Abstract
X-linked adrenoleukodystrophy is a metabolic disorder arising from a mutation/deletion in the ABCD1 gene, leading to a defect in the peroxisomal adrenoleukodystrophy protein (ALDP), which inhibits the oxidation of very long chain fatty acids (VLCFAs). Thus, these VLCFAs accumulate. In a cerebral form of ALD (cALD), VLCFA accumulation induces neuroinflammation that leads to loss of oligodendrocytes and myelin, which ultimately shortens the lifespan. To establish a relationship between the metabolic disease and inflammatory disease induction, we document that small interfering RNA (siRNA)-mediated silencing of Abcd1 (ALDP) and Abcd2 [adrenoleukodystrophy-related protein (ALDRP)] genes in mice primary astrocyte cultures resulted in accumulation of VLCFA and induction of an inflammatory response characteristic of human cALD. Correction of the metabolic defect using monoenoic FAs in Abcd1/Abcd2-silenced cultured astrocytes decreased inducible nitric oxide synthase and inflammatory cytokine expression, suggesting a link between VLCFA accumulation and inflammation. The inflammatory response was found to be mediated by transcription factors NF-κB, AP-1, and C/EBP in Abcd1/Abcd2-silenced mouse primary astrocytes. Although mechanisms of VLCFA-mediated induction of the inflammatory response have been investigated here in vitro, the in vivo mediators remain elusive. Our data represent the first study to suggest a direct link between the accumulation of VLCFA and the induction of inflammatory mediators.
Keywords: peroxisomes, very long chain fatty acids, glia, nitric oxide, cytokines, adrenoleukodystrophy protein, adrenoleukodystrophy-related protein
X-linked adrenoleukodystrophy (X-ALD) is a progressive peroxisomal disease characterized by deficient β-oxidation, leading to accumulation of very long chain fatty acids (VLCFAs), which ultimately affect the central nervous system (CNS) and the adrenal cortex (1–3). Different phenotypes of ALD are generally recognized, distinguished by age of onset and differences in symptoms and course of disease; these range from the rapidly progressing childhood cerebral form (cALD), to the mild adult slow-progressing adrenomyeloneuropathy (AMN), to Addison's disease (affects only the adrenal glands, not myelin), and to the asymptomatic form (or presymptomatic) (1–3). All forms of X-ALD are shown to be caused by deletion or mutations in the ABCD1 gene that belongs to the ATP binding cassette (ABC) superfamily of transmembrane transporters and encodes the adrenoleukodystrophy protein (ALDP), which is located in the peroxisomal membrane.
Peroxisomes contain three additional ABC transporters: ABCD2, adrenoleukodystrophy-related protein (ALDRP); ABCD3 (PMP70, peroxisomal membrane protein); and ABCD4 (PMP70R, PMP70-related protein). Although functions of these half transporters remain unclear, they exhibit considerable sequence similarity and overlapping function(s) in peroxisomal FA metabolism, suggesting promiscuity among the functions of these transporters (4). The ABCD1 gene maps to chromosome Xq23 and encodes a 740-amino acid protein, ALDP. The ABCD2 gene maps to chromosome 12q11 and encodes a 741-amino acid half transporter that is 66% identical at the amino acid level with ABCD1 (5). Both ABCD1 and ABCD2 genes also share the same exon/intron structure (6). The ALDRP is particularly abundant in the brain and adrenal gland (5, 7). Overexpression of the ALDRP in fibroblasts from X-ALD patients at least partially restores the impaired peroxisomal β-oxidation (8). Furthermore, increasing expression of ALDRP by fibrates (peroxisome proliferators) in a peroxisome proliferator-activated receptor α (PPARα)-dependent fashion provides a potential therapeutic strategy for treating X-ALD (9). ABCD2 expression is inducible by pharmacological agents like fibrates, and Abcd2 overexpression has been shown to compensate for ALDP deficiency in Abcd1 knockout (KO) mice, preventing VLCFA accumulation and onset of the neurological phenotype (4). PMP70 overexpression has been shown to partially restore VLCFA β-oxidation in X-ALD fibroblasts (10). Functional gene redundancy is also supported by the fact that relatively high residual VLCFA β-oxidation is present in X-ALD fibroblasts lacking ABCD1. The correction of metabolic defects after statin treatments was also suggested to be due to upregulation of ABCD2 in X-ALD cultured fibroblasts (11). These findings indicate the potential correction of the metabolic disease using pharmacogenomic approaches. Interestingly, cALD has inflammatory components, so we suggest that this ALD therapy might be beneficial for both the metabolic and the neuroinflammatory disease.
VLCFA accumulation is the hallmark of X-ALD and an indication of metabolic disorder, which can subsequently lead to neuroinflammatory disease in cALD. Whether the neuroinflammatory response is directly due to abnormal VLCFA accumulation and, if so, what triggers the transition from the metabolic disorder to the severe neuroinflammatory disease, remains elusive and is the focus of ongoing research. Proinflammatory cytokines and inducible nitric oxide synthase (iNOS) have been implicated in the pathology of most brain diseases, including X-ALD, multiple sclerosis, stroke, neurotrauma, and other inflammatory and infectious neurodegenerative diseases (12–19). Recently, our laboratory observed decreased peroxisomal proteins and increased VLCFA in the CNS of animals with experimental autoimmune encephalomyelitis, which is an animal model of multiple sclerosis (20). Inflammatory mediators are also reported to downregulate peroxisome function, leading to VLCFA accumulation (21–23). In fact, VLCFA accumulation in the inflammatory region was eight to ten times greater than in the histologically normal X-ALD brain, suggesting that induction of inflammatory mediators further downregulates peroxisome function, propagating a cycle (22, 23).
The Abcd1 KO mouse (X-ALD mouse) mimics the human biochemical profile, i.e., accumulation of VLCFA in tissues, decreased VLCFA β-oxidation in fibroblasts, and adrenal dysfunction (16, 24, 25). However, mice do not display the neurological phenotype until 18–20 months of age, at which time they have a mild neuropathological manifestation similar only to AMN (26), which is a late-onset, slower progressing X-ALD phenotype (27). The Abcd2 KO mouse and Abcd1/Abcd2 double KO mice show evidence of oxidative stress at early ages, which is absent or minimal in the Abcd1 KO mouse (28). Myelin destabilization is minimal in Abcd1 KO mice, who do not have cerebral inflammation, only accelerated microglial activation in the spinal cord (29). These observations clearly document that even though Abcd1 KO mice develop metabolic disease, this does not progress to neuroinflammatory disease like that observed in childhood X-ALD patients.
Because deletion or mutation of the ABCD1 gene and the pathognomonic accumulation of VLCFA are used as diagnostic tools both prenatally and postnatally for X-ALD, we investigated the relationship between ABCD1/ABCD2 and the induction of neuroinflammatory disease, focusing on the effects of in vitro silencing of the Abcd1/Abcd2 genes by siRNA in mouse primary astrocyte cultures. We report that silencing of Abcd1/Abcd2 results in downregulation of peroxisomal β-oxidation activity for VLCFA and thus accumulation of VLCFA. Correction of the metabolic defect with monoenoic FAs decreased VLCFA accumulation and downregulated expression of inflammatory mediators in the Abcd1/Abcd2-silenced astrocytes. These data establish a relationship between VLCFA accumulation and increased expression of inflammatory mediators.
MATERIALS AND METHODS
Reagents
DMEM (4.5 g/l) was purchased from Invitrogen Life Technologies; FBS and HBSS were purchased from Gibco (Invitrogen, Carlsbad, CA). ALDP and cycloxygenase-2 (COX-2) antibody were purchased from Chemicon International, Inc. (Temecula, CA). ALDRP antibody was custom-made from ANASPEC against the mouse 20-residue C-terminal sequence: 722 CKILGEDSVLKTIQTPEKTS 741. 5-Lipoxygenase (5-LOX) antibody was purchased from Cayman Chemical (Ann Arbor, MI). Oleic acid and erucic acid were purchased from Sigma-Aldrich, Inc. (Milwaukee, WI). ECL and nitrocellulose membranes were purchased from Amersham Biosciences. Fatty acid methyl ester (FAME) standards were obtained from Supelco (Bellefonte, PA). [1-14C]lignoceric acid was prepared as described earlier (30). [1-14C]palmitic acid and 125I-labeled protein A were obtained from ICN (Cleveland, OH).
Cell culture
C57BL6 mouse breeding pairs were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained at the Medical University of South Carolina (MUSC) animal facility. All animal procedures were approved by the MUSC Animal Review Committee, and all animals received humane care in compliance with the MUSC experimental guidelines and the National Research Council's criteria for humane care (Guide for Care and Use of Laboratory Animals).
Primary astrocyte-enriched cultures were prepared from the whole cortex of 1-day-old C57BL/6 mice as described earlier (31). Briefly, the cortex was rapidly dissected in ice-cold calcium/magnesium-free HBSS at pH 7.4 as described previously (32). The tissue was minced, incubated in HBSS containing trypsin (2 mg/ml) for 20 min, and washed twice in plating medium containing 10% FBS and 10 μg/ml gentamicin and then disrupted by triturating through a Pasteur pipette, after which cells were seeded in 75 cm2 culture flasks (Falcon, Franklin, NJ). After incubation at 37°C in 5% CO2 for 1 day, the medium was completely changed to the culture medium (DMEM containing 10% FBS and 10 μg/ml gentamicin). The cultures received half exchanges with fresh medium twice a week. After 10 days, the cells were shaken for at least 30 min on an orbital shaker to remove the microglia, and flasks were incubated for 1 day, after which they were shaken again for 8 h to remove the oligodendrocytes. The remaining population was used as astrocytes culture. All cultured cells were maintained at 37°C in 5% CO2. Lipids (oleic and erucic acid) were dissolved in ethanol and then diluted in DMEM for treatment of cultures.
siRNA interference of Abcd1 and/or Abcd2 in mouse primary astrocytes
The silencer siRNA (Ambion, Austin, TX) was used for Abcd1 and/or Abcd2 silencing in primary mouse astrocytes. Briefly, mice astrocytes cultured in DMEM with 10% serum and in the presence of antibiotic were transfected with siRNA for Abcd1 and/or Abcd2 using siPORT NeoFX transfection agent (Ambion). Three siRNAs for Abcd1 and Abcd2 (Ambion) each were used (Abcd1 siRNA1, ID 162218; 5′-CCUCUACAACCUAAUUUAUtt-3′; 5′-AUAAAUUAGGUUGUAGAGGtg-3′, siRNA2, ID 60153; 5′-GGUAUUUGAAGAUGUCAAAtt-3′; 5′-UUUGACAUCUUCAAAUACCtg-3′, siRNA3, ID 60064; 5′-GGAAAUUGCCUUCUACGGGtt-3′; 5′-CCCGUAGAAGGCAAUUUCCtc-3′; Abcd2 siRNA1, ID 188185; 5′-GGCUUUAGCUUACCAGAUGtt-3′; 5′-CAUCUGGUAAGCUAAAGCCtt-3′, siRNA2, ID 214996; 5′-GGUAAAUGUCUAGAAAUGGtt-3′; 5′-CCAUUUCUAGACAUUUACCtg-3′, siRNA3, ID 214997, 5′-GCUGUAGAGAUCAAUAGAGtt-3′; 5′- CUCUAUUGAUCUCUACAGCtc-3′). The siRNAs were mixed and diluted in OPTI-MEM1 medium to a final concentration of 30 nM/well. siRNA/transfection agent was dispensed into culture plates as directed by the manufacturer. A positive control using GAPDH siRNA (Ambion) and a negative control with sequence similarity to no known human, mouse, or rat gene were included. Cells were maintained in DMEM with reduced serum (2%). Silencing was observed with Western blot and mRNA quantification. For protein analysis of the transfected cells, two wells per plate were lysed and used for protein measurements and protein levels (Western blot). Cells were maintained for 6 days in DMEM with 2% FBS before harvesting for the analysis.
FA β-oxidation
The peroxisomal oxidation of FAs in control, scrambled RNA (ScrRNA)-silenced, and Abcd1- and/or Abcd2-silenced astrocytes was determined in 6-well plates. β-Oxidation of FAs to acetate (water-soluble product) was determined using [1-14C]FAs as substrate [C24:0, lignoceric acid or C16:0, palmitic acid (ARC, St. Louis, MO); 150,000 dpm suspended in 0.25 mg of α-cyclodextrin/assay] (20). Briefly, plates were washed three times with serum-free medium, and the substrate in 0.250 ml of serum-free media was added to each well. The plates were incubated for 1 h with lignoceric acid or for 30 min with palmitic acid, at 37°C. Blank wells were included in each set of plates. The reaction was stopped with 1 M KOH (0.625 ml) in methanol and the mixture was transferred to capped tubes. The methanolic solution was then incubated at 60°C for 1 h, neutralized with of 6 N HCl (0.125 ml), and partitioned with 1.25 ml of chloroform (20). The amount of radioactivity in the upper phase represents the amount of [1-14C]FA oxidized to acetate. Cells grown in parallel in the same plate were used to determine the protein present in the assays. Experiments were performed in triplicate.
Lipid extraction and FA analysis
Total lipids were extracted from control and treated cells as described previously (33). The FAMEs were analyzed by GC (Shimadzu chromatograph GC-15A attached to a Shimadzu chromatopac C-R3A integrator) using a fused silica capillary column (25 M 007 series methyl silicone, 0.25 mm internal diameter, 0.25 μm film thickness) from Quadrex Corporation (New Haven, CT). The column temperature was programmed at 125°C for 5 min, raised to 280°C at the rate of 5°C per min, and then to 295°C, at the rate of 25°C per min. The temperature was held constant at 295°C for 5 min. The injection block was set at 200°C, and the detector was set at 320°C. The separated components were identified by comparison with retention times of standard FAME. The area under the peaks of identified FAs was set as 100%. The individual FAs were measured as area percent.
Western blot analysis
The cells were washed with cold Tris-buffered saline (20 mM Trizma base and 137 mM NaCl, pH 7.5) and lysed in 1× SDS sample-loading buffer [62.5 mM Trizma base, 2% (w/v) SDS,10% glycerol], and after sonication and centrifugation at 15,000 g for 5 min, the supernatant was used for the immunoblot assay. The protein concentration of samples was determined with a detergent-compatible protein assay reagent (Bio-Rad) using BSA as the standard. The sample was boiled for 3 min with 0.1 vol of 10% β-mercaptoethanol and 0.5% bromphenol blue mix. Then 40 μg of total cellular protein was resolved by electrophoresis on 8% or 12% polyacrylamide gels, electrotransferred to a polyvinylidene difluoride filter, and blocked with Tween 20-containing TBS (TBST; 10 mM Trizma base, pH 7.4, 1% Tween 20, and 150 mM NaCl) with 5% skim milk. After incubation with antiserum raised against mice ALDP, ALDRP, COX-2, 5-LOX, and iNOS, the membranes were then incubated with HRP-conjugated anti-rabbit or mouse IgG for 1 h. The membranes were detected by autoradiography using ECL-plus (Amersham Biosciences) after washing with TBST buffer.
RNA extraction and cDNA synthesis
Following total RNA extraction using TRIzol (Invitrogen) per the manufacturer's protocol, single-stranded cDNA was synthesized from total RNA. Five micrograms of total RNA was treated with 2 units of DNase I (bovine pancreas; Sigma) for 15 min at room temperature in an 18 μl vol containing 1X PCR buffer and 2 mM MgCl2. This was then inactivated by incubation with 25 mM EDTA (2 μl) at 65°C for 15 min. Next, 2 μl of random primers was added and annealed to the RNA according to the manufacturer's protocol. cDNA was synthesized in a 5 μl reaction containing 5 μg of total RNA and 50–100 units of reverse transcriptase by incubating the tubes at 42°C for 60 min.
Real-time PCR
Total RNA isolation from control, ScrRNA-silenced, and Abcd1- and/or Abcd2-silenced astrocytes cultures was performed using TRIzol (Invitrogen) according to the manufacturer's protocol. Real-time PCR was conducted using Bio-Rad iCycler (iCycler iQ Multi-Color Real Time PCR Detection System; Bio-Rad). Single-stranded cDNA was synthesized from total RNA as described. The primer sets for use were designed (Oligoperfect™ designer, Invitrogen) and synthesized from Integrated DNA Technologies (Coralville, IA). The primer sequences for TNF-α: forward, 5′-ctt ctg tct act gaa ctt cgg ggt-3′ and reverse, 5′-tgg aac tga tga gag gga gcc-3′; glyceraldehyde-3-phosphate dehydrogenase: forward, 5′-cct acc ccc aat gta tcc gtt gtg-3′ and reverse, 5′-gga gga atg gga gtt gct gtt gaa-3′; IL-1β: forward, 5′-gag aga caa gca acg aca aaa tcc-3′ and reverse, 5′-ttc cca tct tct tct ttg ggt att g-3′; iNOS: forward, 5′-gga aga gga aca act act gct ggt-3′ and reverse, 5′-gaa ctg agg gta cat gct gga gc-3′; 18S: forward, 5′-gaa aac att ctt ggc aaa tgc ttt-3′ and reverse, 5′-gccgct aga ggt gaa att ctt-3′. IQ™ SYBR Green Supermix was purchased from Bio-Rad. Thermal cycling conditions were as follows: activation of DNA polymerase at 95°C for 10 min, followed by 40 cycles of amplification at 95°C for 30 s and 58.3°C for 30 s. The normalized expression of target gene with respect to glyceraldehyde-3-phosphate dehydrogenase or 18S RNA was computed for all samples using a Microsoft Excel data spreadsheet.
Assay for nitric oxide synthesis
Production of nitric oxide (NO) was determined by assaying culture supernatants for nitrite, a stable reaction product of NO and molecular oxygen. Briefly, 100 μl of culture supernatant was allowed to react with 100 μl of Griess reagent (34) and incubated at room temperature for 15 min. The optical density of the assay samples was measured spectrophotometrically at 570 nm. Fresh culture medium served as the blank in all experiments. Nitrite concentrations were calculated from a standard curve derived from the reaction of NaNO2 in the assay.
Determination of tumor necrosis factor-α and interleukin-1β in culture supernatants
Cells were silenced with Abcd1 and/or Abcd2 siRNA or ScrRNA, and concentrations of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were measured in culture supernatants using high-sensitivity ELISA (R and D Systems).
Measurement of reactive oxygen species
Reactive oxygen species (ROS) were determined using the membrane-permeable fluorescent dye 6-carboxy 20, 70-dichlorodihydrofluorescein diacetate (DCFH2-DA) in serum-free medium as described earlier (35). The cultured cells, with or without treatment, were incubated with 5 mM DCF dye in PBS for 2 h at 37°C. The change in fluorescence was determined at excitation 485 nm and emission 530 nm using a Soft Max Pro spectrofluorometer (Molecular Devices, Sunnyvale, CA).
Preparation of nuclear extracts and electrophoretic mobility shift assay
Nuclear extracts from control, ScrRNA-treated, and Abcd1/Abcd2-silenced primary astrocytes were prepared as described previously (36). Cells were harvested, washed twice with ice-cold PBS, and lysed in 400 μl of buffer A [10 mM HEPES (pH 7.9), 10 mM KCl, 2 mM MgCl2, 0.5 mM dithiothreitol, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml pepstatin A, and 5 μg/ml leupeptin] containing 0.1% Nonidet P-40 for 15 min on ice, vortexed vigorously for 15 s, and centrifuged at 14,000 rpm for 30 s. The pelleted nuclei were resuspended in 40 μl of buffer B [20 mM HEPES (pH 7.9), 25% (v/v) glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 dithiothreitol, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml pepstatin A, and 5 μg/ml leupeptin]. After 30 min on ice, lysates were centrifuged at 14,000 rpm for 10 min. Supernatants containing the nuclear proteins were diluted with 20 μl of modified buffer C [20 mM HEPES (pH 7.9), 20% (v/v) glycerol, 0.05 M KCl, 0.2 mM EDTA, 0.5 mM dithiothreitol, and 0.5 mM PMSF] and stored at −70°C until use.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed using an EMSA “Gel Shift” Kit (Panomics, Inc., Redwood, CA) per the manufacturer's protocol. Briefly, nuclear extracts were normalized for protein (Bio-Rad protein assay), and equal amounts (10 μg) were loaded. DNA-protein complexes were resolved by PAGE on 6% gel (Invitrogen) in 45 mM Tris, 45 mM boric acid, 1 mM EDTA [0.5× tris borate EDTA (TBE)], and run at 11 V/cm. For competition analysis, nuclear extracts were preincubated with cold excess unlabeled competitor DNA for 5 min at room temperature and the samples were processed as for EMSA. At the end of the run, the gel was transferred onto a neutrally charged nylon membrane (GE Healthcare, Piscataway, NJ) in 0.5× TBE for 1 h at 300 mA. The oligos were fixed using ultraviolet crosslinker. The membrane was blocked for 15 min at room temperature and treated with streptavidin-HRP for 15 min and then washed three times for 10 min at room temperature. The membrane was incubated in detection buffer for 5 min at room temperature and then overlaid with substrate solution for 5 min, sandwiched between two plastic sheets. Excess substrate solution was drained, and the membrane was developed using Hyperfilm ECL.
Protein/DNA arrays
To assay the expression of multiple transcription factors in response to Abcd1 and/orAbcd2 gene silencing in the mouse primary astrocytes, we used protein/DNA arrays according to the manufacturer's instructions (Panomics). Briefly, a set of biotin-labeled DNA binding oligonucleotides (TranSignal probe mix) was preincubated with nuclear extract (10 μg) to allow the formation of protein/DNA (or transcription factor/DNA) complexes. The protein/DNA complexes were than separated from the free probes. The probes in the complexes were extracted and hybridized to the TranSignal Array membrane, which contains an array of transcription factor consensus binding sequences, overnight at 42°C in a hybridization oven. The membrane was then incubated with streptavidin-HRP for 15 min at room temperature, and signals were detected using a chemiluminescent imaging system.
Cell viability
Cytotoxic effects of all treatments were determined by measuring the metabolic activity of cells with 3-(4, 5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. The absorbance of viable cells was measured with a test wavelength of 570 nm and a reference wavelength of 630 nm.
Statistical analysis
Using the Student Newman-Keuls test and ANOVA, P values were determined for the respective experiments from three identical experiments using GraphPad software (GraphPad Software, Inc., San Diego, CA). The criterion for statistical significance was P < 0.05.
RESULTS
Silencing of Abcd1 and Abcd2 by RNA interference results in decreased β-oxidation activity and VLCFA accumulation in mouse primary astrocytes
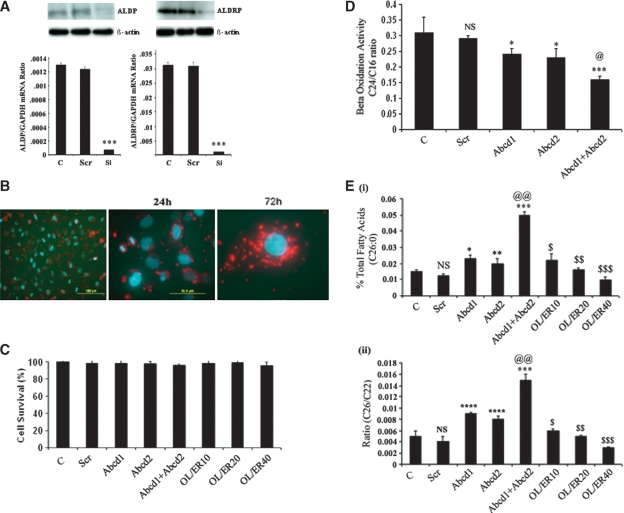
Because ABCD2 transfection complemented the function of ABCD1 in X-ALD cells (37), we investigated the silencing of both Abcd1 and Abcd2 individually and in combination in mouse primary astrocytes. A pool of three siRNA duplexes each for Abcd1 or Abcd2 induced silencing up to 80% when transiently transfected in mouse primary astrocytes, as observed by mRNA expression and Western blot analysis (Fig. 1A). The observation that ScrRNA did not downregulate the expression of either Abcd1 or Abcd2 documents that the silencing was specific. Transfection efficiency was checked by transfecting the astrocytes with Cy3-labeled negative control siRNA (Fig. 1B). Moreover, Cy3-labeled siRNA was also transfected in the presence of oleic acid/erucic acid [Lorenzo's Oil (LO)] to rule out any interference of these FAs in the transfection process for studies with LO. Moreover, Fig. 1C shows that the experimental conditions used in this study had no effect on cell survival.
Fig. 1.
Transient Abcd1 and Abcd2 gene silencing. A: Both of the siRNAs induced gene silencing in mice primary astrocytes, indicated by a significant decrease in Abcd1 and Abcd2 expression analyzed by RT-PCR and Western blot immunoassay detected with monoclonal antibodies against Abcd1 and Abcd2 proteins. The same membranes were stripped and reprobed with anti-actin antibodies as a loading control. B: Transfection efficiency was more than 90% as seen by Cy3-labeled negative control-siRNA transfection. C: siRNA transfection or oleic acid/erucic acid treatment was not cytotoxic. Cell viability of Abcd1- and/or Abcd2-silenced and oleic acid and erucic acid mixture-treated astrocytes are reported as absorbance (A570–A690 nm) obtained with an 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. D: Mouse primary astrocytes were silenced for Abcd1 and/or Abcd2. After 6 days, β-oxidation was measured in the cell suspension as described in Materials and Methods. Lignoceric acid oxidation was significantly decreased upon silencing of Abcd1 or Abcd2 genes in primary astrocytes. Silencing of both Abcd1 and Abcd2 simultaneously further significantly (P < 0.001) inhibited β-oxidation. There was no significant change in palmitic acid oxidation upon silencing with Abcd1/Abcd2. E: Fatty acid methyl ester was prepared directly from cells as described in Material and Methods. FAs were analyzed by GC after adding C27:0 as an internal standard. C26.0, C24.0, and C22:0 were measured as a percent of total FAs and expressed as percent C26:0 of total FAs (i), C26:0/C22:0 (ii). Results represent the means ± SE of duplicates from three different experiments. * P < 0.01, Abcd1 or Abcd2 silencing compared with control; ** P < 0.05, Abcd2 silencing compared with control; *** P < 0.001, Abcd1+Abcd2 silencing compared with control; **** P < 0.001, Abcd1 or Abcd2 silencing compared with control; @@ P < 0.001, Abcd1+Abcd2 silencing compared with Abcd1 or Abcd2 silencing; $ P < 0.001, oleic acid/erucic acid (20 μM) treatment compared with Abcd1+Abcd2 silencing; $$ P < 0.001, oleic acid/erucic acid (20 μM) treatment compared with Abcd1+Abcd2 silencing; $$$ P < 0.001, oleic acid/erucic acid (40 μM) treatment compared with Abcd1+Abcd2 silencing; NS, nonsignificant, control compared with scrambled RNA-treated.
Because peroxisomal VLCFA β-oxidation activity is determined by ALDP expression (38), and because impaired β-oxidation activity results in VLCFA accumulation (39), we also examined the effect of Abcd1 and/or Abcd2 silencing on lignoceric and palmitic acid β-oxidation. Using radiolabeled FAs, we observed that the rate of peroxisomal β-oxidation (lignoceric acid) activity in Abcd1 or Abcd2 silenced individually or in Abcd1/Abcd2 double-silenced astrocytes was reduced compared with control and ScrRNA-treated cells (Fig. 1D). However, there was no significant change in the mitochondrial β-oxidation (palmitic acid) activity in Abcd1- and/or Abcd2-silenced astrocytes. These observations document that deletion of Abcd1/Abcd2 results in decreased peroxisomal β-oxidation in astrocytes.
We further measured VLCFA in the Abcd1 and/or Abcd2 knockdown primary astrocytes. Figure 1E shows the level of saturated C26:0 (i) and ratios of C26:0/C22:0 (ii). The C26:0/C22:0 ratio, along with concentrations of C26:0, is considered a standard diagnostic tool for the assessment of VLCFA in peroxisomal disorders (1–3, 40). The results presented here depict a 3-fold increase in the C26:0/C22:0 ratio as well as in the levels of C26:0 in Abcd1- or Abcd2-silenced astrocytes compared with control and ScrRNA-treated astrocytes. Double silencing of both Abcd1 and Abcd2 increased the C26:0/C22:0 ratio and the levels of C26:0 4-fold compared with control or ScrRNA. Because LO treatment results in normalization of VLCFA levels in cultured X-ALD fibroblasts as well as in plasma of X-ALD patients (2, 41, 42), we evaluated the effect of oleic/erucic acid treatment on Abcd1/Abcd2 double-silenced astrocytes for VLCFA accumulation. As shown in Fig. 1E (i and ii), a significant and dose-dependent decrease was observed in VLCFA levels with LO treatment of Abcd1/Abcd2-silenced astrocytes. These observations document that Abcd1/Abcd2 silencing induced accumulation of VLCFA and that this accumulation can be attenuated by LO treatment.
Silencing of Abcd1 and/or Abcd2 upregulates 5-LOX and COX-2 expression in mouse primary astrocytes
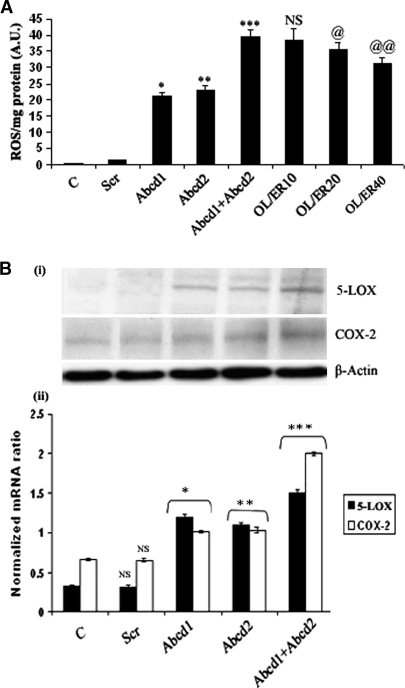
Profound oxidative damage was observed in brains of patients with inflammatory cerebral demyelinating X-ALD (43, 44). We therefore investigated the levels of ROS in Abcd1/Abcd2-silenced astrocyte cultures. The silencing significantly increased ROS in astrocyte cultures (Fig. 2A). ROS were partially but significantly reduced upon treatment of Abcd1/Abcd2-silenced astrocytes with LO. A growing body of evidence suggests that certain leukotrienes, products of 5-LOX (45), and prostaglandin, a product of COX-2 (46), play a critical role in the signaling cascade of inflammatory gene expression. To investigate further the involvement of 5-LOX in the oxidative stress/inflammatory response in Abcd1- and Abcd2-silenced astrocytes cultures, we measured protein [Fig. 2B(i)] and mRNA [Fig. 2B(ii)] of 5-LOX and COX-2 in control, ScrRNA, and Abcd1- and Abcd2-silenced primary astrocyte cultures. As shown in Fig. 2B, 5-LOX expression was significantly enhanced in Abcd1- or Abcd2-silenced cultures (P < 0.001) compared with control or ScrRNA-treated cultures. Expression of 5-LOX and COX-2 genes was upregulated more in double-silenced cells. These observations indicate that VLCFA derangement (accumulation) caused by deletion of Abcd1/Abcd2 leads to upregulation of the proinflammatory mediators 5-LOX and COX-2.
Fig. 2.
Reactive oxygen species (ROS) generation and expression of 5-lipoxygenase (5-LOX) and cycloxygenase-2 (COX-2) is induced in Abcd1/Abcd2-silenced astrocytes. A: ROS generation in primary astrocytes after 6 days of silencing or oleic acid/erucic acid-treated astrocytes. B: The immunoblot (i, ii) and expression of 5-LOX and COX-2 (iii) examined using the iCycler iQ real-time PCR detection system, as discussed in Materials and Methods. The target gene expression was normalized to GAPDH expression, and the result is presented as mean normalized expression. Data are means ± SD of three experiments. * P < 0.001, Abcd1 silencing compared with control; ** P < 0.001, Abcd2 silencing compared with control; *** P < 0.001, Abcd1+Abcd2 silencing compared with Abcd1 or Abcd2 single silencing; @ P < 0.05, oleic acid/erucic acid (20 μM) treatment compared with Abcd1+Abcd2 silencing; @@ P < 0.01 oleic acid/erucic acid (40 μM) treatment compared with Abcd1+Abcd2 silencing; NS, nonsignificant, oleic acid/erucic acid (10 μM) treatment compared with Abcd1+Abcd2 silencing.
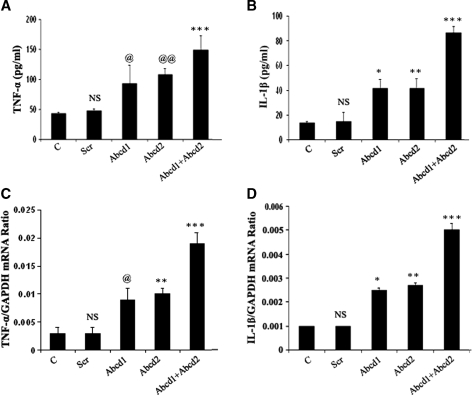
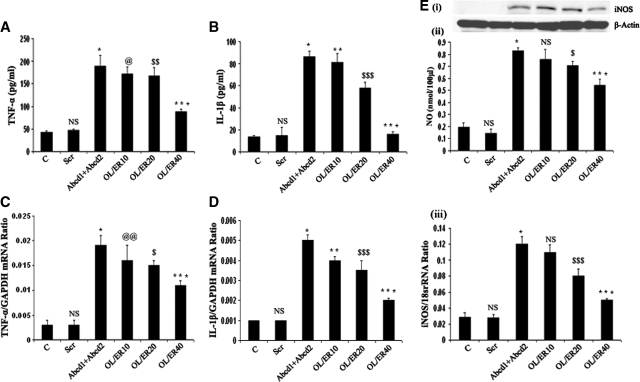
Silencing of Abcd1/Abcd2 induces expression of cytokines and iNOS in mouse primary astrocytes
To establish the relationship between the deletion/mutation of ABCD1 and the derangement of VLCFA to induce an inflammatory response similar to that observed in the CNS of cALD patients, we studied the expression of proinflammatory cytokines and iNOS in astrocytes after silencing of Abcd1/Abcd2. The supernatant from cultured astrocytes silenced for Abcd1 or Abcd2 or Abcd1/Abcd2 were collected for measurement of cytokines and NO. Figure 3 shows that deletion of Abcd1/Abcd2 in primary astrocytes upregulated the expression of proinflammatory cytokines (TNF-α and IL-1β), iNOS, and increased production of NO. Accumulation of VLCFA in X-ALD patients leads to secondary inflammatory demyelination with a marked increase in activation of microglia and astrocytes, with subsequent accumulation of proinflammatory cytokines (TNF-α and IL-1β) (14, 19). Normalization of VLCFA levels correlates with decreased cytokines (31). Expression of mRNA for TNF-α and iNOS is also increased in inflammatory areas compared with normal areas of the cALD brain (22). In the present study, Abcd1 or Abcd2 silencing markedly induced (P < 0.001) the production of proinflammatory cytokines (TNF-α, IL-1β), as determined in media by ELISA. The production of these cytokines was further significantly enhanced (P < 0.001) in Abcd1/Abcd2 double-silenced cultures. RT-PCR for TNF-α and IL-1β had a similar trend of increased gene expression, which correlated with protein levels (Fig. 3). Proinflammatory cytokines were higher in double Abcd1/Abcd2 silencing when compared with silencing of only Abcd1 or only Abcd2, and double silencing was correlated with greater VLCFA and greater VLCFA accumulation, supporting a role for VLCFA derangement in the induction of an inflammatory response.
Fig. 3.
Abcd1/Abcd2 silencing induces the expression of proinflammatory mediators in primary astrocytes. Tumor necrosis factor-α (TNF-α) (A) and interleukin-1β (IL-1β) (B) were measured by ELISA in the supernatant of primary astrocytes after 6 days of silencing. TNF-α (C) and IL-1β (D) expression was examined using the iCycler iQ real-time PCR detection system as discussed in Materials and Methods. The target gene expression was normalized to GAPDH expression, and the result is presented as mean normalized expression. Data are means ± SD of three experiments. *P < 0.001, Abcd1 silencing compared with control; ** P < 0.001, Abcd2 silencing compared with control; *** P < 0.001 Abcd1/Abcd2 silencing compared with Abcd1 or Abcd2 single silencing; @ P < 0.01, Abcd1 silencing compared with control; @@ P < 0.01, Abcd2 silencing compared with control; NS, nonsignificant, scramble-treated compared with control.
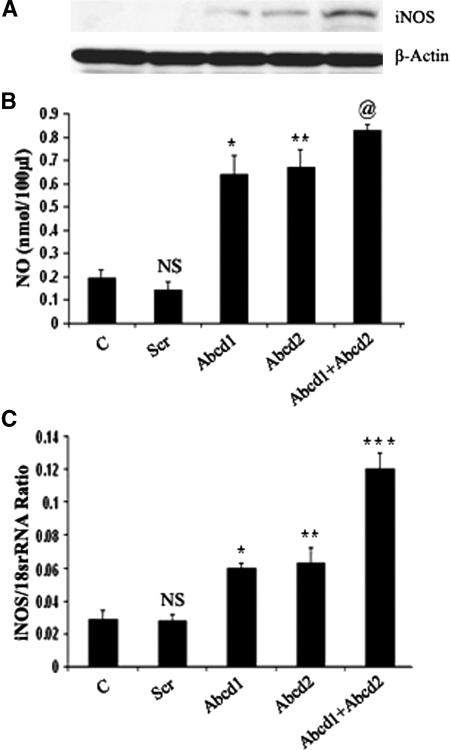
Nitrite production, a stable marker for NO generation, was significantly increased (P < 0.001) in Abcd1- and Abcd2-silenced samples, compared with control and Scr-treated astrocytes. NO production was further significantly enhanced (P < 0.001) in Abcd1/Abcd2 double-silenced astrocytes. These observations indicate that astrocytes with deranged VLCFA due to silencing of Abcd1/Abcd2 predispose these cells to proinflammatory response such as induction of cytokines and iNOS. Previous studies from our laboratory have documented expression of iNOS and NO in the brains of X-ALD patients (18) and augmentation of VLCFA accumulation by NO in C6 cells (23). Consistent with expression of iNOS protein and production of NO, mRNA for iNOS was also upregulated in response to silencing of Abcd1 and/or Abcd2 (Fig. 4A–C), indicating that derangement of VLCFA induces the transcription of iNOS in Abcd1/Abcd2-silenced astrocytes.
Fig. 4.
Abcd1/Abcd2 silencing induces nitric oxide (NO) and inducible nitric oxide synthase (iNOS) expression in primary astrocytes. A: For detection of iNOS protein expression by immunoblot in response to Abcd1/Abcd2 silencing, cell lysate from astrocytes was prepared. B: NO was measured by ELISA in the supernatant of primary astrocytes after 6 days of silencing or oleic acid/erucic acid-treated astrocytes. C: iNOS expression was examined using the iCycler iQ real-time PCR detection system as discussed in Materials and Methods. The target gene expression was normalized to 18S rRNA expression, and the result is presented as mean normalized expression. Data are means ± SD of three experiments. @ P < 0.001, Abcd1 or ABCD2 silencing compared with control; @ P < 0.05, Abcd1 or ABCD2 silencing compared with Abcd1+Abcd2 silencing; * P < 0.001, Abcd1 silencing compared with control; ** P < 0.001, Abcd2 silencing compared with control; *** P < 0.001, Abcd1+Abcd2 silencing compared with Abcd1 or Abcd2 single silencing; NS, nonsignificant, scramble-treated compared with control.
Treatment with LO downregulated inflammatory response in Abcd1/Abcd2-silenced mouse primary astrocytes
Treatment with LO is known to lower the levels of VLCFA in cultured fibroblasts (42) as well as in plasma of X-ALD patients (41, 47); thus, LO is presently being used as therapy for patients with X-ALD (47). We observed that deletion of Abcd1/Abcd2 in cultured astrocytes results in excessive accumulation of VLCFA and induction of inflammatory mediators in cultured primary astrocytes. To further establish the relationship between excessive accumulation of VLCFA and inflammatory response, we examined whether lowering VLCFA after LO treatment of Abcd1/Abcd2-silenced astrocytes downregulates the induction of an inflammatory response. Astrocytes silenced for Abcd1/Abcd2 were treated with different concentrations of LO. Figure 1 depicts reduced VLCFA in Abcd1/Abcd2-silenced cells after treatment with LO. In addition, proinflammatory mediators (TNF-α and IL-1β) were decreased, iNOS expression was downregulated (both at the protein and mRNA levels), and NO production was reduced, as shown in Fig. 5. These observations support the relationship between VLCFA accumulation and induction of a proinflammatory response in cultured primary astrocytes.
Fig. 5.
Lorenzo's Oil treatment downregulates expression of proinflammatory mediators and iNOS in Abcd1/Abcd2-silenced astrocytes. TNF-α (A) and IL-1β (B) were measured by ELISA in the supernatant of primary astrocytes after 6 days of silencing. TNF-α (C) and IL-1β (D) expression was examined using the iCycler iQ real-time PCR detection system as discussed in Materials and Methods. The target gene expression was normalized to GAPDH expression, and the result is presented as mean normalized expression. E: For the detection of iNOS protein expression by immunoblot in response to Abcd1/Abcd2 silencing, cell lysate from astrocytes was prepared (i); NO was measured by ELISA in the supernatant of primary astrocytes after 6 days of silencing or oleic acid/erucic acid-treated astrocytes (ii); iNOS expression was examined using the iCycler iQ real-time PCR detection system as discussed in Materials and Methods (iii). The target gene expression was normalized to 18S rRNA expression, and the result is presented as mean normalized expression. *P < 0.001, Abcd1+Abcd2 silencing compared with control; ** P < 0.001, oleic acid/erucic acid (10 μM) treatment compared with Abcd1+Abcd2 silencing; *** P < 0.001, oleic acid/erucic acid (40 μM) treatment compared with Abcd1+Abcd2 silencing; @ P < 0.01, oleic acid/erucic acid (10 μM) treatment compared with Abcd1+Abcd2 silencing; @@ P < 0.05, oleic acid/erucic acid (10 μM) treatment compared with Abcd1+Abcd2 silencing; $ P < 0.05, oleic acid/erucic acid (20 μM) treatment compared with Abcd1+Abcd2 silencing; $$ P < 0.01, oleic acid/erucic acid (20 μM) treatment compared with Abcd1+Abcd2 silencing; $$$ P < 0.001, oleic acid/erucic acid (20 μM) treatment compared with Abcd1+Abcd2 silencing; NS, nonsignificant, scramble-treated compared with control.
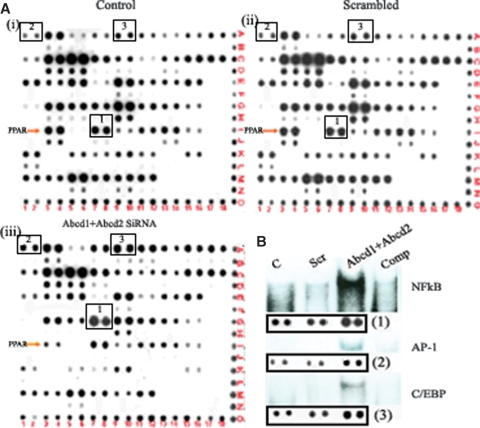
Silencing of Abcd1 and Abcd2 induces NF-κB, AP-1, and C/EBP transcription factors in mouse primary astrocytes
To determine the molecular mechanisms of Abcd1 and/or Abcd2 silencing-mediated upregulation of 5-LOX, COX-2, and a proinflammatory response (iNOS and cytokine expression), the nuclear extracts of primary astrocytes silenced for Abcd1 and/or Abcd2 or ScrRNA were analyzed for transcription factor activation/inhibition using protein/DNA arrays (Panomics). Results presented in Fig. 6A show activation or inhibition of a wide array of transcription factors, notably NF-κB, AP-1, C/EBP (activated), and PPAR (downregulated), in astrocytes after silencing of Abcd1/Abcd2. These transcription factor (NF-κB, AP-1, and C/EBP) binding motifs have been identified in the functional regulatory regions of various proinflammatory genes such as IL-6, IL-1β, TNF-α, IL-8, IL-12, granulocyte colony-stimulating factor, and iNOS (48), suggesting that Abcd1/Abcd2 silencing induced activation of these transcription factors, which, in turn, play a role in the upregulation of the inflammatory response. We therefore investigated the activation of NF-κB, AP-1, and C/EBP by EMSA (Fig. 6B). In agreement with the array data, we observed significantly increased DNA binding activity for NF-κB, AP-1, and C/EBP proteins in Abcd1- and/or Abcd2-silenced primary astrocytes compared with control or ScrRNA-treated astrocytes. Collectively, these observations document that VLCFA derangement induced by Abcd1/Abcd2 silencing participates in the activation of NF-κB, AP-1, and C/EBP, leading to a subsequent inflammatory response.
Fig. 6.
Abcd1/Abcd2 silencing induces expression and DNA binding of NF-κB, AP1, and C/EBP in primary astrocytes. Nuclear extracts were prepared from control, scrambled, and Abcd1/Abcd2-silenced astrocytes (A). Nuclear extracts from control (i), scrambled (ii), and Abcd1/Abcd2-silenced (iii) astrocytes were incubated with a set of biotin-labeled DNA binding oligonucleotides (TranSignal probe mix) to allow the formation of protein/DNA (or transcription factor/DNA) complexes. The protein/DNA complexes were then separated from the free probes. The probes in the complexes were extracted and hybridized to the TranSignal array. Signals were detected using a chemiluminescent imaging system. B: Nuclear extracts were used for electrophoretic mobility shift assay to check the DNA binding activity of NF-κB, AP-1, and C/EBP.
DISCUSSION
Childhood X-ALD pathology progression occurs in two stages: a metabolic phase characterized by excessive accumulation of VLCFA starting in utero, and a subsequent neuroinflammatory disease starting in early childhood (4–8 years of age), leading to loss of oligodendrocytes and myelin, and eventually to death. What triggers the transition from metabolic to neuroinflammatory disease is still unknown. In vitro studies reported here demonstrate that silencing of Abcd1 and/or Abcd2 genes by siRNA in mouse primary astrocytes in culture resulted in reduced β-oxidation and accumulation of VLCFA and, interestingly, induced production of NO and ROS and upregulated proinflammatory mediators (TNF-α, IL-1β, 5-LOX, and COX-2).
Furthermore, the reduction of VLCFA by LO resulted in reduction of expression of inflammatory mediators (iNOS, TNF-α, and IL-1β) in Abcd1/Abcd2-silenced astrocytes, thus establishing the relationship between the ABCD1 gene product, ALDP, resultant accumulation of VLCFA, and induction of the inflammatory disease process.
Although the precise substrates and functions of peroxisomal ABC transporters have yet to be defined, the detrimental effects of a deficient ABCD1 transporter are evident in X-ALD. The lack of a transition from metabolic disease to a neuroinflammatory disease in animal models of ALDP KO mice generated in different laboratories (16, 24, 25) and the absence of a neuroinflammatory disease in AMN, an adult form of ALD, caused by the same mutation in ALDP, suggest that in addition to a deletion/mutation of ALDP, it is likely that factors other than ALDP (modifier gene products) may play a role in the transition of the metabolic to the neuroinflammatory disease of ALD.
Although the precise function of ALDP is still unknown at present, the excessive accumulation of VLCFA in tissues of X-ALD patients (1) and mice (16, 24, 25) and deficient β-oxidation of these FAs in cells derived from X-ALD patients (39, 49) indicate that it is related to the metabolism of VLCFA in peroxisomes (39). Accordingly, siRNA silencing of Abcd1 and/or Abcd2 resulted in decreased β-oxidation activity of VLCFAs and their accumulation in astrocytes. The observed accumulation of VLCFAs due to siRNA silencing of Abcd2, the greater accumulation of VLCFAs after silencing of both Abcd1 and Abcd2 in studies described here, and the correction of VLCFAs in X-ALD fibroblasts after transfection of the ABCD2 gene (37) suggest that both ABCD1 and ABCD2 may function in the metabolism of VLCFA-related compounds. These observations are consistent with a previous in vivo study in which Abcd1/Abcd2 double KO mice had greater VLCFA accumulation than the single KO mice (50).
The inflammatory disease component of X-ALD is quite complex. Two clinical variants (cALD and AMN), caused by the same mutation, have different degrees of inflammation that affect different parts of the nervous system (51). In cALD (the childhood/cerebral form of X-ALD), the neuroinflammatory disease affects the white matter and related cells in the CNS, whereas in AMN, the adult form of X-ALD, axonal tracks of the spinal cord are affected (52). In addition, the neuropathology of cALD is associated with vascular immune cell infiltration (19) and NO-mediated toxicity, as evidenced from vascular infiltration and detection of nitrotyrosine (18) and oxidative stress in the brains of cALD patients (44). Previously, we reported increased iNOS expression in astrocytes and macrophage/microglia in cALD brain tissue (18). However, the molecular events associated with the transition from a relatively benign metabolic disease to a fatal neuroinflammatory disease are not understood at present. Myelin breakdown products, due to myelin instability from excessive amounts of VLCFA, are believed to initiate the inflammatory disease via activation of resident immune cells (microglia and astrocytes) for the expression of cytokines, chemokines, iNOS, and other proinflammatory mediators. Infiltrating vascular immune cells may further amplify the inflammatory disease. NO and/or peroxtnitrite (ONOO−), produced by the reaction of NO with superoxide (O2−), are considered to play a role in cytotoxic effects, suggesting a role of ROS and reactive nitrogen species in the pathobiology of X-ALD. Previous studies from our laboratory have demonstrated that NO supplied by exogenous donors (e.g., S-nitroso-N-acetylpenicillamine) or endogenously produced by treatment of C6 glial cells with proinflammatory cytokines does alter the status of antioxidant enzymes in the cell in favor of producing higher levels of ROS by downregulating the activity of catalase, glutathione peroxidase, and Mn superoxide dismutase, suggesting a possible role of cellular redox in the inflammatory response (34). We therefore analyzed the expression of 5-LOX and COX-2 in Abcd1- and/or Abcd2-silenced astrocytes. 5-LOX-mediated ROS generation has been implicated in the activation of NF-κB (53, 54). Oxygen-glucose deprivation-induced oxidative stress induces COX-2 via NF-κB activation in astrocytes (55). Recent studies also reported that PLA2 and 5-LOX are involved in lipopolysaccharide-induced iNOS gene expression via NF-κB-dependent and -independent pathways in glial cells (56). Accumulating evidence suggests that COX, especially inducible cyclooxygenase-2 (COX-2), plays a key role in neuroinflammation (46), and that prostaglandins produced by COX-2 (57) are reported to be a positive regulator for iNOS gene expression in macrophages (58). Silencing of Abcd1 and/or Abcd2 in astrocytes increased the expression of 5-LOX and COX-2. The fact that 5-LOX and COX-2 expression and NF-κB activation were induced in wild-type astrocytes after ABCD1/ABCD2 silencing suggests that Abcd1/Abcd2 silencing may stimulate 5-LOX and COX-2 transcription through NF-κB signaling pathways.
Proinflammatory cytokines such as TNF-α and IL-1β are involved in the induction of iNOS in astrocytes through NF-κB activation (59). Consistent with these findings, Abcd1 and Abcd2 silencing resulted in increased NF-κB activation observed by DNA/protein arrays and EMSA compared with control and ScrRNA-silenced cultures. In addition, AP-1 and CCAAT/enhancer binding protein (C/EBP) transcription and DNA binding activity were also enhanced in Abcd1/Abcd2-silenced cultures compared with control and ScrRNA-treated primary astrocytes cultures. These transcription factors are also known to be activated in astrocytes in response to proinflammatory cytokines such as TNF-α, IL-1β, and IL-6, and thus probably play a role in amplification of inflammation, because C/EBP binding sites are found in the regulatory regions of genes encoding the inflammatory cytokines IL-6, IL-1β, and TNF-α (60–63). Astrocytes expressing C/EBP may thus produce these cytokines, which, in turn, can induce the expression of C/EBP in the surrounding cells. This feed-forward mechanism could therefore favor the spreading of the inflammation. The preceding discussion suggests that Abcd1/Abcd2-mediated activation of NF-κB, AP-1, and C/EBP transcription factors in astrocytes followed by release of cytokines is the driving force for inflammation in our culture system. The observed expression of inflammatory mediators in Abcd1/Abcd2-silenced astrocytes establishes, for the first time, the relationship of induction of inflammatory disease to loss of Abcd1. Decreased induction of inflammatory mediators by Abcd1/Abcd2-silenced astrocytes following treatment with LO and reduction of VLCFA further establishes the relationship between ABCD1, metabolism of VLCFA, and expression of inflammatory mediators in astrocytes. Oleic acid treatment was reported to activate PKA and PPARα-DNA binding activity and to upregulate expression of the Pgc-1α gene involved in FA oxidation (64), suggesting that reduction of VLCFA by FA may be due to both decreased VLCFA synthesis as well as increased oxidation. In addition, our laboratory has previously shown that PKA activation inhibits the induction of iNOS and cytokines in microglia and astrocytes (65), suggesting that the lowering of VLCFA and inhibition of inflammatory mediators are related to each other. However, it is unclear why astrocytes with Abcd1/Abcd2 deletion in vivo in the CNS of KO mice do not express inflammatory molecules, whereas silencing of Abcd1/Abcd2 in vitro in astrocyte cultures expresses inflammatory mediators. The observed downregulation of an inflammatory response in Abcd1/Abcd2-silenced cells after LO treatment suggests that correction of FA derangement should have a therapeutic value for X-ALD. LO therapy for ALD patients for the purpose of decreasing VLCFA may be beneficial.
Understanding the complex mechanisms that influence demyelination in ALD patients remains elusive. The variable phenotype of patients within individual families with identical mutations suggests a modifier gene(s) or environmental factor(s) that tips the balance toward the severe clinical phenotype, cALD. Considering environmental factors, cells in culture have been shown to behave differently from those in vivo. The stress of the isolation procedure and the foreign environment may predispose the cultured cells to oxidative stress (66). The accumulation of VLCFA upon Abcd1 and/or Abcd2 silencing presumably “primes” these cells to be susceptible to oxidative insult compared with the control and ScrRNA-treated astrocytes under unknown stressful environments of the culture system. Although we still do not know what triggers the Abcd1- and/or Abcd2-silenced cells to produce an inflammatory response in culture, which is conspicuously absent in Abcd1- or Abcd1/Abcd2-silenced mice, these studies do document, for the first time, the relationship of VLCFA derangement to an inflammatory response in astrocytes.
Acknowledgments
The authors thank their laboratory for valuable comments and help during this study. They greatly appreciate the help of Dr. M. A. Contreras in setting up the β-oxidation assay and the technical assistance of Ms. Joyce Bryan.
Abbreviations
ALDP, adrenoleukodystrophy protein
ALDRP, adrenoleukodystrophy-related protein
AMN, adrenomyeloneuropathy
cALD, cerebral adrenoleukodystrophy
CNS, central nervous system
COX-2, cycloxygenase-2
EMSA, electrophoretic mobility shift assay
FAME, fatty acid methyl ester
IL-1β, interleukin-1β
iNOS, inducible nitric oxide synthase
LO, Lorenzo's Oil
5-LOX, 5-lipoxygenase
NO, nitric oxide
PPAR, peroxisome proliferator-activated receptor
ROS, reactive oxygen species
Scr, scrambled
TNF-α, tumor necrosis factor-α
VLCFA, very long chain fatty acid
X-ALD, X-linked adrenoleukodystrophy
Published, JLR Papers in Press, August 31, 2008.
Footnotes
This study was supported in part by The National Institutes of Health, Grants NS-22576, NS-34741, NS-37766, NS-40810, C06 RR-018823, and C06 RR-015455.
References
- 1.Moser H. W. 1995. Adrenoleukodystrophy. Curr. Opin. Neurol. 8 221–226. [DOI] [PubMed] [Google Scholar]
- 2.Moser H. W. 1995. Clinical and therapeutic aspects of adrenoleukodystrophy and adrenomyeloneuropathy. J. Neuropathol. Exp. Neurol. 54 740–745. [DOI] [PubMed] [Google Scholar]
- 3.Singh I. 1997. Biochemistry of peroxisomes in health and disease. Mol. Cell. Biochem. 167 1–29. [DOI] [PubMed] [Google Scholar]
- 4.Pujol A., I. Ferrer, C. Camps, E. Metzger, C. Hindelang, N. Callizot, M. Ruiz, T. Pampols, M. Giros, and J. L. Mandel. 2004. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum. Mol. Genet. 13 2997–3006. [DOI] [PubMed] [Google Scholar]
- 5.Lombard-Platet G., S. Savary, C. O. Sarde, J. L. Mandel, and G. Chimini. 1996. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl. Acad. Sci. USA. 93 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broccardo C., N. Troffer-Charlier, S. Savary, J. L. Mandel, and G. Chimini. 1998. Exon organisation of the mouse gene encoding the Adrenoleukodystrophy related protein (ALDRP). Eur. J. Hum. Genet. 6 638–641. [DOI] [PubMed] [Google Scholar]
- 7.Savary S., N. Troffer-Charlier, G. Gyapay, M. G. Mattei, and G. Chimini. 1997. Chromosomal localization of the adrenoleukodystrophy-related gene in man and mice. Eur. J. Hum. Genet. 5 99–101. [PubMed] [Google Scholar]
- 8.Netik A., S. Forss-Petter, A. Holzinger, B. Molzer, G. Unterrainer, and J. Berger. 1999. Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): implications for therapy. Hum. Mol. Genet. 8 907–913. [DOI] [PubMed] [Google Scholar]
- 9.Fourcade S., S. Savary, S. Albet, D. Gauthe, C. Gondcaille, T. Pineau, J. Bellenger, M. Bentejac, A. Holzinger, J. Berger, et al. 2001. Fibrate induction of the adrenoleukodystrophy-related gene (ABCD2): promoter analysis and role of the peroxisome proliferator-activated receptor PPARalpha. Eur. J. Biochem. 268 3490–3500. [DOI] [PubMed] [Google Scholar]
- 10.Braiterman L. T., S. Zheng, P. A. Watkins, M. T. Geraghty, G. Johnson, M. C. McGuinness, A. B. Moser, and K. D. Smith. 1998. Suppression of peroxisomal membrane protein defects by peroxisomal ATP binding cassette (ABC) proteins. Hum. Mol. Genet. 7 239–247. [DOI] [PubMed] [Google Scholar]
- 11.Singh I., K. Pahan, and M. Khan. 1998. Lovastatin and sodium phenylacetate normalize the levels of very long chain fatty acids in skin fibroblasts of X- adrenoleukodystrophy. FEBS Lett. 426 342–346. [DOI] [PubMed] [Google Scholar]
- 12.Abbas N., I. Bednar, E. Mix, S. Marie, D. Paterson, A. Ljungberg, C. Morris, B. Winblad, A. Nordberg, and J. Zhu. 2002. Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J. Neuroimmunol. 126 50–57. [DOI] [PubMed] [Google Scholar]
- 13.Eng L. F., R. S. Ghirnikar, and Y. L. Lee. 1996. Inflammation in EAE: role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem. Res. 21 511–525. [DOI] [PubMed] [Google Scholar]
- 14.McGuinness M. C., D. E. Griffin, G. V. Raymond, C. A. Washington, H. W. Moser, and K. D. Smith. 1995. Tumor necrosis factor-alpha and X-linked adrenoleukodystrophy. J. Neuroimmunol. 61 161–169. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira S. A., G. M. Castro, F. Papes, M. L. Martins, F. Rogerio, F. Langone, L. M. Santos, P. Arruda, G. de Nucci, and M. N. Muscara. 2002. Expression and activity of nitric oxide synthase isoforms in rat brain during the development of experimental allergic encephalomyelitis. Brain Res. Mol. Brain Res. 99 17–25. [DOI] [PubMed] [Google Scholar]
- 16.Lu J. F., A. M. Lawler, P. A. Watkins, J. M. Powers, A. B. Moser, H. W. Moser, and K. D. Smith. 1997. A mouse model for X-linked adrenoleukodystrophy. Proc. Natl. Acad. Sci. USA. 94 9366–9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanislaus R., A. K. Singh, and I. Singh. 2001. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental allergic encephalomyelitis. J. Neurosci. Res. 66 155–162. [DOI] [PubMed] [Google Scholar]
- 18.Gilg A. G., A. K. Singh, and I. Singh. 2000. Inducible nitric oxide synthase in the central nervous system of patients with X-adrenoleukodystrophy. J. Neuropathol. Exp. Neurol. 59 1063–1069. [DOI] [PubMed] [Google Scholar]
- 19.Powers J. M., Y. Liu, A. B. Moser, and H. W. Moser. 1992. The inflammatory myelinopathy of adreno-leukodystrophy: cells, effector molecules, and pathogenetic implications. J. Neuropathol. Exp. Neurol. 51 630–643. [DOI] [PubMed] [Google Scholar]
- 20.Singh I., A. S. Paintlia, M. Khan, R. Stanislaus, M. K. Paintlia, E. Haq, A. K. Singh, and M. A. Contreras. 2004. Impaired peroxisomal function in the central nervous system with inflammatory disease of experimental autoimmune encephalomyelitis animals and protection by lovastatin treatment. Brain Res. 1022 1–11. [DOI] [PubMed] [Google Scholar]
- 21.Paintlia M. K., A. S. Paintlia, M. A. Contreras, I. Singh, and A. K. Singh. 2008. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp. Neurol. 210 560–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paintlia A. S., A. G. Gilg, M. Khan, A. K. Singh, E. Barbosa, and I. Singh. 2003. Correlation of very long chain fatty acid accumulation and inflammatory disease progression in childhood X-ALD: implications for potential therapies. Neurobiol. Dis. 14 425–439. [DOI] [PubMed] [Google Scholar]
- 23.Khan M., K. Pahan, A. K. Singh, and I. Singh. 1998. Cytokine-induced accumulation of very long-chain fatty acids in rat C6 glial cells: implication for X-adrenoleukodystrophy. J. Neurochem. 71 78–87. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi T., N. Shinnoh, A. Kondo, and T. Yamada. 1997. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochem. Biophys. Res. Commun. 232 631–636. [DOI] [PubMed] [Google Scholar]
- 25.Forss-Petter S., H. Werner, J. Berger, H. Lassmann, B. Molzer, M. H. Schwab, H. Bernheimer, F. Zimmermann, and K. A. Nave. 1997. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J. Neurosci. Res. 50 829–843. [DOI] [PubMed] [Google Scholar]
- 26.Pujol A., C. Hindelang, N. Callizot, U. Bartsch, M. Schachner, and J. L. Mandel. 2002. Late onset neurological phenotype of the X-ALD gene inactivation in mice: a mouse model for adrenomyeloneuropathy. Hum. Mol. Genet. 11 499–505. [DOI] [PubMed] [Google Scholar]
- 27.Bezman L., A. B. Moser, G. V. Raymond, P. Rinaldo, P. A. Watkins, K. D. Smith, N. E. Kass, and H. W. Moser. 2001. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann. Neurol. 49 512–517. [PubMed] [Google Scholar]
- 28.Lu J. F., E. Barron-Casella, R. Deering, A. K. Heinzer, A. B. Moser, K. L. deMesy, G. S. Bentley, C. M. M. Wand, Z. Pei, P. A. Watkins, et al. 2007. The role of peroxisomal ABC transporters in the mouse adrenal gland: the loss of Abcd2 (ALDR), not Abcd1 (ALD), causes oxidative damage. Lab. Invest. 87 261–272. [DOI] [PubMed] [Google Scholar]
- 29.Dumser M., J. Bauer, H. Lassmann, J. Berger, and S. Forss-Petter. 2007. Lack of adrenoleukodystrophy protein enhances oligodendrocyte disturbance and microglia activation in mice with combined Abcd1/Mag deficiency. Acta Neuropathol. (Berl.). 114 573–586. [DOI] [PubMed] [Google Scholar]
- 30.Hoshi M., and Y. Kishimoto. 1973. Synthesis of cerebronic acid from lignoceric acid by rat brain preparation. Some properties and distribution of the -hydroxylation system. J. Biol. Chem. 248 4123–4130. [PubMed] [Google Scholar]
- 31.Pahan K., F. G. Sheikh, M. Khan, A. M. Namboodiri, and I. Singh. 1998. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J. Biol. Chem. 273 2591–2600. [DOI] [PubMed] [Google Scholar]
- 32.Won J. S., M. R. Choi, and H. W. Suh. 2001. Stimulation of astrocyte-enriched culture with C2 ceramide increases proenkephalin mRNA: involvement of cAMP-response element binding protein and mitogen activated protein kinases. Brain Res. 903 207–215. [DOI] [PubMed] [Google Scholar]
- 33.Wilson R., and J. R. Sargent. 1993. Lipid and fatty acid composition of brain tissue from adrenoleukodystrophy patients. J. Neurochem. 61 290–297. [DOI] [PubMed] [Google Scholar]
- 34.Dobashi K., K. Pahan, A. Chahal, and I. Singh. 1997. Modulation of endogenous antioxidant enzymes by nitric oxide in rat C6 glial cells. J. Neurochem. 68 1896–1903. [DOI] [PubMed] [Google Scholar]
- 35.Khan M., E. Haq, S. Giri, I. Singh, and A. K. Singh. 2005. Peroxisomal participation in psychosine-mediated toxicity: implications for Krabbe's disease. J. Neurosci. Res. 80 845–854. [DOI] [PubMed] [Google Scholar]
- 36.Giri S., M. Jatana, R. Rattan, J. S. Won, I. Singh, and A. K. Singh. 2002. Galactosylsphingosine (psychosine)-induced expression of cytokine-mediated inducible nitric oxide synthases via AP-1 and C/EBP: implications for Krabbe disease. FASEB J. 16 661–672. [DOI] [PubMed] [Google Scholar]
- 37.Kemp S., H. M. Wei, J. F. Lu, L. T. Braiterman, M. C. McGuinness, A. B. Moser, P. A. Watkins, and K. D. Smith. 1998. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nat. Med. 4 1261–1268. [DOI] [PubMed] [Google Scholar]
- 38.Braiterman L. T., P. A. Watkins, A. B. Moser, and K. D. Smith. 1999. Peroxisomal very long chain fatty acid beta-oxidation activity is determined by the level of adrenodeukodystrophy protein (ALDP) expression. Mol. Genet. Metab. 66 91–99. [DOI] [PubMed] [Google Scholar]
- 39.Singh I., A. E. Moser, H. W. Moser, and Y. Kishimoto. 1984. Adrenoleukodystrophy: impaired oxidation of very long chain fatty acids in white blood cells, cultured skin fibroblasts, and amniocytes. Pediatr. Res. 18 286–290. [DOI] [PubMed] [Google Scholar]
- 40.Poulos A. 1995. Very long chain fatty acids in higher animals—a review. Lipids. 30 1–14. [DOI] [PubMed] [Google Scholar]
- 41.Rizzo W. B., R. T. Leshner, A. Odone, A. L. Dammann, D. A. Craft, M. E. Jensen, S. S. Jennings, S. Davis, R. Jaitly, and J. A. Sgro. 1989. Dietary erucic acid therapy for X-linked adrenoleukodystrophy. Neurology. 39 1415–1422. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo W. B., P. A. Watkins, M. W. Phillips, D. Cranin, B. Campbell, and J. Avigan. 1986. Adrenoleukodystrophy: oleic acid lowers fibroblast saturated C22–26 fatty acids. Neurology. 36 357–361. [DOI] [PubMed] [Google Scholar]
- 43.Khan M., J. Singh, and I. Singh. 2008. Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. J. Neurochem. 106 1766–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers J. M., Z. Pei, A. K. Heinzer, R. Deering, A. B. Moser, H. W. Moser, P. A. Watkins, and K. D. Smith. 2005. Adreno-leukodystrophy: oxidative stress of mice and men. J. Neuropathol. Exp. Neurol. 64 1067–1079. [DOI] [PubMed] [Google Scholar]
- 45.Sala A., S. Zarini, and M. Bolla. 1998. Leukotrienes: lipid bioeffectors of inflammatory reactions. Biochemistry (Mosc.). 63 84–92. [PubMed] [Google Scholar]
- 46.Minghetti L. 2004. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 63 901–910. [DOI] [PubMed] [Google Scholar]
- 47.Deon M., M. P. Garcia, A. Sitta, A. G. Barschak, D. M. Coelho, G. O. Schimit, M. Pigatto, L. B. Jardim, M. Wajner, R. Giugliani, et al. 2008. Hexacosanoic and docosanoic acids plasma levels in patients with cerebral childhood and asymptomatic X-linked adrenoleukodystrophy: Lorenzo's oil effect. Metab. Brain Dis. 23 43–49. [DOI] [PubMed] [Google Scholar]
- 48.Eberhardt W., D. Kunz, R. Hummel, and J. Pfeilschifter. 1996. Molecular cloning of the rat inducible nitric oxide synthase gene promoter. Biochem. Biophys. Res. Commun. 223 752–756. [DOI] [PubMed] [Google Scholar]
- 49.Singh I., H. W. Moser, A. B. Moser, and Y. Kishimoto. 1981. Adrenoleukodystrophy: impaired oxidation of long chain fatty acids in cultured skin fibroblasts an adrenal cortex. Biochem. Biophys. Res. Commun. 102 1223–1229. [DOI] [PubMed] [Google Scholar]
- 50.Ferrer I., J. P. Kapfhammer, C. Hindelang, S. Kemp, N. Troffer-Charlier, V. Broccoli, N. Callyzot, P. Mooyer, J. Selhorst, P. Vreken, et al. 2005. Inactivation of the peroxisomal ABCD2 transporter in the mouse leads to late-onset ataxia involving mitochondria, Golgi and endoplasmic reticulum damage. Hum. Mol. Genet. 14 3565–3577. [DOI] [PubMed] [Google Scholar]
- 51.Moser H. W. 2006. Therapy of X-linked adrenoleukodystrophy. NeuroRx. 3 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powers J. M., and H. W. Moser. 1998. Peroxisomal disorders: genotype, phenotype, major neuropathologic lesions, and pathogenesis. Brain Pathol. 8 101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonizzi G., J. Piette, S. Schoonbroodt, R. Greimers, L. Havard, M. P. Merville, and V. Bours. 1999. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol. Cell. Biol. 19 1950–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christman J. W., T. S. Blackwell, and B. H. Juurlink. 2000. Redox regulation of nuclear factor kappa B: therapeutic potential for attenuating inflammatory responses. Brain Pathol. 10 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee Y. S., Y. S. Song, R. G. Giffard, and P. H. Chan. 2006. Biphasic role of nuclear factor-kappa B on cell survival and COX-2 expression in SOD1 Tg astrocytes after oxygen glucose deprivation. J. Cereb. Blood Flow Metab. 26 1076–1088. [DOI] [PubMed] [Google Scholar]
- 56.Won J. S., Y. B. Im, M. Khan, A. K. Singh, and I. Singh. 2005. Involvement of phospholipase A2 and lipoxygenase in lipopolysaccharide-induced inducible nitric oxide synthase expression in glial cells. Glia. 51 13–21. [DOI] [PubMed] [Google Scholar]
- 57.Chen C. C., K. T. Chiu, Y. T. Sun, and W. C. Chen. 1999. Role of the cyclic AMP-protein kinase A pathway in lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages. Involvement of cyclooxygenase-2. J. Biol. Chem. 274 31559–31564. [DOI] [PubMed] [Google Scholar]
- 58.Kim S. F., D. A. Huri, and S. H. Snyder. 2005. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 310 1966–1970. [DOI] [PubMed] [Google Scholar]
- 59.Nomura Y. 2001. NF-kappaB activation and IkappaB alpha dynamism involved in iNOS and chemokine induction in astroglial cells. Life Sci. 68 1695–1701. [DOI] [PubMed] [Google Scholar]
- 60.Akira S., H. Isshiki, T. Sugita, O. Tanabe, S. Kinoshita, Y. Nishio, T. Nakajima, T. Hirano, and T. Kishimoto. 1990. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 9 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pope R. M., A. Leutz, and S. A. Ness. 1994. C/EBP beta regulation of the tumor necrosis factor alpha gene. J. Clin. Invest. 94 1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shirakawa F., K. Saito, C. A. Bonagura, D. L. Galson, M. J. Fenton, A. C. Webb, and P. E. Auron. 1993. The human prointerleukin 1 beta gene requires DNA sequences both proximal and distal to the transcription start site for tissue-specific induction. Mol. Cell. Biol. 13 1332–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wedel A., G. Sulski, and H. W. Ziegler-Heitbrock. 1996. CCAAT/enhancer binding protein is involved in the expression of the tumour necrosis factor gene in human monocytes. Cytokine. 8 335–341. [DOI] [PubMed] [Google Scholar]
- 64.Coll T., E. Eyre, R. Rodríguez-Calvo, X. Palomer, R. M. Sánchez, M. Merlos, J. C. Laguna, and M. Vázquez-Carrera. 2008. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J. Biol. Chem. 283 11107–11116. [DOI] [PubMed] [Google Scholar]
- 65.Pahan K., M. Khan, and I. Singh. 1998. Therapy for X-adrenoleukodystrophy: normalization of very long chain fatty acids and inhibition of induction of cytokines by cAMP. J. Lipid Res. 39 1091–1100. [PubMed] [Google Scholar]
- 66.Wright W. E., and J. W. Shay. 2002. Historical claims and current interpretations of replicative aging. Nat. Biotechnol. 20 682–688. [DOI] [PubMed] [Google Scholar]