Abstract
Background
A challenge in the reconstruction of periodontal structures is the targeted delivery of growth-promoting molecules to the tooth root surface. Polypeptide growth factors such as platelet-derived growth factor (PDGF) stimulate both cementogenesis and osteogenesis. Recent advances in gene therapy offer the advantage of delivering recombinant proteins to tissues for extended periods of time in vivo.
Methods
Recombinant adenoviral vectors encoding for the PDGF-A gene were constructed to allow delivery of PDGF transgenes to cells. The recombinant adenoviruses were assembled using the viral backbone of Ad2/CMV/EGFP and replacing GFP (reporter gene encoding green fluorescent protein driven by the cytomegalovirus promoter [CMV] within adenovirus type 2) with the PDGF-A gene. Root lining cells (cloned cementoblasts) were transduced with Ad2/PDGF-A and evaluated for gene expression, DNA synthesis, and cell proliferation. PDGF-inducible genes, c-myc and osteopontin, were also evaluated following gene delivery of Ad2/PDGF-A.
Results
The results revealed high level transduction of cementoblasts by gene transfer for 7 days as evidenced by flow cytometry and Northern blotting. Cementoblast DNA synthesis and subsequent proliferation were stimulated by Ad2/PDGF-A at levels equal to or greater than continuous rhPDGF-AA application. Strong message for the PDGF-A gene and protein as evidenced by Northern blotting and immunocytochemistry was noted. Furthermore, the potent induction of c-myc and osteopontin mRNA was found after PDGF gene delivery to cementoblasts.
Conclusions
These findings demonstrate that gene delivery of platelet-derived growth factor stimulates cementoblast activity that is sustained above that of rhPDGF-AA application. The use of gene therapy as a mode of growth factor delivery offers a novel approach to periodontal tissue engineering.
Keywords: Growth factors, platelet-derived; periodontium/growth; osteogenesis, dental cementum/growth and development; gene therapy
The success of tissue engineering relies on the large-scale purification and production of signaling molecules, as well as methods to deliver these factors to their targets.1 One problem with current growth factor delivery to periodontal wounds is the extremely short half-life of the factors. Topically-administered growth factors remain in the periodontal defect for a limited duration, presumably due to proteolytic breakdown, receptor-mediated endocytosis, and the solubility of the delivery vehicle.2 Therefore, the use of DNA delivery systems may serve as an alternative method of targeting proteins to periodontal wounds, since existing protein delivery systems provide such a transient action of the administered growth factor. Gene therapy has been applied to several diseases that display tissue deficiencies (e.g., skin and bone injury).3,4 Therefore, the use of gene therapy to promote repair and regeneration has become an active area of research.5 In the context of wound repair, a transient expression of a putative transgene may be advantageous to restore the tissue defect. Examples of methods of gene delivery for short-term expression of genes include adenovirus and DNA-lipid complexes.6 Since the regulation of wound repair occurs in a controlled fashion over a defined period of time, the use of gene therapeutics in chronic wounds (e.g., periodontal disease) may stimulate an elevated and sustained production of growth factors to promote tissue regeneration.7
Recombinant adenoviral expression in vivo has been demonstrated to extend for up to 35 days depending on the tissue transduced and route of administration.8 Adenovirus has a transient expression pattern due to ability of the virus to remain extrachromosomal (i.e., episomal) as a nonintegrating virus. The total level of recombinant virus in vivo decreases over time depending on the lifespan of the cells infected and the attenuation of the virus expression by host immunologic mechanisms.9
Platelet-derived growth factor (PDGF) was studied for gene delivery given its consistent results in stimulating periodontal tissue regeneration.10-12 PDGF elicits its biologic effects by binding to cell-surface receptors (PDGF-αR and -βR) possessing intrinsic tyrosine kinase activity which signal the cell to undergo mitosis, chemotaxis or matrix synthesis.13 Our strategy was to insert the PDGF gene into a transiently expressing viral vector (i.e., adenovirus) for gene targeting to quiescent and replicating cells. By using a “longer-term” delivery of PDGF we can use the extended expression pattern of PDGF receptors following tissue injury to our advantage.14,15 Several groups have delivered growth factor genes to healing skin,16,17 bone,4,18 and periodontal19 wounds using plasmid DNA. The use of biodegradable polymers to deliver PDGF DNA offers promise in tissue repair.20 Other investigators have delivered bone morphogenetic protein (BMP) genes by adenovirus to orthotopic wounds by ex vivo approaches with impressive results.21-23 Eriksson et al. have developed unique methods of transducing wounds by the in vivo microseeding technique3,24 which we have adapted to periodontal wounds.19 Furthermore, with the recent characterization of root lining cells that possess phenotypic characteristics similar to cementoblasts, we are now able to investigate the delivery of PDGF transgenes to cementoblasts.25
The objective of this study was to determine the ability of a recombinant adenovirus encoding PDGF-A to transduce and modulate the activity of cementoblasts. The investigation of growth factor gene therapy may provide a better understanding of cementogenesis and periodontal tissue regeneration, which could prove useful in future therapeutics.
MATERIALS AND METHODS
Construction of Recombinant Adenoviruses
We have previously described the construction of adenoviruses encoding PDGF genes which is shown in diagrammatic form in Figure 1.26 In brief, the full-length murine PDGF-A cDNA (gift of Dr. Charles D. Stiles, Dana-Farber/Harvard Cancer Center) was sub-cloned into a shuttle plasmid (pAD2/CMV/SVIX),‡ under the control of the cytomegalovirus (CMV) promoter. The viral backbone DNA Ad2/EGFP (adenovirus encoding the gene for green fluorescent protein [GFP]) was precut with PshAI and the shuttle plasmid containing either PDGF-A or PDGF-1308 cDNA was linearized with XbaI. The linearized shuttle plasmid and viral backbone DNA were co-transfected into 293 packaging cells (human embryonic kidney cells transformed with wild-type adenovirus) using calcium phosphate transfection. Recombination between the shuttle plasmid and the GFP viral backbone resulted in substitution of the GFP cDNA with PDGF-A cDNA (Fig. 1). At 8 to 10 days post-transfection, recombinant viral plaques were readily identified under inverted fluorescent microscopy by lack of fluorescence. Recombinant plaques were plaque purified and expanded for large-scale purification of the viral stocks using ultracentrifugation with a cesium chloride gradient. The cesium chloride-containing viral stocks were desalted and viral stocks were stored at −80°C. Titers of the virus stocks were determined on 293 cells by plaque formation assay and expressed as the number of plaque forming units (pfu) per ml.
Figure 1.
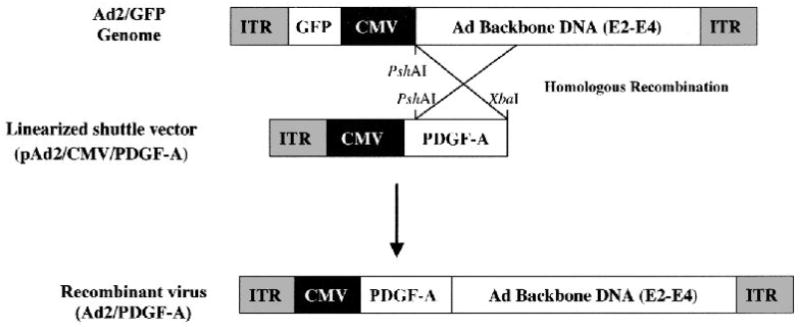
Schematic representation of the constructs used to synthesize the replication-deficient recombinant adenovirus containing the full-length murine PDGF-A cDNA. Note 5’ CMV promoter, adenoviral genes, which include E2, E3, and E4, and flanking ITR. Recombinant adenoviruses are generated after homologous recombination between pAd2/CMV/PDGF-A and linearized, Ad2/GFP genome as outlined in the materials and methods. ITR: inverted terminal repeat; CMV: cytomegalovirus promoter.
Cell Culture
The cementoblast cell cultures used in these experiments are described in detail in several reports by Somerman and colleagues.27-30 Briefly, cells were obtained from the root surface of first mandibular molars of OC-TAg transgenic mice. These mice contain the SV40 large T-antigen (TAg) under control of the osteocalcin (OC) promoter. Therefore, only cells that express OC also express TAg and are immortalized in vitro. Based on results of prior in situ studies, OC is expressed by cementoblasts during root development, but not by cells within the periodontal ligament (PDL). Consequently, when cell populations are isolated from developing molars using enzyme digestion, only cementoblasts (not PDL cells) are immortalized and thus will survive in culture. The resulting immortalized cementoblast population (OC/CM) express bone sialoprotein (BSP), osteopontin (OPN), and OC, markers selective to cells lining the root surface. These cells also promote mineral nodule formation both in vitro and in vivo.25,31 The cells utilized were from OC/CM clone 30, which were tested to confirm the expression of osteocalcin and bone sialoprotein by Northern blotting prior to utilization in experiments.
Gene Transfer to Cementoblasts
Cementoblasts were plated at subconfluence (~80%) in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% bovine calf serum (BCS) and antibiotics. Cells were then transduced with recombinant adenoviruses (Ad2/GFP or Ad2/PDGF-A) at a multiplicity of infection (MOI) = 100 (i.e., one MOI unit is that amount of virus required to form a plaque on a standard virus plaque forming assay; therefore, a MOI = 100 would equal 100 plaque forming units for each cell). After 4 of 5 hours of shaking, the medium was replenished with fresh 10% BCS and the cells maintained and evaluated for subsequent experiments (below). Depending on the experiment, cells were transferred to 5% platelet-poor plasma to negate the effects of PDGF and other growth factors found in bovine calf serum.
Flow Cytometry
Flow cytometry was performed to determine transduction efficiency of the Ad2/CMV/GFP control vector, which was used to generate the PDGF-A recombinant adenovirus, to cementoblasts. The cells were plated at a seeding density of 1 × 106 cells/dish in 60 mm culture dishes. Cells received either no treatment or exposure to an ascending range of Ad2/GFP (MOI = 0.1 to 100). The cell monolayers were harvested at days 1, 2, 4, and 7 after transduction. Single cell suspensions were collected and assessed by cell sorting at the University of Michigan Flow Cytometry Facility. Fluorescence intensity was plotted against total cell number to calculate the proportion of cells exhibiting fluorescence (i.e., percentage of cells expressing GFP).
Northern Blotting
Cementoblasts were plated at a density of 4 ×105 cells/well in 6 well plates. After 24 hours the cells were transduced with either Ad2/PDGF-A or Ad2/GFP (at MOI = 100) for 4 to 5 hours. After specified timepoints, total RNA was isolated from the cells and quantified by spectrophotometry as previously described.32 Three μg of total RNA was electrophoresed on 6% formaldehyde-1.2% agarose gels, transferred to nylon memranes§ and immobilized by UV crosslinking.§ Membranes were hybridized with the murine PDGF-A cDNA probe (gift of Dr. Charles D. Stiles, Dana-Farber/Harvard Cancer Center), murine osteopontin cDNA probe (gift of Dr. Marian Young, NIH/NIDCR), or murine c-myc cDNA (gift of Dr. Craig Logsdon, University of Michigan) and labeled using a horseradish peroxidase chemiluminescence technique.∥ Blots were exposed to autoradiographic film∥ for 20 minutes to 24 hours. Relative loading of wells was evaluated by ethidium bromide staining of the original agarose gel or hybridization of blots with 18S rRNA.33 Transduction efficiency of Ad2/PDGF-A gene delivery was compared at 24 hours and 7 days after gene transfer to evaluate prolonged gene expression. The induction of the immediate-early gene c-myc was assessed at timepoints ranging from 30 minutes to 48 hours. The induction of osteopontin gene expression was determined at 24 hours and 48 hours.
Immunohistochemistry
Cementoblasts were plated at a density of 2.0 ×104 cells/well in 4-well RS-coated chamber slides.¶ After 24 hours the medium was changed to DMEM supplemented with 5% platelet-poor plasma. The cells were subsequently transduced with either Ad2/GFP (MOI = 100), or Ad2/PDGF-A (MOI = 100). After 24 hours, the cells were fixed with 2% paraformaldehyde, permeabilized with 0.5% IGEPAL,# and blocked with 2% normal goat serum. Following treatment with primary antibody (1:50 dilution) specific for PDGF-AA, ** the cells were processed for peroxidase staining using a commercial avidin-biotin complex method kit†† and VIP substrate.††
Alkaline Phosphatase Activity
OC-CM cells were plated at a density of 1 × 105 cells/well in 24-well plates containing in 10% BCS. After 12 hours, the medium was changed to 5% platelet poor plasma. Twelve hours later, cementoblasts were treated with 20 ng/ml of rhPDGF-AA or transduced with either Ad2/GFP or Ad2/PDGF-A at MOI =100. Alkaline phosphatase activity was assessed 24 hours following treatment. Cells were evaluated for the ability to convert p-nitrophenylphosphate to p-nitrophenol as described previously.34 Monolayers of cells were released from tissue culture dishes and aliquots of 100,000 cells were counted. The cells were washed and suspended in 1 ml of 10 mM magnesium sulfate in saline to activate the enzyme, pelleted, and subjected to the addition of 0.5 ml of alkaline phosphatase substrate incubated for 5 minutes at 37°C. The reaction was stopped with 10 ml of 0.05 N NaOH. The absorbance of the samples was determined at a wavelength of 400 nm using a spectrophotometer and compared with a standard curve generated from prepared standards. Triplicate wells were evaluated in 3 separate experiments.
DNA Synthesis Assay
Mitogenic activity due to adenoviral delivery was evaluated as previously described.35 Cementoblasts were plated at a seeding density of 5,000 cells/well in 96-well plates for 5 days. Cells were then washed 3 times with PBS and stimulated in serum-free medium with either negative control (Ad2/GFP MOI =100); positive control (10% BCS); rhPDGF-AA‡‡ (at 20 ng/ml); or Ad2/PDGF-A (MOIs 1-100) for a period of 24 hours. One μCi of [3H]thymidine (NEN/)/§§well (5 μCi/ml) was added to the cells during the last 6 hours of incubation. Each well was fixed with ice-cold trichloroacetic acid (TCA) for 20 minutes. The wells were then washed 3 times with TCA. Next, 0.25 N NaOH (37°C) was added for 15 to 30 minutes followed by neutralization by 0.75 N HCl. The contents from each well were transferred to scintillation vials for measurement of [3H]thymidine uptake. Tritium levels were measured on a liquid scintillation counter. The results were expressed as CPM per 5 wells of 3 separate experiments.
Cell Proliferation
Cementoblasts were plated in 24-well plates at a density of 2.5 × 104 cells/well in 10% BCS. After a period of 12 hours to allow cell attachment, the cells were treated with either 20 ng/ml rhPDGF-AA (in 5% platelet poor plasma), Ad2/GFP (MOI =100) or Ad2/PDGF-A (MOI =100). After viral infection the medium was changed to 5% platelet poor plasma to avoid potential neutralizing antibodies to adenovirus found in the human-derived platelet poor plasma. The medium was changed every other day and cells were harvested at days 1, 2, 4, and 7 after treatment. Cell counts were made using a hemocytometer on triplicate cell culture wells.
Statistical Analyses
An analysis of variance with the Bonferroni-Dunn Multiple comparison procedure was performed to evaluate DNA synthesis and proliferation comparing treatments with corresponding controls. An alpha level of 0.05 was used to determine statistical differences between groups.
RESULTS
This study demonstrates the effective transduction of cementoblasts by Ad2/GFP and Ad2/PDGF-A recombinant adenoviruses with strong effects on the stimulation of DNA synthesis, cell proliferation and gene expression.
Flow Cytometry
Cementoblast transduction using the control virus Ad2/GFP demonstrated a dose-dependent effect on GFP expression (from MOI 0.1 to 100) for up to 7 days following gene delivery. The MOI of 100 was found to be optimal in achieving nearly 100% transduction of cementoblasts and was used for subsequent experiments. The percentage of cells exhibiting fluorescence as an indicator of GFP protein expression was maintained at >90% for the 7-day observation period (Fig. 2). MOI = 100 was chosen for subsequent gene transfer experiments since the highest transduction efficiency was shown by this MOI without altering cell growth (data not shown).
Figure 2.

Effective, prolonged gene transfer of Ad2/GFP to cementoblasts. Subconfluent cementoblasts were transduced with Ad2/GFP at an MOI = 100. After 24 hours the cells were subsequently visualized under phase contrast (left panel; A) and fluorescence microscopy (right panel; A). The cementoblasts were then subjected to FACS analysis to determine the percentage of cells transduced by the adenovirus encoding the jellyfish protein green fluorescent protein (GFP) after 1 and 7 days (B). Note the stability of protein expression in the cells at 1 and 7 days after gene transfer with >90% of the cells exhibiting fluorescence intensity above threshold (middle and right panels, respectively; B). Cells that were not transduced by Ad2/GFP fail to fluoresce (left panel; B) The B designation gives the total cell count on the cell sorter, while the C designation gives the number of cells which surpassed the fluorescence threshold (i.e., cells producing GFP).
PDGF Gene Expression
Strong expression of the PDGF-A gene was noted following gene transfer of Ad2/PDGF-A to cementoblasts (Fig. 3). Furthermore, prolonged gene expression of PDGF-A was also seen at 7 days following gene delivery. In contrast, no detectable PDGF-A gene expression was found in Ad2/GFP-transduced cells at any timepoint.
Figure 3.
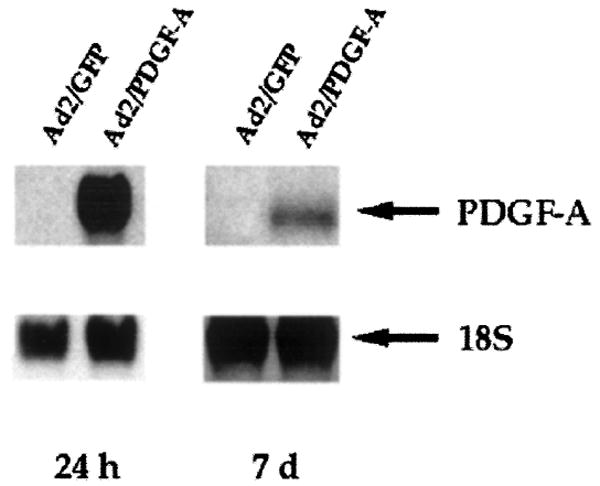
A representative Northern blotting analysis demonstrating dose-dependent gene expression of PDGF-A by infected cementoblasts 24 hours and 7 days after transduction. Cementoblasts were transduced with Ad2/GFP or Ad2/PDGF-A at MOI = 100 as described in the materials and methods. Prolonged gene expression was found 7 days after PDGF-A gene transfer. 18S is shown to indicate relative loading of RNA.
Immunohistochemistry
Immunocytochemical analysis of cementoblasts transduced with Ad2/PDGF-A revealed positive staining with the monospecific PDGF-AA antibody (Fig. 4) within 24 hours of gene transfer. For comparison, cells transduced with Ad2/GFP failed to demonstrate measurable levels of PDGF-AA protein.
Figure 4.

Immunohistochemical detection of PDGF-AA protein by cementoblasts transduced with Ad2/PDGF-A. Cementoblasts were plated at subconfluence for 24 hours in DMEM supplemented with 10% BCS. The medium was changed to DMEM supplemented with 5% platelet-poor plasma and cells transduced with either Ad2/GFP (A) or Ad2/PDGF-A (B) at MOI = 100. After 24 hours, the cells were fixed with 2% paraformaldehyde, permeabilized with 0.5% IGEPAL and blocked with 2% normal goat serum. Following treatment with primary antibody specific for PDGF-A, the cells were processed for peroxidase staining using a commercial avidin-biotin complex method kit and VIP substrate. Cementoblasts transduced with Ad/PDGF-A display positive staining consistent with PDGF-AA, while cells exposed to Ad2/GFP show minimal staining (original magnification ×20; phase contrast).
DNA Synthesis and Alkaline Phosphatase Activity
Assessment of cementoblast mitogenesis was measured by [3H]thymidine uptake as shown in Table 1. Ad2/PDGF-A exposure of cementoblasts significantly stimulated DNA synthesis above that of control virus (Ad2/GFP). Furthermore, when Ad2/PDGF-A was compared to 20 ng/ml of rhPDGF-AA, the stimulation was indistinguishable (P <0.05) at MOI = 100. In contrast, PDGF-A gene transfer and rhPDGF-AA application significantly downregulated ALP activity within 24 hours of application (Table 1).
Table 1.
Regulation of Cementoblast Mitogenesis and Alkaline Phosphatase Activity (ALP) by PDGF-A Gene Transfer
| Group | Counts per Minute ± SD (× 103) | ALP ± SD (× 10−6) |
|---|---|---|
| Ad2/GFP (control) | 225.5 ± 22.7 | 115.9 ± 16.4 |
| rhPDGF-AA | 288.7 ± 18.4* | 68.1 ± 15.9‡ |
| Ad2/PDGF-A (MOI = 1) | 240.1 ± 14.3* | ND§ |
| Ad2/PDGF-A (MOI = 10) | 290.4 ± 19.0* | ND |
| Ad2/PDGF-A (MOI = 100) | 301.5 ± 9.5* | 75.2 ± 16.8‡ |
| Ad2/PDGF-A (MOI = 500) | 150.8 ± 20.7† | ND |
| 10% Serum | 325.8 ± 30.7* | 100.8 ± 15.6 |
P <0.001 increase compared to Ad2/GFP.
P <0.001 reduction compared to Ad2/GFP.
P <0.05 reduction compared to Ad2/GFP.
not determined.
rhPDGF-AA = 20 ng/ml.
Cell Proliferation
Since PDGF has been shown to be a strong stimulator of cementoblasts in vitro,36 continuous exposure of cells to PDGF was compared to PDGF gene transfer using adenovirus. All cells responded similarly following continuous PDGF protein application or by Ad2/PDGF-A (Fig. 5). A statistically significant increase in cell number was noted for cementoblasts following treatment with rhPDGF-AA or Ad2/PDGF-A at days 4 and 7. Furthermore, cells treated by Ad2/PDGF-A also showed significant elevations in cell number at days 4 and 7 above that of continuous rhPDGF-AA application (P <0.01).
Figure 5.

Proliferation of cementoblasts following PDGF gene transfer. Cementoblasts were plated at subconfluence in DMEM supplemented with 10% BCS for 12 hours. At day 0, the medium was changed to DMEM containing either 5% platelet-poor plasma containing Ad2/GFP (circles), rhPDGF-AA (triangles), or Ad2/PDGF-A (squares). The cells were counted at days 1, 2, 4, and 7 after treatment. The experiment was repeated twice with typical results shown above. Statistically significant stimulation of cell growth above that of control virus (Ad2/GFP) and for continuous PDGF-AA application with Ad2/PDGF-A gene delivery at day 4 and 7. Error bars represent standard deviation. *P <0.01.
Expression of PDGF-Inducible Genes
Northern blotting was performed to ascertain the induction of genes expressed following exposure to PDGF Figure 6A reveals the induction in the immediate-early gene c-myc 6 hours following gene transfer of Ad2/PDGF-A, while minimal basal levels of c-myc were noted for Ad2/GFP transduced cells. The positive control lane demonstrates total RNA from carbachol-stimulated pancreatic acinar cells. In addition, no significant differences in c-myc gene expression were noted for later timepoints up to 48 hours post-gene transfer. Figure 6B illustrates the potent induction of osteopontin mRNA following gene delivery of Ad2/PDGF-A 48 hours after transduction. Gene expression for OPN was induced within 24 hours (not shown and was increased even further at 48 hours (Fig. 6B). When 50 μg/ml ascorbic acid was added to the medium, the induction in OPN mRNA was even more pronounced.
Figure 6.

PDGF gene transfer alters gene expression of immediate-early and osteoblast-associated genes in cementoblasts. A. Confluent cementoblasts cultured in 5% platelet-poor plasma were treated with either Ad2/GFP or Ad2/PDGF-A. RNA harvested from the cells were run on agarose gels, transferred to nylon membranes and then probed with the murine c-myc probe 6 hours after gene delivery. Note induction in the immediate-early gene c-myc in Ad2/PDGF-A transduced cells, while Ad2/GFP transduced cells reveal minimal c-myc gene expression by Northern blot analysis. The control lane represents RNA harvested from c-myc expressing pancreatic acinar cells. B. Confluent cementoblasts were plated in the presence (+) or absence (−) of ascorbic acid (AA) for 48 hours following gene transfer of PDGF. Northern analysis reveals the potent induction in osteopontin (OPN) mRNA by PDGF gene transfer with and without AA addition. Human gingival fibroblasts (hGF) failed to show measurable OPN gene expression, while differentiated MC3T3 E1 osteoblasts were used as a positive control. The blots were normalized with an 18S probe or ethidium bromide staining of the gels.
DISCUSSION
These results demonstrate for the first time the use of growth factor gene delivery to root lining cells. Our findings show that PDGF-A gene transfer is stable in vitro and that genes known to be modulated by PDGF are induced by Ad2/PDGF-A, and in a prolonged fashion.
The data suggest that gene transfer to a putative cell type that participates in periodontal regeneration (i.e., the cementoblast) can be effectively transduced at high levels (>90% of cells) for extended periods of time in vitro. However, the gene expression profile would likely be decreased in vivo by the mounting of a cytotoxic T-lymphocyte response following adenoviral protein release by Ad2/PDGF-A.37 Furthermore, the risks of employing this technique in periodontal wounds, such as viral load, the immune response, and the fate of the transduced cells in vivo, will need to be carefully assessed. Ongoing studies in both ex vivo and in vivo gene transfer to the periodontium seek to examine the extent of the above effects in modulating periodontal wound healing.
PDGF has pleiotropic effects on cells derived from the periodontium including osteoblasts, periodontal ligament cells, and cementoblasts.36,38,39 Topical application of PDGF accelerates tissue repair in a multitude of wound model systems such as skin,40 bone,41 and periodontium.12 Despite encouraging results using recombinant growth factors in many of these clinical situations, limitations exist with topical protein delivery such as short half-life, protease inactivation, and poor bioavailability from the available delivery vehicles. Therefore, our approach was to develop a methodology to optimize growth factor delivery to maximize the therapeutic efficacy. Using a genetic approach to tissue engineering may provide a means to achieve delivery of growth factors directly from the target cells to overcome many of the shortcomings of protein delivery.42,43
The results of this study establish the stable expression of the PDGF-A gene as shown by Northern blotting (Fig. 3); the production of PDGF-AA protein as identified by immunohistochemistry (Fig. 4); and that the PDGF-AA released by cementoblasts possesses biological activity as demonstrated by DNA synthesis assay and proliferation (Table 1 and Fig. 5). Furthermore, we show that the PDGF-AA delivered by gene transfer affects cementoblasts in a manner consistent with its response by protein delivery (e.g., stimulation of the immediate-early gene c-myc). The proto-oncogene c-myc is an important member of the early responsive nuclear oncogene family that is induced typically within minutes of growth factor stimulation. In the case of gene transfer, there is a lag in the induction of c-myc due to the steps of virus entry into the cell followed by transcription and subsequent translation of PDGF protein to induce c-myc mRNA. It was interesting to note that the biological effects are relatively rapid considering the steps required for viral infection. The biologic response (i.e., increase in [3H]thymidine uptake) is equivalent between PDGF-AA protein and Ad2/PDGF-A gene transfer within 24 hours of gene delivery, while over time, Ad2/PDGF-A gene transfer results in increased cementoblast cell numbers above that of rhPDGF-AA application after 4 and 7 days (Fig.5).
The induction of osteopontin mRNA following PDGF-A gene transfer further illustrates the consistency of effects of viral versus protein delivery of PDGF-AA. Figure 6B shows the potent induction of OPN gene expression alone and when combined with ascorbic acid, an important co-factor in extracellular matrix production. This result is comparable to the findings of Saygin et al. who demonstrated an increase in OPN gene expression following PDGF-BB application to cultured cementoblasts.36 Osteopontin, which has been identified in multiple connective tissues,44 promotes adhesion of osteoblasts and osteoclasts and regulates mineral crystal growth.45
Prolonged PDGF delivery may have differing effects on the mineralization process. Studies by Graves and colleagues46,47 have shown that long-term exposure of osteoblastic cells to PDGF stimulates proliferation, while decreasing the expression of the osteoblast phenotype with subsequent inhibition of mineralization in vitro. The finding that PDGF gene delivery inhibits ALP activity is not unexpected since rapidly proliferating cells by definition are not expressing the differentiated cell phenotype (Table 1). Therefore, regulation of the spatial and temporal levels of PDGF gene expression in vivo is likely to influence the composition of newly regenerating periodontal tissues.
In conclusion, the present study demonstrates effective gene transfer of PDGF-A to cementoblasts in vitro. The transduction of cementoblasts by PDGF-A results in stimulation of mitogenesis, proliferation, and expression of PDGF-inducible genes. Current investigations are seeking to determine the long-term effects of PDGF gene transfer on cementogenesis in vitro and in vivo. Future studies plan to elucidate the role of PDGF gene therapy in the modulation of periodontal tissue regeneration in vivo.
Acknowledgments
The authors acknowledge the helpful discussions with Dr. Martha Somerman and Ms. Jan Berry. The technical support of Robert Robke and Emily Watters is greatly appreciated. This study was funded by NIH/NIDCR grants DE11960, DE13397 to Dr. Giannobile and NIH/NIDCR 5T35 DE07101 to Mr. Tomala.
Footnotes
Genzyme Corporation, Cambridge, MA.
Strategene, Inc., LaJolla, CA.
Amersham Life Science, Bucks, UK.
Lab-Tek, Nagle Nunc International, Naperville, IL.
Sigma Chemical Co., St. Louis, MO.
Santa Cruz Biotechnology, Santa Cruz, CA.
Vector Laboratories, Burlingame, CA.
Upstate Biotechnology, Inc., Lake Placid, NY.
NEN Life Sciences, Boston, MA.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson E, Yao F, Svensjö T, et al. In vivo gene transfer to skin and wound by microseeding. J Surg Res. 1998;78:85–91. doi: 10.1006/jsre.1998.5325. [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Zhu YY, Smiley E, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci (USA) 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonadio J, Goldstein SA, Levy RJ. Gene therapy for tissue repair and regeneration. Adv Drug Del Rev. 1998;33:53–69. doi: 10.1016/s0169-409x(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 6.Schmid SI, Hearing P. Adenovirus DNA packaging: Construction and analysis. In: Wold WSM, editor. Adenovirus Methods and Protocols. Totowa, New Jersey: Humana Press; 1999. pp. 47–60. [Google Scholar]
- 7.Giannobile WV. Periodontal tissue regeneration by polypeptide growth factors and gene transfer. In: Lynch SE, Genco RJ, Marx RE, editors. Tissue Engineering: Applications in Maxillofacial Surgery and Periodontics. Vol. 1. Chicago: Quintessence; 1999. pp. 231–243. [Google Scholar]
- 8.Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinate of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Therapy. 1995;2:107–115. [PubMed] [Google Scholar]
- 9.Hackett NR, Crystal RG. Adenovirus vectors for gene therapy. In: Templeton NS, Lasic DD, editors. Gene Therapy: Therapeutic Mechanisms and Strategies. New York: Marcel Dekker, Inc.; 2000. pp. 17–40. [Google Scholar]
- 10.Park JB, Matsuura M, Han KY, et al. Periodontal regeneration in Class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J Periodontol. 1995;66:462–477. doi: 10.1902/jop.1995.66.6.462. [DOI] [PubMed] [Google Scholar]
- 11.Giannobile WV, Hernandez RA, Finkelman RD, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodont Res. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 12.Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. Evaluation of a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68:1186–1193. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201–216. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 14.Antoniades HN, Galanopolous T, Neville-Golden J, Kiritsy CP, Lynch SE. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci (USA) 1991;88:565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green RJ, Usui ML, Hart CE, Ammons WF, Narayanan AS. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-alpha and beta receptors in human gingival wounds. J Periodont Res. 1997;32:209–214. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 16.Andree C, Swain WF, Page CP, et al. In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci (USA) 1994;91:12188–12192. doi: 10.1073/pnas.91.25.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eming SA, Whitsitt JS, He L, Krieg T, Morgan JR, Davidson JM. Particle-mediated gene transfer of PDGF isoforms promotes wound repair. J Invest Dermatol. 1999;112:297–302. doi: 10.1046/j.1523-1747.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–9. doi: 10.1038/10473. see comments. [DOI] [PubMed] [Google Scholar]
- 19.Giannobile WV, Pomahac B, Eriksson E. Gene transfer to periodontal wounds. J Dent Res. 1998;77(Spec Issue):2026. Abstr. 1572. [Google Scholar]
- 20.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. see comments. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman JR, Le LQ, Wu L, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman JR, Daluiski A, Stevenson S, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene directed osteogenesis: BMP-transduced human fibroblasts form bone in vivo. Human Gene Therapy. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 24.Slama J, Andree C, Svensjö T, Swain WF, Macklin MD, Eriksson E. In vivo gene transfer with microseeding. Surg Forum. 1995;46:702–705. doi: 10.1006/jsre.1998.5325. [DOI] [PubMed] [Google Scholar]
- 25.Somerman MJ, Ouyang HJ, Berry JE, et al. Evolution of periodontal regeneration: from the root’s point of view. J Periodont Res. 1999;34:420–424. doi: 10.1111/j.1600-0765.1999.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z, Lee CS, Tejeda KM, Giannobile WV. Gene transfer and expression of platelet-derived growth factor. J Dent Res. 2001;80:892–897. doi: 10.1177/00220345010800030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone. 1997;20:117–126. doi: 10.1016/s8756-3282(96)00348-1. [DOI] [PubMed] [Google Scholar]
- 28.D’Errico JA, Ouyang H, Berry JE, et al. Immortalized cementoblasts and periodontal ligament cells in culture. Bone. 1999;25:39–47. doi: 10.1016/s8756-3282(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang H, McCauley LK, Berry JE, D’Errico JA, Strayhorn CL, Somerman MJ. Response of immortalized murine cementoblasts/periodontal ligament cells to parathyroid hormone and parathyroid hormone-related protein in vitro. Arch Oral Biol. 2000;45:293–303. doi: 10.1016/s0003-9969(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 30.D’Errico JA, Berry JE, Ouyang H, Strayhorn CL, Windle JJ, Somerman MJ. Employing a transgenic animal model to obtain cementoblasts in vitro. J Periodontol. 2000;71:63–72. doi: 10.1902/jop.2000.71.1.63. [DOI] [PubMed] [Google Scholar]
- 31.Saygin NE, Giannobile WV, Somerman MJ. Cell and molecular biology of cementum. Periodontol 2000. 2000;24:73–98. doi: 10.1034/j.1600-0757.2000.2240105.x. [DOI] [PubMed] [Google Scholar]
- 32.Xie WQ, Rothblum LI. Rapid, small-scale RNA isolation from tissue culture cells. Biotechniques. 1991;11:324–327. [PubMed] [Google Scholar]
- 33.Renkawitz R, Kunz W. Independent replication of the ribosomal RNA genes in the polytrophic-meroistic ovaries of Calliphora erythrocephala, Drosophila hydei, and Sarcophaga barbata. Chromosoma. 1975;53:131–140. doi: 10.1007/BF00333041. [DOI] [PubMed] [Google Scholar]
- 34.Whitson SW, Whitson MA, Bowers DE, Jr, Falk MC. Factors influencing synthesis and mineralization of bone matrix from fetal bovine bone cells grown in vitro. J Bone Miner Res. 1992;7:727–741. doi: 10.1002/jbmr.5650070703. [DOI] [PubMed] [Google Scholar]
- 35.Giannobile WV, Whitson SW, Lynch SE. Non-coordinate control of bone formation displayed by growth factor combinations with IGF-I. J Dent Res. 1997;76:1569–1578. doi: 10.1177/00220345970760090901. [DOI] [PubMed] [Google Scholar]
- 36.Saygin NE, Tokiyasu Y, Giannobile WV, Somerman MJ. Growth factors regulate expression of mineral associated genes in cementoblasts. J Periodontol. 2000;71:1591–1600. doi: 10.1902/jop.2000.71.10.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci (USA) 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piché JE, Carnes DL, Jr, Graves DT. Initial characterization of cells derived from human periodontia. J Dent Res. 1989;68:761–767. doi: 10.1177/00220345890680050201. [DOI] [PubMed] [Google Scholar]
- 39.Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J Periodontol. 1993;64:142–148. doi: 10.1902/jop.1993.64.2.142. [DOI] [PubMed] [Google Scholar]
- 40.Grotendorst GR, Martin GR, Pencev D, Sodek J, Harvey AK. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985;76:2323–2329. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitlak BH, Finkelman RD, Hill EL, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res. 1996;11:238–247. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- 42.Langer R. Tissue engineering. Mol Ther. 2000;1:12–15. doi: 10.1006/mthe.1999.0003. [DOI] [PubMed] [Google Scholar]
- 43.Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 44.Butler WT, Ridall AL, McKee MD. Osteopontin. In: Bilezikian JP, Raizs LG, Rodan GA, editors. Principles of Bone Biology. San Diego: Academic Press; 1996. pp. 167–181. [Google Scholar]
- 45.Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Hsieh SC, Bao W, Graves DT. Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am J Physiol. 1997;272:C1709–C1716. doi: 10.1152/ajpcell.1997.272.5.C1709. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh SC, Graves DT. Pulse application of platelet-derived growth factor enhances formation of a mineralizing matrix while continuous application is inhibitory. J Cell Biochem. 1998;69:169–180. doi: 10.1002/(sici)1097-4644(19980501)69:2<169::aid-jcb7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]


