Abstract
We examined whether the risk factors for increased brachial pulse pressure (PP) are similar for Blacks and Whites. Many studies have reported the strong association of increased brachial PP and the prevalence of cardiovascular disease. Participants were from 4 major United States epidemiologic studies (26,083 subjects): Charleston Heart Study, Evans County Heart Study, the NHANES I study, and the NHANES II study. At baseline, there was no history or clinical evidence of coronary heart disease (CHD). The CHD mortality as a function of brachial PP and the association of traditional risk factors for CHD with PP were analyzed for the 4 studies individually and for the 4 studies combined. Multiple regression analysis showed that the most significant predictors of high brachial PP are body mass index ≥ 30 kg/m2 (regression coefficient 3.79, p<0.0001), diabetes mellitus (5.14, p<0.0001, serum total cholesterol ≥ 240 mg/dl (0.51,<0.0157), age (0.60, p<0.0001), gender (-1.77, p<0.0001), and race (3.75, p<0.0001). The same risk factors for CHD (namely, increase in body mass index ≥ 30 kg/m2, diabetes mellitus, hypercholesterolemia and age) are significantly associated with high brachial PP for Blacks and Whites. These risk factors were stronger among Whites compared to Blacks. However, female gender and age variables were even more associated with brachial PP among Blacks. Smoking was significant but not reflected in peripheral brachial PP as it is in aortic pulse pressure.
Keywords: aortic stiffness, risk factors, brachial pulse pressure, race
In the present study, we have investigated the relation of traditional cardiovascular risk factors, namely, hypertension, diabetes mellitus, obesity, cigarette smoking, and lipid abnormalities for CHD to brachial PP in a long term follow-up of Whites and Blacks.
Methods
Participants were from 4 major United States epidemiologic studies (26,083 subjects) that at baseline examination had no history or clinical evidence of coronary heart disease (CHD).
Charleston Heart Study
The study population from Charleston, South Carolina, was a random sample of black and white men and women who were 35 years of age or older in 1960 (1). Among those who were sampled, a high consent rate was obtained: 78% in black men, 85% in white men, 84% in black women, and 86% in white women. In 1990, 30 years after the initial baseline measures, the vital status of 98% of the white participants and 99% of the black participants was known. The total population was 2,282 and data from 1,928 participants were used for this current analysis.
Evans County Heart Study
In 1960-1962, all non-institutionalized residents of Evans County, Georgia, ≥ 40 years and 50% of those 15-39 years were invited to participate in an epidemiologic, closed community-based cohort study (2). With 90% or higher consent rate across races and genders, 3,102 people participated. Excluding those who were missing any of the variables, data from 2,593 were used for this analysis. Vital status was assessed for 30-year follow-up.
NHANES I Epidemiologic Follow-up Study
This study used a probability sample of the civilian non-institutionalized population of the Unites States (3,4). The baseline survey was conducted during 1971-1975 on 20,749 persons 1-74 years of age, but the follow-up survey was only conducted on those 25-74 years at baseline (N=14,407). As of 1993, after those with missing data were excluded, 12,722 were used for the current analysis.
NHANES II Mortality Study
Baseline data for this study were collected during 1976 to 1980 from 20,322 individuals 6 months to 74 years of age (5). The follow-up as of 1992 was conducted on 9,252 above 30 years of age. Data from 8,840 participants were used for the current analysis.
When these 4 study samples were combined, a sample size of 26,083 was obtained. Among them, 12,058 were white women, 9,586 were white men, 2,610 were black women, and 1,829 were black men. The average age at baseline was 51 years (std=14 years) and the average follow-up period was 16 years (std=7 years). All participants had systolic and diastolic blood pressures and serum cholesterol measurements. For those who died the underlying cause was ascertained at the end of the study. Death occurred in 8,801 of these participants and 2,717 of the deaths were due to CHD.
Differences in means and proportions were assessed using 2-sample t-tests and chi-squared tests respectively. Multiple linear regression models were used to examine the relationship that cholesterol, smoking, obesity, diabetes mellitus, race, gender, and age have with increasing PP. In addition, logistic regression models were implemented to describe the association that increased brachial PP categories have on the odds of occurrence of cardiovascular risk factors. The brachial PP categories used in the logistic regression analyses were <40, 40-50, 51-60, and >60 mmHg. In all regression models a race interaction term was included to determine if the associations varied between blacks and whites. Regression models adjusted for study by including study-specific indicator variables. Data management and analyses were performed using SAS Software Version 9.0 (SAS Institute, Cary, NC). All statistical tests were performed using a two-sided alpha level of 0.05.
Results
Table 1 presents age, diabetes mellitus, smoking, obesity and high cholesterol by brachial PP category for Whites, Blacks and the combined races. Age was associated with increasing PP for both race groups. The prevalence of diabetes mellitus, obesity and high cholesterol each increased significantly with PP for both race groups. Cigarette smoking was inversely related to PP for both Whites and Blacks. Blacks were more likely to have diagnosed diabetes mellitus. The prevalence of obesity was higher for Blacks than Whites. The reverse was seen for high cholesterol (≥240) where Whites had significantly higher prevalence compared to Blacks. In general, the smoking prevalence was higher for Blacks.
Table 1.
Percentages, Sample Size, and Trend Test P-values for Risk Factors for Four Categories of Brachial Pulse Pressure
| Pulse Pressure Category (mmHg) | |||||
|---|---|---|---|---|---|
| Races Combined
|
<40
(n=6209) |
40-50
(n=8544) |
51-60
(n=4912) |
>60
(n=6418) |
P-value |
| Age (Years) | 43.2 | 47.7 | 54.0 | 61.3 | <0.0001 |
| Diabetes Mellitus | 2.0% | 3.1% | 4.5% | 8.6% | <0.0001 |
| Current Smokers | 39.0% | 36.6% | 33.1% | 27.3% | <0.0001 |
| BMI ≥ 30 kg/m2 | 12.0% | 15.1% | 19.1% | 23.4% | <0.0001 |
| Total Cholesterol 200-239 mg/dL | 32.5% | 33% | 34.9% | 34.0% | 0.0387 |
| Total Cholesterol ≥ 240 mg/dL | 25.6% | 31.0% | 36.7% | 42.9% | <0.0001 |
|
Whites
|
<40
(n=5352) |
40-50
(n=7313) |
51-60
(n=4903) |
>60
(n=4886) |
P-value
|
| Age | 43.3 | 47.9* | 54.5* | 62.1* | <0.0001 |
| Diabetes Mellitus | 1.8%* | 2.7%* | 4.4%* | 8.6% | <0.0001 |
| Current Smokers | 38.1%* | 35.6%* | 32.1%* | 26.7% | <0.0001 |
| BMI ≥ 30 kg/m2 | 10.6%* | 13.4%* | 17.6%* | 21.7%* | <0.0001 |
| Total Cholesterol 200-239 mg/dL | 32.6% | 33.5% | 35.2% | 33.5% | 0.1549 |
| Total Cholesterol ≥ 240 mg/dL | 25.8% | 31.6%* | 37.8%* | 44.8%* | <0.0001 |
|
Blacks
|
<40
(n=857) |
40-50
(n=1231) |
51-60
(n=819) |
>60
(n=1532) |
P-value
|
| Age | 42.7 | 46.3* | 51.5* | 58.8* | <0.0001 |
| Diabetes mellitus | 3.4% | 5.2% | 5.0% | 8.7% | <0.0001 |
| Current Smokers | 44.7% | 42.7% | 38.0% | 29.1% | <0.0001 |
| BMI ≥ 30 kg/m2 | 20.5% | 25.0% | 26.6% | 28.6% | <0.0001 |
| Total Cholesterol 200-239 mg/dL | 31.6% | 30.1% | 33.3% | 35.4% | 0.0086 |
| Total Cholesterol ≥ 240 mg/dL | 23.9% | 27.7% | 30.9% | 36.9% | <0.0001 |
BMI=body mass index
p-value < 0.05 for race comparison
Table 2 presents the age-adjusted odds ratios for diabetes mellitus, obesity and high cholesterol increase with brachial PP for combined and individual races. The increased risks for diabetes mellitus and high cholesterol with increased PP are most evident among Whites. A strong linear relationship for obesity and increasing PP was detected for both race groups. Similarly, the inverse relationship of smoking and PP was observed for Whites and Blacks.
Table 2.
Age-adjusted Odds Ratios and 95% Confidence Intervals for Risk Factors Controlling for Study Cohort
| Pulse Pressure Category (mmHg) | ||||
|---|---|---|---|---|
| Races Combined
|
<40
(n=6209) |
40-50
(n=8544) |
51-60
(n=4912) |
>60
(n=6418) |
| Diabetes mellitus | 1.00 | 1.27 (1.02, 1.58) | 1.50 (1.19, 1.89) | 2.40 (1.94, 2.99) |
| Current Smokers | 1.00 | 0.99 (0.93, 1.06) | 0.99 (0.91, 1.08) | 0.89 (0.81, 0.97) |
| BMI ≥ 30 kg/m2 | 1.00 | 1.33 (1.21, 1.47) | 1.80 (1.62, 2.01) | 2.39 (2.14, 2.66) |
| Total Cholesterol 200-239 mg/dL | 1.00 | 1.00 (0.93, 1.07) | 0.94 (0.87, 1.02) | 1.01 (0.93, 1.10) |
| Total Cholesterol ≥ 240 mg/dL | 1.00 | 1.11 (1.03, 1.20) | 1.14 (1.05, 1.24) | 1.15 (1.05, 1.25) |
|
Whites
|
<40
(n=5352) |
40-50
(n=7313) |
51-60
(n=4093) |
>60
(n=4886) |
| Diabetes Mellitus | 1.00 | 1.22 (0.95, 1.57) | 1.57 (1.21, 2.04) | 2.50 (1.95, 3.20)* |
| Current Smokers | 1.00 | 0.99 (0.92, 1.06) | 0.99 (0.90, 1.08) | 0.90 (0.82, 1.00)* |
| BMI ≥ 30 kg/m2 | 1.00 | 1.32 (1.18, 1.48) | 1.84 (1.62, 2.08) | 2.40 (2.12, 2.72)* |
| Total Cholesterol 200-239 mg/dL | 1.00 | 1.02 (0.95, 1.10) | 1.07 (0.98, 1.17) | 0.96 (0.88, 1.06) |
| Total Cholesterol ≥ 240 mg/dL | 1.00 | 1.11 (1.02, 1.20) | 1.15 (1.05, 1.26) | 1.17 (1.07, 1.29) |
|
Blacks
|
<40
(n=857) |
40-48
(n=1231) |
49-60
(n=819) |
>60
(n=1532) |
| Diabetes Mellitus | 1.00 | 1.42 (0.90, 2.24) | 1.16 (0.70, 1.91) | 1.79 (1.14, 2.81) |
| Current Smokers | 1.00 | 1.03 (0.86, 1.23) | 1.00 (0.81, 1.23) | 0.85 (0.69, 1.04) |
| BMI ≥ 30 kg/m2 | 1.00 | 1.37 (1.11, 1.69) | 1.57 (1.24, 1.99) | 1.91 (1.52, 2.39) |
| Total Cholesterol 200-239 mg/dL | 1.00 | 0.91 (0.75, 1.10) | 1.03 (0.83, 1.27) | 1.08 (0.88, 1.32) |
| Total Cholesterol ≥ 240 mg/dL | 1.00 | 1.10 (0.90, 1.36) | 1.10 (0.88, 1.38) | 1.18 (0.95, 1.47) |
BMI=body mass index
p-value < 0.05 for race comparison
Table 3 presents the results of multiple linear regression analyses for predictors of brachial PP and a comparison of the estimate by race. Race, gender, age, high cholesterol, diabetes mellitus and obesity were significant predictors of PP for the total study cohort as well as the race stratified analysis. The analysis was repeated with cholesterol 200-239 mg with similar but somewhat less significant association than cholesterol ≥ 240 mg. However, the magnitude of the association of the predictors varied significantly by race. The association of obesity, diabetes mellitus and high cholesterol with PP was significantly greater among Whites compared with Blacks. In addition, female gender and age variables were significantly more associated with PP among Blacks. A 10-year age increase is associated with a 5.7 mmHg increase in PP among Whites compared with a 7.4 mmHg in increase among Blacks. After controlling for other risk factors and study cohort, smoking is no longer a significant predictor if only brachial PP is measured.
Table 3.
Multiple Linear Regression with Brachial Pulse Pressure as Dependent Variable Controlling for Study Cohort, Ethnicity, Gender, Age, Elevated Cholesterol, Smoking Status, Diabetes Mellitus, and Obesity
| Variable | Parameter Estimate | P-value | |
|---|---|---|---|
| Blacks (Compared to Whites) | 3.7464 | <0.0001 | |
| Men (Compared to Women) | -1.76547 | <0.0001 | |
| Age (years) | 0.59695 | <0.0001 | |
| Total Cholesterol ≥ 240 mg/dl | 0.50695 | 0.0157 | |
| Cigarette Smoking | -0.285 | 0.1739 | |
| Diabetes Mellitus | 5.14205 | <0.0001 | |
| BMI ≥ 30 kg/m2 | 3.78873 | <0.0001 | |
| Stratified by Race | |||
| Variable | Whites Estimate | Blacks Estimate | Interaction P-value |
| Men (Compared to Women) | -1.27971 | -4.73544 | <0.0001 |
| BMI ≥ 30 kg/m2 | 4.09406 | 2.43839 | <0.0001 |
| Diabetes Mellitus | 5.86774 | 2.59018 | 0.0004 |
| Age (years) | 0.56913 | 0.74382 | <0.0001 |
| Cigarette Smoking | -0.06918 | -0.06336 | 0.2796 |
| Total Cholesterol ≥ 240 mg/dl | 0.35554 | 1.33273 | 0.0714 |
Discussion
The media of the aorta and large arteries is often altered by atherosclerosis. Atherosclerosis in some cases may be a contributing factor to aortic stiffness and wide brachial PP. If atherosclerosis is present, the atheroma is most pronounced immediately adjacent and extending into the inner portion of the media and it may amplify stiffening of the aorta (6,7). This condition leads to degeneration of elastic tissue, eventual loss of interstitial glucosaminoglycans (GAGs), and accumulation of collagen (fibrosis). At the edge of the atheroma inflammatory infiltrates, mostly composed of small lymphocytes are found. In addition, the atheroma can have lipids, hemorrhage, thrombosis, and calcification. Figure 1 shows a cross section of atherosclerotic thoracic aorta of a 55-year-old insulin-dependent diabetic with a brachial PP of >60 mmHg showing haphazard distribution of disease. The four enlargements depict changes which would produce differing degrees of loss of elasticity. The figures marked by capital letters correspond to the boxes marked by lower case letters. In A, there is moderate intimal and medial sclerosis (arrow). In B, there is a thicker old atheroma with cholesterol clefts (arrow), dense fibrosis, and medial thinning. In C, there is also intimal fibrosis but the media between the arrows is intact. In D, calcification has proceeded to ossification with bone marrow elements (arrow). Between B and D is a small zone of severe mural atrophy (MA). (Verhoeff-van Gieson stain – elastic black, muscle brown, collagen and bone red). However, atherosclerotic changes of the aorta may not be present in all cases of medial sclerosis with aortic stiffness with wide PP as has been noted in the Chinese (8).
Figure 1. A cross section of atherosclerotic thoracic aorta of a 55-year-old insulin dependent diabetic with a wide pulse pressure.
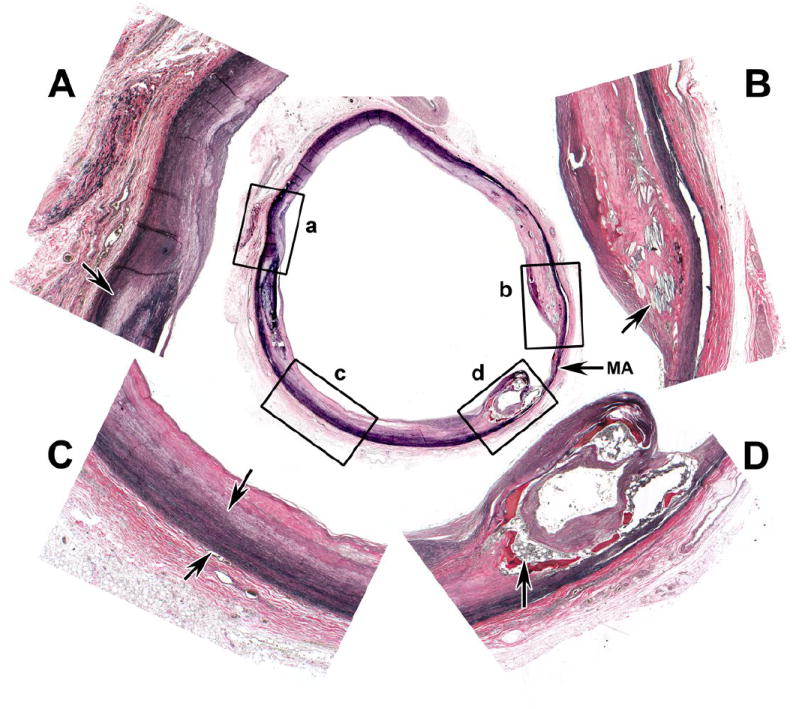
A. Intimal and medial sclerosis (arrow). B. Old atheroma with cholesterol clefts (arrow) dense fibrosis and medial thinning. C. Intimal fibrosis with intact media between arrows. D. Calcification and ossification with bone marrow elements (arrow). Figures marked by capital letters corresponds to the boxes marked by lower case letter (a,b,c,d). MA indicates severe mural atrophy between b and d. (Verhoeff-van Gieson stains-elastic black, muscle brown, collagen and bone red)
The risk factors for high brachial PP in most cases are the same as those for CHD. However, as noted in Table 3 the strength of this association varies by race. The significance was greater among Whites. Female gender and age were significantly more associated with brachial PP among Blacks. The negative association with smoking can be explained by several studies. Mahmud and Feely (9) reported that the aortic-brachial PP amplification is calculated by subtracting aortic from brachial PP. This amplification is another measure of aortic elasticity and when reduced indicates aortic stiffness. Radial applanation tonometry and pulse wave analysis were used to calculate derived aortic central blood pressure. Chronic smoking increases aortic systolic pressure and decreases PP amplification significantly as compared to non smokers. It also reduces the oscillatory arterial compliance but does not significantly change large artery compliance (10,11). The oscillatory diastolic wave form is generated principally by pulse wave reflection from small arteries and arterioles. In smokers, it is associated with a decrease in amplitude and duration of the diastolic wave and altered timing of wave reflection which arrives early in systole, superimposes on the forward wave, and further increases the aortic systolic pressure. The diminution of the diastolic oscillation is an early change in wave form occurring before significant augmentation of the systolic pressure wave becomes apparent. The augmentation index is the percentage of the arterial reflection of the aortic systolic pressure which includes the initial onset with ventricular ejection and the arterial reflection wave. This index and aortic systolic pressure are higher but not the brachial pressure in smokers compared with non smokers. As a result, the aortic-brachial PP amplification is reduced. Therefore, the deleterious effect of chronic smoking may be overlooked if only brachial blood pressure measurements are made and aortic systolic blood pressure and PP amplification are not included.
The sphygmomanometry blood pressure fails to measure indices of aortic stiffness such as aortic PP, pulse velocity or augmentation index which have to be measured invasively or non-invasively (12). Most of the effects of drugs such as nitrates, calcium blockers, angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists which dilate the medium sized peripheral muscular arteries and the smaller arteries (6,13-16) reduce wave reflection. This favorable effect on the proximal aorta and central arteries cannot be measured by the brachial blood pressure. Several studies with statin drugs and lifestyle intervention have shown a trend toward reduction of systolic, diastolic blood pressure and brachial PP unrelated to changes in cholesterol (17-20).
Acknowledgments
This work was supported in part from the Black Pooling Project NIH 1R01HL072377
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keil JE, Sutherland SE, Knapp RG, Lackland DT, Gazes PC, Tyroler HA. Mortality rates and risk factors for coronary disease in black as compared to white men and women. N Engl J Med. 1993;329:73–78. doi: 10.1056/NEJM199307083290201. [DOI] [PubMed] [Google Scholar]
- 2.Hames CG. Evans County Cardiovascular and Cerebrovascular Epidemiologic Study. Introduction. Arch Int Med. 1971;128:883–886. doi: 10.1001/archinte.128.6.883. [DOI] [PubMed] [Google Scholar]
- 3.Madans JH, Kleinman JC, Cox CS, Barbano HE, Feldmann JJ, Cohen B, Finucane FF, Cornoni-Huntley J. 10 years after NHANES I: report of initial follow-up, 1982-1984. Public Health Rep. 1986;101:465–473. [PMC free article] [PubMed] [Google Scholar]
- 4.Cox CS, Mussalano ME, Rothwell ST, Lane MA, Golden CD, Madans JH, Feldman JJ. Office of Analysis, Epidemiology, and Health Promotion. Plan and operation of the NHANES I epidemiologic follow-up study 1992. Vital and Health Statistics, Series 1: Programs and Collection Procedures. 1997;35:1–231. [PubMed] [Google Scholar]
- 5.Liao Y, McGee DL, Cooper RS, Sutkowski MB. How generalizable are coronary risk predictor models? Comparison of Framingham and two national cohorts. Am Heart J. 1999;137:837–845. doi: 10.1016/s0002-8703(99)70407-2. [DOI] [PubMed] [Google Scholar]
- 6.Dart AM, Kingwell BA. Pulse pressure – A review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 7.Groenink M, Langerak SE, Vanbavel E, van der Wall EE, Mulder BJ, van der Wal AC, Spaan JA. The influence of aging and aortic stiffness on permanent dilation and breaking stress of the thoracic descending aorta. Cardiovasc Res. 1999;43:471–480. doi: 10.1016/s0008-6363(99)00095-4. [DOI] [PubMed] [Google Scholar]
- 8.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. 2003;41:183–187. doi: 10.1161/01.hyp.0000047464.66901.60. [DOI] [PubMed] [Google Scholar]
- 10.McVeigh GE, Morgan DJ, Finkelstein SM, Lemay LA, Cohn JN. Vascular abnormalities associated with long-term cigarette smoking identified by arterial waveform analysis. Am J Med. 1997;102:227–231. doi: 10.1016/S0002-9343(96)00454-8. [DOI] [PubMed] [Google Scholar]
- 11.McVeigh G, Brennan G, Hayes R, Cohn J, Finkelstein S, Johnston D. Vascular abnormalities in non-insulin-dependent diabetes mellitus identified by arterial waveform analysis. Am J Med. 1993;95:424–430. doi: 10.1016/0002-9343(93)90313-e. [DOI] [PubMed] [Google Scholar]
- 12.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 13.O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension. 1990;15:339–347. doi: 10.1161/01.hyp.15.4.339. [DOI] [PubMed] [Google Scholar]
- 14.O’Rourke MF, Kelly RP. Wave reflection in the systemic circulation and its implications in ventricular function. J Hypertension. 1993;11:327–337. doi: 10.1097/00004872-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Karpanou EA, Vyssoulis GP, Stefanadis CI, Cokkinos DV. Differential pulse pressure response to various antihypertensive drug families. J Hum Hypertens. 2006;20:765–771. doi: 10.1038/sj.jhh.1002069. [DOI] [PubMed] [Google Scholar]
- 16.The CAFE Investigators, for the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Investigators. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes. Principal results of the Conduit Artery Function Evaluation (CAFE) Study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 17.Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, Kingwell BA. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39:1020–1024. doi: 10.1016/s0735-1097(02)01717-5. [DOI] [PubMed] [Google Scholar]
- 18.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J ASCOT Investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomized controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 19.Strazzullo P, Kerry SM, Barbato, Versiero M, D’Elia L, Cappuccio FP. Do statins reduce blood pressure?: A meta-analysis of randomized, controlled trial. Hypertension. 2007;49:792–798. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- 20.Cameron JD, Dart AM. Exercise testing increases total systemic arterial compliance in humans. Am J Physiol. 1994;266:H693–701. doi: 10.1152/ajpheart.1994.266.2.H693. [DOI] [PubMed] [Google Scholar]


