimond C, van der Flier A, van Delft S, Brakebusch C, Kuikman I, Collard JG, Fässler R, Sonnenberg A. Induction of Cell Scattering by Expression of β1 Integrins in β1-deficient Epithelial Cells Requires Activation of Members of the Rho Family of GTPases and Downregulation of Cadherin and Catenin Function. J of Cell Biol. 1999;147:1325–1340. doi: 10.1083/jcb.147.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimond C, Aumailley M. Cellular interactions with the extracellular matrix are coupled to diverse transmembrane signaling pathways. Exp Cell Res. 1992;203:365–373. doi: 10.1016/0014-4827(92)90010-6. [DOI] [PubMed] [Google Scholar]
- 14.Cali G, Mazzarella C, Chiacchio M, Negri R, Retta SF, Zannini M, Gentile F, Tarone G, Nitsch L, Garbi C. RhoA activity is required for fibronectin assembly and counteracts beta1B integrin inhibitory effect in FRT epithelial cells. J Cell Sci. 1999;112:957–965. doi: 10.1242/jcs.112.6.957. [DOI] [PubMed] [Google Scholar]
- 15.Cali G, Retta SF, Negri R, Damiano I, Gentile R, Tarone G, Nitsch L, Garbi C. Beta1B integrin interferes with matrix assembly but not with confluent monolayer polarity, and alters some morphogenetic properties of FRT epithelial cells. Eur J Cell Biol. 1998;75:107–117. doi: 10.1016/s0171-9335(98)80053-8. [DOI] [PubMed] [Google Scholar]
- 16.Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J Cell Sci. 1994;107:561–576. [PubMed] [Google Scholar]
- 17.Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by alpha3beta1 integrin and regulated by insulin-like growth factor-1 and CD98. J. Biol. Chem. 1999;274:11408–11416. doi: 10.1074/jbc.274.16.11408. [DOI] [PubMed] [Google Scholar]
- 18.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:15–17. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- 19.Fenczik CA, Zent R, Dellos M, Calderwood DA, Satriano J, Kelly C, Ginsberg MH. Distinct domains of CD98hc regulate integrins and amino acid transport. J. Biol. Chem. 2001;276:8746–8752. doi: 10.1074/jbc.M011239200. [DOI] [PubMed] [Google Scholar]
- 20.Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, Ginsberg MH. CD98hc (SLC3A2) mediates integrin signaling. Proc. Nat. Acad. Sci. 2005;102:355–360. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson NC, Collis EA, Mackinnon AC, Simpson KJ, Haslett C, Zent R, Ginsberg M, Sethi T. CD98hc (SLC3A2) interaction with beta 1 integrins is required for transformation. J. Biol Chem. 2004;279(52):54731–54741. doi: 10.1074/jbc.M408700200. [DOI] [PubMed] [Google Scholar]
- 22.Kolesnikova TV, Mannion BA, Berditchevski F, Hemler ME. Beta1 integrins show specific association with CD98 protein in low density membranes. BMC Biochem. 2001;2:10–19. doi: 10.1186/1471-2091-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 2000;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 24.Merlin D, Sitaraman S, Liu X, Eastburn K, Sun J, Kucharzik T, Lewis B, Madara JL. CD98-mediated links between amino acid transport and beta 1 integrin distribution in polarized columnar epithelia. J Biol Chem. 2001;276:39282–39289. doi: 10.1074/jbc.M105077200. [DOI] [PubMed] [Google Scholar]
- 25.Zent R R, Fenczik CA, Calderwood DA, Liu S, Dellos M M, Ginsberg MH. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J. Biol. Chem. 2000;275:5059–5064. doi: 10.1074/jbc.275.7.5059. [DOI] [PubMed] [Google Scholar]
- 26.Dave MH, Schulz N, Zecevic M, Wagner CA. Expression of heteromeric amino acid transporters along the murine intestine. J Physiol. 2004;558:597–610. doi: 10.1113/jphysiol.2004.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossier G, Meir C, Bauch C, Summa V, Sordat B, Verrey F, Kuhn CJ. LAT2, a New Basolateral 4F2hc/CD98-associated Amino Acid Transporter of Kidney and Intestine. J Biol Chem. 1999;274:39948–39954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- 28.Quackenbush E, Clabby M, Gottesdiener KM, Barbosa J, Jones NH, Strominger JL, Speck S, Leiden JM. Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell-surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. Proc. Nat. Acad. Sci. 1987;84:6526–6530. doi: 10.1073/pnas.84.18.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quackenbush EJ, Gougos A, Baumal R, Letarte M. Differential localization within human kidney of five membrane proteins expressed on acute lymphoblastic leukemia cells. The Journal of Immunology. 1986;136:118–124. [PubMed] [Google Scholar]
- 30.Teixeira S, Di Grandi SS, Kuhn LC. Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J Biol Chem. 1987;262:9574–9580. [PubMed] [Google Scholar]
- 31.Lumadue JA, Glick AB, Ruddle FH. Cloning, sequence analysis, and expression of the large subunit of the human lymphocyte activation antigen 4F2. Proc Natl Acad Sci U S A. 1987;84:9204–9208. doi: 10.1073/pnas.84.24.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Y, Vasudevan S, Bork U, Sitaraman SV, Merlin D. Extracellular interaction between hCD98 and the PDZ class II domain of hCASK in intestinal epithelia. J. Memb. Biol. 2007;215:15–26. doi: 10.1007/s00232-007-9001-8. [DOI] [PubMed] [Google Scholar]
- 33.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 34.Mastroberardino L L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer R, Rossier G, Spindler B, Meier C, Kühn LC, Verrey F. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1998;18:49–57. doi: 10.1093/emboj/18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pineda M, Fernandez E, Torrents D, Estevez RCL, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- 37.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 38.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 39.Yagita H, Masuko T, Hashimoto Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res. 1986;46:1478–1484. [PubMed] [Google Scholar]
- 40.Ohkame H, Masuda H, Ishii Y, Kanai Y. Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in liver tumor lesions of rat models. J Surg Oncol. 2001:265–271. doi: 10.1002/jso.1165. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto Y, Sakata M, Ogura K, Yamamoto T, Yamaguchi M, Tasaka K, Kurachi H, Tsurudome M, Murata Y. Expression and regulation of 4F2hc and hLAT1 in human trophoblasts. Am J Physiol Cell Physiol. 2002;282(1):C196–C204. doi: 10.1152/ajpcell.2002.282.1.C196. [DOI] [PubMed] [Google Scholar]
- 42.Fraga S, Pinho MJ, Soares-da-Silva P. Expression of LAT1 and LAT2 amino acid transporters in human and rat intestinal epithelial cells. Amino Acids. 2005;29:229–233. doi: 10.1007/s00726-005-0221-x. [DOI] [PubMed] [Google Scholar]
- 43.Torrents D, Estevez R, Pineda M M, Fernandez E, Lloberas J, Shi YB, Zorzano A, Palacin M. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J Biol Chem. 1998;273:32437–32445. doi: 10.1074/jbc.273.49.32437. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura E, Sato M, Yang H, Miyagawa F, Harasaki M, Tomita K, Matsuoka S, Noma A, Iwai K, Minato N. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J. Biol. Chem. 1999;274:3009–3016. doi: 10.1074/jbc.274.5.3009. [DOI] [PubMed] [Google Scholar]
- 45.Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM, Wood DF. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daniels DL, Cohen AR, Anderson JM, Brunger AT. Crystal structure of the hCASK PDZ domain reveals the structural basis of class II PDZ domain target recognition. Nat Struct Biol. 1988;5:317–325. doi: 10.1038/nsb0498-317. [DOI] [PubMed] [Google Scholar]
- 47.Sanford JL, Mays TA, Rafael-Fortney JA. CASK and Dlg form a PDZ protein complex at the mammalian neuromuscular junction. Muscle Nerve. 2004;20:164–171. doi: 10.1002/mus.20073. [DOI] [PubMed] [Google Scholar]
- 48.Nix SL, Chishti AH, Anderson JM, Walther Z. hCASK and hDlg associate in epithelia, and their src homology 3 and guanylate kinase domains participate in both intramolecular and intermolecular interactions. J. Biol. Chem. 2000;275:41192–41200. doi: 10.1074/jbc.M002078200. [DOI] [PubMed] [Google Scholar]
- 49.Walter J, Schindzielorz A, Hartung B, Haass C. Phosphorylation of the β-Amyloid Precursor Protein at the Cell Surface by Ectocasein Kinases 1 and 2. J. Biol. Chem. 2000;275:23523–23529. doi: 10.1074/jbc.M002850200. [DOI] [PubMed] [Google Scholar]
- 50.Redegeld FA, Caldwell CC, Sitkovsky MV. Ecto-protein kinases: ecto-domain phosphorylation as a novel target for pharmacological manipulation? Trends Pharmacol Sci. 1999;20:453–459. doi: 10.1016/s0165-6147(99)01399-1. [DOI] [PubMed] [Google Scholar]
- 51.Ehrlich YH, Davis TB, Bock E, Kornecki E, Lenox RH. Ecto-protein kinase activity on the external surface of neural cells. Nature. 1986;320:67–70. doi: 10.1038/320067a0. [DOI] [PubMed] [Google Scholar]
- 52.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 53.Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987;48:913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- 54.Skubitz KM, Ehresmann DD, Ducker TP. Characterization of human neutrophil ecto-protein kinase activity released by kinase substrates. J. Immunol. 1991;147:638–650. [PubMed] [Google Scholar]
- 55.Calderwood DA, Zent R, Grant R, Rees DJG, Hynes RO, Ginsberg MH. The Talin Head Domain Binds to Integrin β Subunit Cytoplasmic Tails and Regulates Integrin Activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 56.Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811–3815. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- 57.Thorne RF, Marshall JF, Shafren DR, Gibson PG, Hart IR, Burns GF. The integrins alpha3beta1 and alpha6beta1 physically and functionally associate with CD36 in human melanoma cells Requirement for the extracellular domain of CD36. J Biol Chem. 2000;275:35264–35275. doi: 10.1074/jbc.M003969200. [DOI] [PubMed] [Google Scholar]
- 58.Wang XQ, Lindberg FP, Frazier WA. Integrin-associated protein stimulates alpha2beta1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. J Cell Biol. 1999;147:389–400. doi: 10.1083/jcb.147.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson KE, Li Z Z, Kara M, Gardner KL, Roberts DD. Beta 1 integrin- and proteoglycan-mediated stimulation of T lymphoma cell adhesion and mitogen-activated protein kinase signaling by thrombospondin-1 and thrombospondin-1 peptides. J. Immunol. 1999;163:3621–3628. [PubMed] [Google Scholar]
- 60.Yanez-Mo M, Tejedor R, Rousselle P, Sanchez -Madrid F F. Tetraspanins in intercellular adhesion of polarized epithelial cells: spatial and functional relationship to integrins and cadherins. J Immunol. 1999;163:3621–3628. doi: 10.1242/jcs.114.3.577. [DOI] [PubMed] [Google Scholar]
- 61.Cai S, Bulus N, Fonseca-Siesser PM, Chen D, Hanks SK, Pozzi A, Zent R. CD98 modulates integrin beta1 function in polarized epithelial cells. J Cell Sci. 1005;118:889–899. doi: 10.1242/jcs.01674. [DOI] [PubMed] [Google Scholar]
- 62.Shishido T, Uno S, Kamohara M, Tsuneoka-Suzuki T, Hashimoto Y, Enomoto T, Masuko T. Transformation of BALB3T3 cells caused by over-expression of rat CD98 heavy chain (HC) requires its association with light chain: mis-sense mutation in a cysteine residue of CD98HC eliminates its transforming activity. Int J Cancer. 2000;87:311–316. doi: 10.1002/1097-0215(20000801)87:3<311::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 63.Armulik A, Nilsson I, von Heijne G, Johansson S. Determination of the border between the transmembrane and cytoplasmic domains of human integrin subunits. J Biol Chem. 1999;274:37030–37034. doi: 10.1074/jbc.274.52.37030. [DOI] [PubMed] [Google Scholar]
- 64.Féral CC, Zijlstra A, Tkachenko E, Prager G, Gardel ML, Slepak M, Ginsberg MH. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J Cell Biol. 2007;178:701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Charrier L, Gewirtz A, Sitaraman S, Merlin D. CD98 and intracellular adhesion molecule I regulate the activity of amino acid transporter LAT-2 in polarized intestinal epithelia. J Biol Chem. 2003;278:23672–23677. doi: 10.1074/jbc.M302777200. [DOI] [PubMed] [Google Scholar]
- 66.Chubb S, Kingsland AL, Broer A, Broer S. Mutation of the 4F2 heavy-chain carboxy terminus causes y+ LAT2 light-chain dysfunction. Mol Membr Biol. 2006;23:255–267. doi: 10.1080/09687860600652968. [DOI] [PubMed] [Google Scholar]
- 67.Patarroyo M, Prieto J, Rincon J, Timonen T, Lundberg C, Lindbom L, Asjö B, Gahmberg CG. Leukocyte-cell adhesion: a molecular process fundamental in leukocyte physiology. Immunol Rev. 1990;114:67–108. doi: 10.1111/j.1600-065x.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 68.Vainer B, Horn T, Nielsen OH. Colonic epithelial cell expression of ICAM-1 relates to loss of surface continuity: a comparative study of inflammatory bowel disease and colonic neoplasms. Scand J Gastroenterol. 2006;41:318–325. doi: 10.1080/00365520510024241. [DOI] [PubMed] [Google Scholar]
- 69.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 70.van Sluijters DA, Dubbelhuis PF, Blommaart EF, Meijer AJ. Amino-acid-dependent signal transduction. J Biol Chem. 2003;278:23672–23677. doi: 10.1042/0264-6021:3510545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 72.Iiboshi Y, Papst PJ, Kawasome H, Hosoi H, Abraham RT, Houghton PJ, Terada N. Amino acid-dependent control of p70(s6k). Involvement of tRNA aminoacylation in the regulation. J Biol Chem. 1999;274:1092–1099. doi: 10.1074/jbc.274.2.1092. [DOI] [PubMed] [Google Scholar]
- 73.Fais S, Capobianchi MR, Silvestri M, Mercuri F, Pallone F, Dianzani F. Interferon expression in Crohn's disease patients: increased interferon-gamma and -alpha mRNA in the intestinal lamina propria mononuclear cells. J Interferon Res. 1994;14:235–238. doi: 10.1089/jir.1994.14.235. [DOI] [PubMed] [Google Scholar]
- 74.Fais S, Pallone F. Ability of human colonic epithelium to express the 4F2 antigen, the common acute lymphoblastic leukemia antigen, and the transferrin receptor. Studies in inflammatory bowel disease and after in vitro exposure to different stimuli. Gastroenterology. 1989;97:1435–1441. doi: 10.1016/0016-5085(89)90387-9. [DOI] [PubMed] [Google Scholar]
- 75.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 76.Nii T, Segawa H, Taketani Y, Tani Y, Ohkido M, Kishida S, Ito M, Endou H, Kanai Y, Takeda E, Miyamoto E. Ki Molecular events involved in up-regulating human Na+-independent neutral amino acid transporter LAT1 during T-cell activation. Biochem J. 2001;358:693–704. doi: 10.1042/0264-6021:3580693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; 77 Kucharzik T, Lugering A, Yan Y, Driss A, Charrier L, Sitaraman S, Merlin D. Activation of epithelial CD98 glycoprotein perpetuates colonic inflammation. Lab. Invest. 2005;85:932–941. doi: 10.1038/labinvest.3700289. [DOI] [PubMed] [Google Scholar]
- 78.Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820–2828. doi: 10.1111/j.1572-0241.2002.07029.x. [DOI] [PubMed] [Google Scholar]
- 79.Yan Y, Dalmasso G, Sitaraman SV, Merlin D. Characterization of the Human Intestinal CD98 Promoter and its Regulation by Interferon Gamma. Am J Physiol Gastrointest Liver Physiol. 2006;292:G535–G545. doi: 10.1152/ajpgi.00385.2006. [DOI] [PubMed] [Google Scholar]
- 80.Karpinski BA, Yang LH, Cacheris P, Morle GD, Leiden JM. The first intron of the 4F2 heavy-chain gene contains a transcriptional enhancer element that binds multiple nuclear proteins. Mol. Cell. Biol. 1989;9:2588–2597. doi: 10.1128/mcb.9.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deng TL, Li DW, Jenh CH, Johnson LF. Structure of the gene for mouse thymidylate synthase. Locations of introns and multiple transcriptional start sites. J. Biol. Chem. 1986;261:16000–16005. [PubMed] [Google Scholar]
- 82.Azizkhan JC, Vaughn JP, Christy RJ, Hamlin JL. Nucleotide sequence and nuclease hypersensitivity. Biochemistry. 1986;25:6228–6236. doi: 10.1021/bi00368a059. [DOI] [PubMed] [Google Scholar]
- 83.Valerio D, Duyvesteyn MG, Dekker BM, Weeda G, Berkvens TM, van der Voorn L, van Ormondt LH, van der Eb AJ. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985;4:437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]