Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-activated transcription factor, which belongs to the family of nuclear hormone receptors. Recent in vitro studies have shown that PPARγ can regulate the transcription of phosphatase and tensin homolog on chromosome ten (PTEN), a known tumor suppressor. PTEN is a susceptibility gene for a number of disorders, including breast and thyroid cancer. Activation of PPARγ through agonists increases functional PTEN protein levels that subsequently induces apoptosis and inhibits cellular growth, which suggests that PPARγ may be a tumor suppressor. Indeed, several in vivo studies have demonstrated that genetic alterations of PPARγ can promote tumor progression. These results are supported by observations of the beneficial effects of PPARγ agonists in the in vivo cancer setting. These studies signify the importance of PPARγ and PTEN's interaction in cancer prevention.
1. INTRODUCTION
1.1. PPARγ
Peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-activated transcription factor, belonging to the nuclear hormone receptor family, whose ligand-binding domain is located at the carboxy-terminus. There are several known natural and synthetic PPARγ agonists with 15-deoxy-delta 12, 14-prostaglandin-J2 (15d-PG-J2) being the most notable natural PPARγ agonist. Additionally, linoleic, linolenic, and arachidonic acids are other commonly recognized natural agonists. Synthetic PPARγ agonists, such as the thiazolidinediones (TZDs), are some of the most commonly prescribed medications for the treatment of type II diabetes mellitus. The four commercially recognized TZDs are ciglitazone (Alexis), pioglitazone (Actos), rosiglitazone (Avandia), and troglitazone (Rezulin).
After ligand-activation, PPARγ forms a heterodimer complex with retinoic acid receptor (RXR). This PPARγ/RXR complex subsequently translocates to the nucleus and binds to a peroxisome proliferator response element (PPRE) within a target gene thereby initiating transcription. The primary, and most studied, targets of PPARγ are involved in metabolic pathways and adipocyte differentiation. However, in recent years it has been suggested that PPARγ has a role in cancer development. Indeed, initial studies demonstrated alterations of cellular differentiation, indicative of apoptosis in a breast cancer setting, after PPARγ agonist stimulation. This indicates that PPARγ and its agonists may play an important role in cancer development, prevention, and treatment.
In 1998, Mueller et al. performed one of the first PPARγ agonist studies in a cancer setting [1]. They demonstrated that both 15d-PG-J2 and rosiglitazone (Rosi) could induce changes in epithelial gene expression associated with a more differentiated, less malignant state. Moreover, they described a reduction in the overall growth rate of breast cancer cells when treated with a PPARγ agonist. These data suggest that PPARγ can contribute to the prevention of breast cancer development and its agonists may be a novel therapy for cancer treatment [1]. These results stimulated further studies investigating PPARγ-mediated tumor suppression. One protein, that may play a role in PPARγ-mediated tumor suppression, is phosphatase and tensin homolog on chromosome ten (PTEN), which has an established role in breast cancer development. Interestingly, Mueller et al. characterization of breast cancer cells after PPARγ activation demonstrated a striking resemblance to cells with active PTEN expression [1]. Taken together, these results suggested that PTEN and PPARγ, together, may modulate breast cancer progression.
1.2. PTEN
In 1995, PTEN was identified as the susceptibility gene for Cowden syndrome (CS), which is characterized by breast, thyroid, and endometrial carcinoma as well as macrocephaly [2–8]. Patients diagnosed with CS have a 25–50% lifetime risk of developing female breast cancer, compared to the general population risk of ~13% [9, 10] Additionally, patients have ~10% lifetime risk of developing thyroid cancer, compared to <1% in the general population and have a ~5–10% lifetime risk of endometrial cancer compared to ~2–4% in the general population [9, 11]. Since its identification, research has detected a PTEN mutation in 85% of CS patients [11]. Furthermore, somatic alterations in PTEN, whether by genetic or epigenetic mechanisms, play some role in the pathogenesis of a broad range of solid tumors, including sporadic carcinomas of the breast, thyroid, endometrium, and colon.
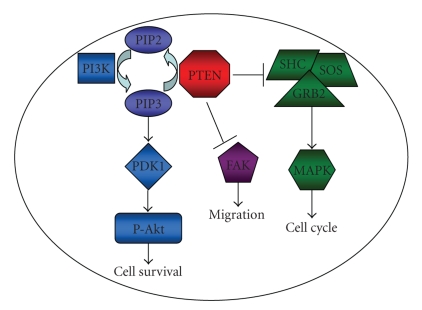
PTEN’s protein, PTEN, is a unique phosphatase that has the ability to dephosphorylate both proteins and lipids (Figure 1) [4]. Its lipid phosphatase activity functions as a negative regulator of Akt phosphorylation (P-Akt). PTEN dephosphorylates phosphatidylinositol-3,4,5-triphosphate (PIP3) at the D3 position generating phosphatidylinositol 4,5-biphosphate (PIP2), decreasing cellular PIP3 levels. Since PIP3 is required for Akt phosphorylation, active PTEN leads to a decrease in the levels of P-Akt and consequently a decrease in Akt-mediated proliferation pathways. PTEN’s protein phosphatase activity has been shown to inhibit the SHC/SOS/GRB2 and mitogen-activated protein kinase (MAPK) pathways. The dephosphorylation of SHC by PTEN indirectly decreases the phosphorylated form of MAPK levels, reducing MAPK’s activity. Additionally, PTEN’s protein phosphatase activity upregulates p27 with a concomitant downregulation of cyclin D1 which coordinates G1 arrest [12]. By regulating these key-signaling pathways, PTEN downregulates cell division and upregulates apoptosis. Additionally, PTEN’s protein phosphatase activity has been shown to dephosphorylate focal adhesion kinase (FAK), which inhibits cell spreading and migration [4].
Figure 1.
PTEN protein signaling pathways. PTEN’s lipid phosphatase activity dephosphorylates PIP3 to PIP2 inhibiting PDK1-mediated Akt phosphorylation and downregulating Akt-mediated cell survival. PTEN’s protein phosphatase activity inhibits the phosphorylation of FAK to prevent cell migration. PTEN’s protein phosphatase activity also dephosphorylates the SHC/SOS/GRB2 complex resulting in the decreased phosphorylation of MAPK and inhibition of the cell cycle.
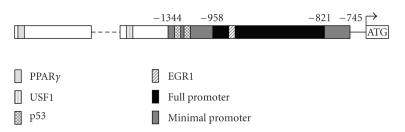
Transcriptional regulation of PTEN is only beginning to be elucidated. To date, analysis of PTEN’s promoter suggests that there are at least eight regulatory factors that modulate PTEN’s transcription (Figure 2). In 2001, Stambolic et al. identified a functional p53 binding site, located at nucleotide positions −1190 to −1157 in PTEN’s promoter, which was required for PTEN’s upregulation [13]. Additionally, early growth response-1 (Egr-1) has been shown to bind to the PTEN promoter at −947 to −939 and induce PTEN expression [14]. Recently, our laboratory has identified a USF1 binding site ~2 kb (−2237 and −2058) upstream of the ATG site [15]. CBF-1, Sp1, and c-Jun have also recently been suggested as PTEN transcription factors [11, 16–18]. The majority of PTEN promoter analyses have been focused on transcription factors that increase PTEN levels. However, recently, suppression of PTEN gene expression has been shown by the tumor necrosis factor-alpha/nuclear factor-kappa B (NF-κB) [19], however the precise mechanism of this inhibition remains unclear.
Figure 2.
PTEN promoter and its transcription factors. PTEN’s full-length promoter lies between −1344 and −745 (gray bar), while the minimal promoter lies between −958 and −821 (black bar). Four transcription factors are known to directly bind upstream of PTEN: PPARγ (solid gray bar), USF1 (stripped gray bar), p53 (dotted gray bar), and EGR1 (dashed gray bar).
1.3. PPARγ and PTEN in vitro
In 2001, Patel et al. first showed that PPARγ can be a PTEN transcription factor [20]. They observed that Rosi induced PTEN protein expression in both MCF-7 breast and CoCa2 colon cancer cell lines. In addition to the increase in PTEN expression, they observed an inhibition of both Akt phosphorylation and cellular proliferation. They also identified two putative PPREs within the PTEN promoter approximately 15 and 13 kb upstream of the ATG site (Figure 2). While this study was significant in demonstrating a potential link between PPARγ and PTEN, it remained correlative.
In 2005, two independent laboratories confirmed Patel’s suspicion that PPARγ induces PTEN transcription in a breast cancer setting [21, 22]. We demonstrated that of the four TZDs, only Rosi had the ability to induce PTEN transcription and subsequently its protein expression in MCF-7 cells [21]. Furthermore, we showed that stimulation with Rosi induces a PTEN protein that is both protein- and lipid-phosphatase active, as evidenced by decreased phosphorylation of Akt and MAPK concomitant with PTEN expression. Additionally, Rosi treatment induced G1 arrest that paralleled with PTEN expression. By using a Rosi analog, Compound 66, that is incapable of activating PPARγ, we confirmed that Rosi induced PTEN expression via a PPARγ-dependent mechanism in several reporter assays [21].
Additionally, in 2005, Bonofiglio et al. also demonstrated that PPARγ could upregulate PTEN’s transcription in a breast cancer setting [22]. After cells were stimulated with Rosi, an increase in PTEN protein was observed as well as an inhibition of Akt phosphorylation and cellular growth. More importantly, they were able to observe for the first time the specific binding of PPARγ to the PTEN promoter (−15376 to −15364; Figure 2). Interestingly, this interaction was enhanced by Rosi treatment. Further analysis indicated that PPARγ and estrogen receptor (ER) could bind to the PPRE both independently and simultaneously. The ER’s association with the PPRE inhibited PPARγ’s ability to induce transcription as demonstrated by cotreatment of MCF-7 breast cancer cells with both Rosi and 17ß-estradiol. This cotreatment inhibited the induction of PTEN protein that was observed by Rosi stimulation alone [22]. This is an important observation as it is appealing to postulate that this crosstalk, between PPARγ and ER, may significantly affect breast cancer therapeutics as well as lead the way to the discovery of future novel treatment therapies.
In 2006, Zhang et al. showed that Rosi stimulation of hepatocarcinoma cells results in the upregulation of PTEN and PTEN-dependent inhibition of cell migration [23]. This is significant because PTEN expression is decreased or absent in approximately half of all primary hepatocarcinoma patients. As similarly demonstrated by others, Rosi treatment of hepatocarcinoma cells resulted in an increase in PTEN mRNA. They further speculated that there may be three other potential PPREs within the PTEN promoter, located at −2874 to −2854, −1615 to −1596, and −1594 to −1574, however, it has not yet been determined if these are functional PPREs. Interestingly, Zhang et al. do not observe an increase in transcriptional activity of the PTEN promoter in response to Rosi treatment [23]. We observed similar results when examining the full-length PTEN promoter, using a luciferase reporter assay and Rosi stimulation (Teresi, Waite, and Eng; unpublished observations). This may suggest that elements beyond the full-length PTEN promoter are required for Rosi-mediated PTEN transcription.
These initial studies concretely demonstrated that PPARγ acts as a tumor suppressor in a cancer setting by upregulating PTEN transcription. However, these studies were performed solely in breast cancer cell lines, leaving the speculation that these observations are cancer-type dependent. To this end, several groups have studied PPARγ’s ability to regulate PTEN levels in other cancer backgrounds. Lee et al. observed an inhibition of cellular proliferation and Akt phosphorylation in accord with an increase in G1 arrest and PTEN protein expression in A549 lung cancer cells [24]. Subsequently, PPARγ has been shown to upregulate PTEN expression in nonsmall cell lung cancer, neuroblastoma, adrenocortical, pancreatic, heptocarcinoma, and thyroid cell lines [23, 25, 26].
Interestingly, the majority of these studies utilized Rosi as the PPARγ agonist. This may be due to the combination of our initial study, which demonstrated that of the TZDs only Rosi was capable of inducing PTEN expression, and the fact that natural ligands can be difficult to work with in vitro [21]. Despite this, Chen et al. demonstrated that both ciglitazone and 15d-PG-J2 could upregulate PTEN expression in W-2 thyroid cells [27], which raises the possibility that of the TZDs, Rosi stimulation is limited to breast cancer. This remains to be determined.
1.4. PPARγ and PTEN in vivo
Despite the growing amount of in vitro data supporting the role of PPARγ as a tumor suppressor, only a small number of cancers have had their PPARγ status characterized in vivo and there are very few studies of clinical PPARγ agonist treatment. Nonetheless, current studies provide some essential and encouraging information. One of the first studies to analyze PPARγ status in an in vivo cancer setting examined 55 unrelated sporadic colon cancer samples and revealed 4 PPARγ mutations [28]. Moreover, these mutations produced an inactive PPARγ protein. This study demonstrated that PPARγ can act as a tumor suppressor in vivo and when its normal activity is altered it can lead to cancer development [29]. Subsequent studies have confirmed these results showing the reduction of PPARγ expression in both acrometaly [30] and ulcerative colitis [31], two predisposing conditions of colon cancer. In contrast to these studies, Ikezoe et al. did not observe any PPARγ alterations in their colon cancer study; however they limited their study to only exons 3 and 5 of PPARγ [32]. These studies indicate that PPARγ is indeed a tumor suppressor in the colon cancer setting; however none of these studies tested if the TZDs could effect the cancer’s progression.
To date, the majority of studies correlating PPARγ with PTEN have been performed in vitro and these studies suggest that PPARγ agonists may be beneficial to PTEN in vivo. Moreover, in vitro data suggest that PPARγ agonists have the potential to be highly effective PTEN transcriptional inducers for patients who have one of the following: a hemizygous deletion, a germline nucleotide alteration within the promoter, and potentially in the circumstance, where a PTEN mutation is not identified but a decrease in protein expression is observed.
Despite the potential beneficial effects of TZD treatment, in particular Rosi, one must be aware that the use of these medications may lead to more harm than good. For example, treatment of patients with germline intragenic PTEN mutations or those with neoplasias containing somatic intragenic mutations may see a raise of mutant, inactive protein. Recently, PTEN has been shown to induce gain-of-function p53 protein suggesting that TZD treatment in this setting may subsequently induce mutant, nonbeneficial p53 protein. Additionally, our work and others have suggested that not all of TZDs signal through the same pathways, at least in cell culture conditions [21]. Rosi is the only TZD that is known to increase PTEN in breast cancer lines, which indicates that each TZD may lead to its own individual side effects. Indeed in 2000, troglitazone (Rezulin) was pulled off of the market due to liver toxicity. Interestingly, to date, this has not been observed with other TZDs [33]. A recent study demonstrated that Rosi (Avandia) increases the risk of heart complications, specifically heart attacks; however these results have yet to be replicated [34]. This indicates that the significance of Rosi treatment on cardiac function needs to be examined further. Indeed, in this first study, important results, which came to the opposite conclusions, were not included in the meta-analysis. In spite of this, a deeper understanding of the signaling mechanisms behind these side effects should open the door to both new avenues of cancer treatment and personalized health care, allowing physicians to properly weigh the benefits against the known side effects prior to prescribing such a treatment.
Drug-drug interactions are another aspect that physicians will need to be aware of. Bonofiglio’s PPARγ-ER-PTEN results are significant in the context of breast cancer and hormone therapies [22]. Their data suggest that women treated with hormones, either through birth control or hormonal therapies, may not benefit from cotreatment with a PPARγ agonist. This further suggests that hormone treatment may actually be detrimental by inhibiting naturally occurring PTEN transcription.
1.5. The translation of PPARγ and PTEN into the clinic
A recent study has suggested that Rosi treatment could be beneficial to patients with Gefitinib-resistant lung cancer [24], a cancer which is typically correlated with the loss of PTEN protein. Lee et al. have shown that in the human lung cancer cell line, A549, the combined treatment of Rosi and Gefitinib was more beneficial than Gefitinib treatment alone [24]. Taken together, these data provide support that the upregulation of PTEN levels with Rosi treatment may reverse the Gefitinib resistance in these patients. Such a treatment could have the potential to be advantageous to patients with both sporadic and familial cancer.
PPARγ status is only now beginning to be examined in the in vivo cancer setting, however the TZDs have been used in a variety of clinical trials, although not directly related to PPARγ activation. Seemingly, out of the ordinary, polycystic ovary syndrome (PCOS) is the most commonly studied syndrome with regards to the effects of TZD treatment [35]. While there is still much debate on what treatment is best for these patients, the majority believe that Rosi treatment is beneficial. Studies have demonstrated that Rosi treatment raises insulin and androgen levels in the obese PCOS population, thereby inhibiting tumor progression. Furthermore, Yee et al. recently performed a pilot study in women with breast cancer to determine if Rosi treatment would be beneficial. Thirty-eight women with early stage breast cancer were treated with Rosi for 2–6 weeks with tumor growth inhibition or progression as an end point [36]. The data indicate that short-term Rosi therapy in early-stage breast cancer patients has both local and systemic effects on PPARγ signaling. Both of these studies suggest that Rosi may be used clinically to benefit cancer patients.
1.6. PPARγ and PTEN’s future
The culmination of these data strongly suggests that Rosi stimulation may be advantageous to the cancer patient. However, lacking in many of these studies is the role of PTEN. To date, in vitro data has demonstrated a connection between PPARγ and PTEN, yet no in vivo study has concretely confirmed these results. The results obtained from these studies would concretely determine if Rosi treatment is advantageous for cancer patients by upregulating PTEN expression through PPARγ.
While clinical trials are necessary to determine if Rosi treatment is truly beneficial for cancer patients and which patients it is most advantageous for, much remains to be learned at the molecular level. The relevance of the putative PPRE in the PTEN promoter identified by Bonofiglio et al. remains to be determined (Figure 2) [22]. This PPRE is located a long distance from the ATG site, thus making it unclear if this site is functional in regulating PTEN expression. It will be interesting to find out the role of this unique site.
While evidence suggests that TZDs induce PTEN expression through PPARγ, further studies are warranted to determine the exact mechanism of action. Evidence by our group suggests that PPARγ may regulate PTEN expression through both transcriptional-dependent and -independent mechanisms [37]. While this may add to the complexity of the role of PPARγ, with regards to PTEN, it may also provide other areas for therapeutic advances. Interestingly, while studying the ability of statins to induce PTEN expression, we observed that statins increase PTEN transcription via an unknown PPARγ-mediated mechanism [37]. Retrospectively, we observed a similar response with Rosi stimulation indicating that PPARγ is necessary; however its transcriptional activity is not. These results suggest that PPARγ may induce PTEN transcription through an unknown mechanism and an unrecognized transcription factor; however this remains to be determined.
2. CONCLUSION
In recent years, there has been a growing accumulation of data implicating the importance of both PPARγ and PTEN in cancer prevention, development, and treatment. In vitro data has demonstrated that PPARγ agonists can induce functional PTEN protein that controls cellular growth. In vivo data has suggested that PPARγ genetic alteration can lead to cancer development, while its agonists can inhibit tumor progression. Despite this progress, we are only beginning to determine the roles of these two proteins and their complex interactions. Undoubtedly, future studies will clarify the PPARγ-PTEN connection providing a variety of targets that may lead to novel therapeutic treatments for cancer patients.
ACKNOWLEDGMENT
The authors would like to thank Dr. Charis Eng and members of the Eng lab, both past and present, for their support on the research contributing to this paper.
References
- 1.Mueller E, Sarraf P, Tontonoz P, et al. Terminal differentiation of human breast cancer through PPARγ . Molecular Cell. 1998;1(3):465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 2.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. Journal of Medical Genetics. 2000;37(11):828–830. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eng C. Genetics of Cowden syndrome: through the looking glass of oncology. International Journal of Oncology. 1998;12(3):701–710. doi: 10.3892/ijo.12.3.701. [DOI] [PubMed] [Google Scholar]
- 4.Waite KA, Eng C. Protean PTEN: form and function. The American Journal of Human Genetics. 2002;70(4):829–844. doi: 10.1086/340026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nature Genetics. 1997;16(1):64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 6.Marsh DJ, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Human Molecular Genetics. 1999;8(8):1461–1472. doi: 10.1093/hmg/8.8.1461. [DOI] [PubMed] [Google Scholar]
- 7.Witman PM. More than just a bump: the hamartoma syndromes. Advances in Dermatology. 2006;22:157–180. doi: 10.1016/j.yadr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Zbuk KM, Eng C. Cancer phenomics: RET and PTEN as illustrative models. Nature Reviews Cancer. 2007;7(1):35–45. doi: 10.1038/nrc2037. [DOI] [PubMed] [Google Scholar]
- 9.Ries LAG, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2000. Bethesda, Md, USA: National Cancer Institute; 2003. [Google Scholar]
- 10.Pilarski R, Eng C. Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. Journal of Medical Genetics. 2004;41(5):323–326. doi: 10.1136/jmg.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X-P, Waite KA, Pilarski R, et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. The American Journal of Human Genetics. 2003;73(2):404–411. doi: 10.1086/377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planchon SM, Waite KA, Eng C. The nuclear affairs of PTEN. Journal of Cell Science. 2008;121(3):249–253. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]
- 13.Stambolic V, MacPherson D, Sas D, et al. Regulation of PTEN transcription by p53. Molecular Cell. 2001;8(2):317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 14.Virolle T, Adamson ED, Baron V, et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nature Cell Biology. 2001;3(12):1124–1128. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- 15.Pezzolesi MG, Zbuk KM, Waite KA, Eng C. Comparative genomic and functional analyses reveal a novel cis-acting PTEN regulatory element as a highly conserved functional E-box motif deleted in Cowden syndrome. Human Molecular Genetics. 2007;16(9):1058–1071. doi: 10.1093/hmg/ddm053. [DOI] [PubMed] [Google Scholar]
- 16.Whelan JT, Forbes SL, Bertrand FE. CBF-1 (RBP-Jκ) binds to the PTEN promoter and regulates PTEN gene expression. Cell Cycle. 2007;6(1):80–84. doi: 10.4161/cc.6.1.3648. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Dong Z, Liu Y, Chen Q, Hashimoto K, Zhang J-T. Regulation of constitutive expression of mouse PTEN by the 5′-untranslated region. Oncogene. 2003;22(34):5325–5337. doi: 10.1038/sj.onc.1206783. [DOI] [PubMed] [Google Scholar]
- 18.Hettinger K, Vikhanskaya F, Poh MK, et al. c-Jun promotes cellular survival by suppression of PTEN. Cell Death & Differentiation. 2007;14(2):218–229. doi: 10.1038/sj.cdd.4401946. [DOI] [PubMed] [Google Scholar]
- 19.Vasudevan KM, Gurumurthy S, Rangnekar VM. Suppression of PTEN expression by NF-κB prevents apoptosis. Molecular and Cellular Biology. 2004;24(3):1007–1021. doi: 10.1128/MCB.24.3.1007-1021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH. Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Current Biology. 2001;11(10):764–768. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 21.Teresi RE, Shaiu C-W, Chen C-S, Chatterjee VK, Waite KA, Eng C. Increased PTEN expression due to transcriptional activation of PPARγ by Lovastatin and Rosiglitazone. International Journal of Cancer. 2006;118(10):2390–2398. doi: 10.1002/ijc.21799. [DOI] [PubMed] [Google Scholar]
- 22.Bonofiglio D, Gabriele S, Aquila S, et al. Estrogen receptor α binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor γ signaling in breast cancer cells. Clinical Cancer Research. 2005;11(17):6139–6147. doi: 10.1158/1078-0432.CCR-04-2453. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Wu N, Li Z, Wang L, Jin J, Zha X-L. PPARγ activator rosiglitazone inhibits cell migration via upregulation of PTEN in human hepatocarcinoma cell line BEL-7404. Cancer Biology and Therapy. 2006;5(8):1008–1014. doi: 10.4161/cbt.5.8.2887. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Hur GY, Jung KH, et al. PPAR-γ agonist increase gefitinib's antitumor activity through PTEN expression. Lung Cancer. 2006;51(3):297–301. doi: 10.1016/j.lungcan.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARγ-dependent and PPARγ-independent signal pathways. Molecular Cancer Therapeutics. 2006;5(2):430–437. doi: 10.1158/1535-7163.MCT-05-0347. [DOI] [PubMed] [Google Scholar]
- 26.Aiello A, Pandini G, Frasca F, et al. Peroxisomal proliferator-activated receptor-γ agonists induce partial reversion of epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology. 2006;147(9):4463–4475. doi: 10.1210/en.2005-1610. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Wang S-M, Wu J-C, Huang S-H. Effects of PPARγ agonists on cell survival and focal adhesions in a Chinese thyroid carcinoma cell line. Journal of Cellular Biochemistry. 2006;98(4):1021–1035. doi: 10.1002/jcb.20839. [DOI] [PubMed] [Google Scholar]
- 28.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Molecular Cell. 1999;3(6):799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 29.Thompson EA. PPARγ physiology and pathology in gastrointestinal epithelial cells. Molecules and Cells. 2007;24(2):167–176. [PubMed] [Google Scholar]
- 30.Bogazzi F, Ultimieri F, Raggi F, et al. Peroxisome proliferator activated receptor γ expression is reduced in the colonic mucosa of acromegalic patients. The Journal of Clinical Endocrinology & Metabolism. 2002;87(5):2403–2406. doi: 10.1210/jcem.87.5.8625. [DOI] [PubMed] [Google Scholar]
- 31.Dubuquoy L, Dharancy S, Nutten S, Pettersson S, Auwerx J, Desreumaux P. Role of peroxisome proliferator-activated receptor γ and retinoid X receptor heterodimer in hepatogastroenterological diseases. The Lancet. 2002;360(9343):1410–1418. doi: 10.1016/S0140-6736(02)11395-X. [DOI] [PubMed] [Google Scholar]
- 32.Ikezoe T, Miller CW, Kawano S, et al. Mutational analysis of the peroxisome proliferator-activated receptor γ in human malignancies. Cancer Research. 2001;61(13):5307–5310. [PubMed] [Google Scholar]
- 33.Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. American Journal of Gastroenterology. 2000;95(1):272–276. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 34.Nissen SE. Perspective: effect of rosiglitazone on cardiovascular outcomes. Current Cardiology Reports. 2007;9(5):343–344. doi: 10.1007/BF02938358. [DOI] [PubMed] [Google Scholar]
- 35.Banning M. Symptoms and treatments of polycystic ovary syndrome. British Journal of Nursing. 2006;15(12):635–639. doi: 10.12968/bjon.2006.15.12.21393. [DOI] [PubMed] [Google Scholar]
- 36.Yee LD, Williams N, Wen P, et al. Pilot study of rosiglitazone therapy in women with breast cancer: effects of short-term therapy on tumor tissue and serum markers. Clinical Cancer Research. 2007;13(1):246–252. doi: 10.1158/1078-0432.CCR-06-1947. [DOI] [PubMed] [Google Scholar]
- 37.Teresi RE, Planchon SM, Waite KA, Eng C. Regulation of the PTEN promoter by statins and SREBP. Human Molecular Genetics. 2008;17(7):919–928. doi: 10.1093/hmg/ddm364. [DOI] [PubMed] [Google Scholar]