Abstract
Objective
The zebrafish is an established model system for studying the embryonic emergence of tissues and organs, including the hematopoietic system. We hypothesized that key signaling pathways controlling embryonic hematopoiesis continue to be important in the adult, and we sought to develop approaches to test this in zebrafish, focused on the BMP signaling pathway. Functions for this pathway in adult hematopoiesis have been challenging to probe in other models.
Methods
Several approaches tested the function of BMP signaling during adult zebrafish hematopoiesis. First, we evaluated steady-state hematopoiesis in adult fish that are heterozygous for mutant alleles of Smad5, or are homozygous for mutant alleles, and rescued to adulthood by injection of RNA encoding Smad5. Second, we tested the relative ability of smad5 mutant fish to recover from hemolytic anemia. Third, we generated a transgenic line that targets the expression of a dominant-negative BMP receptor to adult-stage Gata1+ progenitor cells.
Results
Adult fish with a strong mutant smad5 allele are anemic at steady state, and in addition respond to hemolytic anemia with kinetics that are altered compared to wild-type fish. Fish expressing a mutant BMP receptor in early Gata1+ definitive progenitors generate excessive eosinophils.
Conclusions
Our study provides proof of principle that regulation of adult hematopoiesis can be studied in zebrafish by altering specific pathways. We show that the BMP signaling pathway is relevant for adult hematopoiesis to maintain steady state erythropoiesis, control the erythropoietic response following stress anemia, and to generate normal numbers of eosinophils.
Keywords: definitive erythropoiesis, genetics, anemia, transgenic, kidney marrow
Introduction
The zebrafish system provides a powerful model for investigating the establishment of progenitor cells, tissues, and organs [1]. Genetic screens can identify key hematopoietic regulatory genes; for example vlad tepes is a mutant in the gene encoding Gata1 [2,3]. Mutants that affect earlier stages of mesoderm development are also relevant, since the hemato-vascular system is derived from lateral mesoderm that is dependent on ventralizing signals, including those from the Bone Morphogenetic Protein (BMP) pathway. BMP signaling is required to specify the fate of the mesoderm from which embryonic blood and vascular progenitors develop. For example, knockout of mouse Bmp4 [4] or Smad5 [5,6] causes early embryonic lethality and severe hematopoietic defects. The dependency of embryonic hematopoiesis on BMP signaling is a conserved feature in the early vertebrate embryo that is independent of the initial steps of mesoderm induction [7-9]. Indeed, a number of zebrafish mutants that display a dorsalized phenotype and defects in hematopoiesis are known to affect specifically the function of BMP signaling components [10,11]. We showed that during embryogenesis Smad1/Smad5 signaling has both early redundant functions for progenitor specification, and later distinct functions for primitive hematopoietic lineage differentiation [12].
Given the importance of BMP signaling during embryonic hematopoiesis, the pathway is likely involved in stem or progenitor cell biology relevant to adult (definitive) stages of blood development [13]. While this has been supported by experiments using in vitro cell culture models [14,15], it is challenging to investigate the issue in mice due to early embryonic lethality caused by manipulating components of the pathway. The zebrafish system has recently been adapted to study adult stages of hematopoiesis [16,17]. In adult zebrafish the site of hematopoiesis is the kidney [18,19]. The separation of distinct cell types from whole kidney marrow (WKM) can be achieved through the use of flow cytometry (FACS), which is further enhanced using transgenic reporter lines, for example, by sorting GFP+ cells from peripheral blood or WKM of fish that are transgenic for the gata1:gfp reporter gene [17,20]. Here we tested several approaches to evaluate the effect of altered BMP signaling in adult animals. Our study provides proof of principle that the zebrafish system can be used to study signaling pathways relevant to adult hematopoiesis.
Materials and Methods
Zebrafish
Zebrafish were raised and staged as described by Westerfield [21]. Wild-type fish were a hybrid of AB and TUB strains or +/+ siblings when comparing to mutant strains. Pgy and sbn heterozygotes were crossed to gata1:gfp homozygous fish to generate smad5 mutants on the gata1:gfp background. Due to poor survival of embryos derived from sbn/+ (male) × gata1:gfp (female), fertilized eggs were injected with 75 pg capped smad5 mRNA to facilitate growth to adulthood. Capped mRNA was prepared from linearized template DNA using the Sp6 mMessage mMachine kit (Ambion) according to the manufacturer's instructions. The pgy, sbn, and m169 mutants were genotyped by restriction digestion of fin-clipped DNA. The tc227 homozygous mutants were identified as juveniles by a consistent facial deformity. The gata1:urod-gfp transgene [22] was provided by Shuo Lin (UCLA). The ΔBR sequences [23,24] were amplified by PCR to incorporate an EcoRI site at the 5′ end and a BamHI site at the 3′ end, which facilitated replacement of the UROD sequences and in-frame fusion of the ΔBR sequences with GFP. The purified transgene was injected into fertilized eggs (100 pg) as described [25]. Embryos were raised to adulthood and transgenic founders identified by germline transmission of GFP expression.
Preparation of whole kidney marrow or peripheral blood from adult zebrafish
WKM was prepared as described [17]. Cells were resuspended in 200 μl PBF (0.9× PBS + 5% FCS) + 1 μg/ml propidium iodide or 0.1 μg/ml DAPI (Molecular Probes) and analyzed by flow cyometry. Data analysis was performed using FlowJo software (Tree Star Inc.). Gating to identify polychromatic erythroblasts and proerythroblasts was done as described [17]. To determine the number of apoptotic cells within a sample of cells prepared from WKM, the cells were incubated 5 min with Alexa647-conjugated annexin V (5 μl per 1 ml sample, as described by the manufacturer, Invitrogen). Cells were co-stained with 0.1 μg/ml DAPI. Peripheral blood was obtained via cardiac puncture and 1 μl counted on a Beckman Coulter Z1 Particle Counter.
Stress anemia induction by phenylhydrazine treatment
Genotyped adult fish (6-18 months; compared samples were always siblings) were placed in a solution of 0.003 mg/ml phenylhydrazine with 0.2 mM Tris pH 7.2 in fish system water for 20 minutes. Fish were then rinsed by four net changes in clean system water and returned to the system. Fish were sacrificed on days 2, 5, 12 or 20 for analysis by flow cytometry of hematopoietic cells from whole blood and WKM.
Real-time quantitative PCR
Total RNA was extracted using the RNeasy Micro Kit (Qiagen) according to the manufacturer's instructions. First-strand cDNA synthesis was performed using Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen). The cDNA was used for quantitative real-time PCR analysis (Opticon DNA Engine 2, MJ Research). The PCR reaction was performed using Qiagen SYBR Green mixture, and the data was analyzed by the 2-ΔΔCT method [26]. Primers were:
β-actin [27] F:5′CGCGCAGGAGATGGGAACC; R:5′CAACGGAAACGCTCATTGC
gata1 F:5′GTTGTAGTACTAGTGTGGCAGTTGG; R:5′AACTGCGCAACAAGATGAGC
pu.1 F:5′CCACATCAGCTCTACAACAGGA; R:5′GCAGAAGGTCAAGCAGGAAC
c/ebpα F:5′ACCCATCTACGACAGCCAAG; R:5′GAGTCGCCAAGCTCATCTTC
smad1 F:5′GGCACAGTCAGTCAATCACG; R:5′ATCCTGCCGATGGTACTCTG
smad5 F:5′CTTTGAGGCCGTCTATGAGC; R:5′GGGTGCTTGTCACATCTTGT
ef1α F:CTGGAGGCCAGCTCAAACAT; R:5′ATCAAGAAGAGTAGTACCGCTAGCATTAC
Results
Flow cytometry quantifies hematopoietic cell types from adult zebrafish WKM
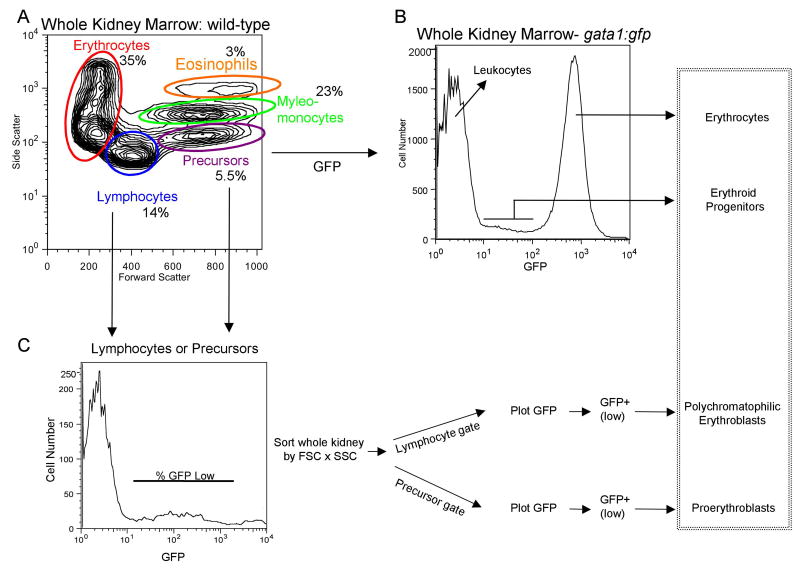
Traver et al. [17] described an approach using forward scatter (FSC) and side scatter (SSC) to separate four major (definitive) blood cell populations from adult zebrafish WKM. We tested this protocol to evaluate steady state hematopoiesis in the wild-type fish used in our laboratory, which are of a distinct genetic background. As shown in Fig. 1A, the following cell populations were purified by FACS: erythrocytes (35%), lymphocytes (14%), precursor cells, consisting of a mixed population of early lineages (6%), and a myelomonocyte population, including granulocytes and monocytes (23%). A small population of myelomonocytic cells (3%) is also distinguished at high side scatter that is comprised of eosinophils (described also in ref. 17). We next confirmed that defined subsets of erythroid progenitors can be purified by FACS using fish that are transgenic for the gata1:gfp reporter [20]. Adult erythrocytes in the WKM (Fig. 1B) and in the peripheral blood (not shown) are strongly GFP positive. In the WKM a second, GFP+ “low” population can be distinguished that contains the erythroid progenitor populations. As described [17] the relative number of proerythroblasts or polychromatophilic erythroblasts can be measured by gating on the percentage of GFP+ (low) cells in the “precursor” or “lymphocyte” populations, respectively (Fig. 1C). The identity of the purified cell types was confirmed by standard histology (Fig. 1D). All relative cell numbers in our wild-type fish are consistent with those from the previous study [17], suggesting that the protocols developed by Traver and colleagues can be generalized across different zebrafish strains.
Fig. 1. Purification of hematopoietic cellular subsets and erythroid progenitors from adult zebrafish.
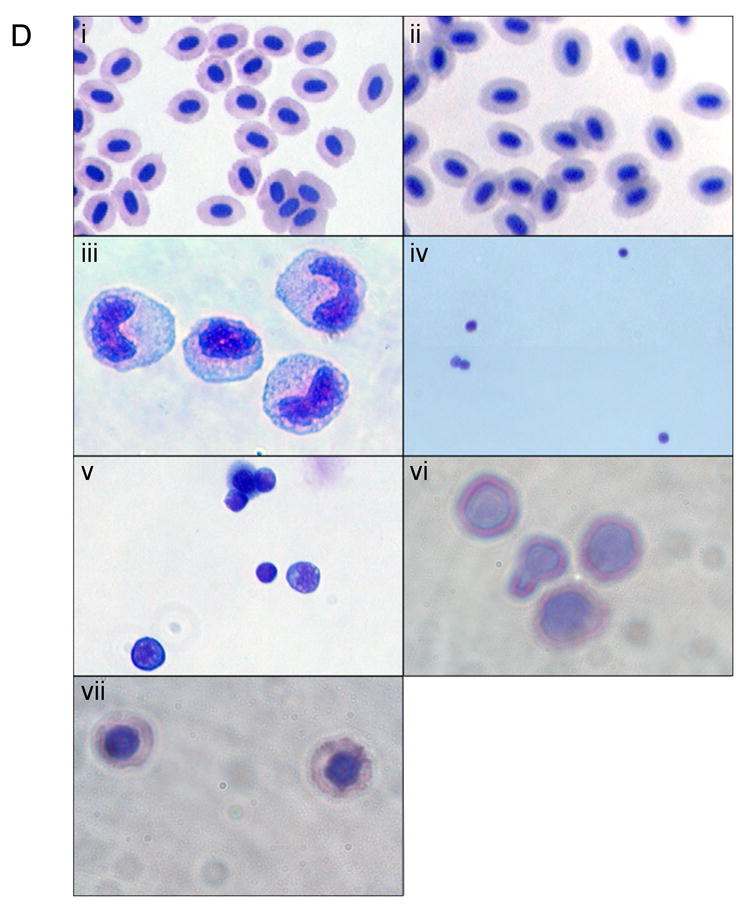
We applied general approaches described by Traver and colleagues [16,17] to purify cellular subsets from wild-type fish in our system, which are of distinct genetic backgrounds compared to those used in the published protocols. (A) Whole kidney marrow (WKM) was separated by flow cytometry (FACS). Dead cells were gated out by propidium iodide (PI) staining and the live cells analyzed by forward and side scatter profiles. The contour plot demonstrates that distinct populations can be identified: erythrocytes, myelo-monocytes, lymphocytes, and precursors. The average percentage of WKM for the populations are shown (n=11): erythrocytes: 35% ±9.2, lymphocytes: 14% ±3.1, precursors: 5.5% ±2.2, myelo-monocytes: 23% ±5.8. In addition, there is a minor eosinophil gate component that gates with higher relative side scatter from the myelo-monocyte group, approximately 3%. (B) FACS analysis of WKM from gata1:gfp reporter fish. A histogram representing relative GFP expression levels in cells from WKM is shown. Three distinct populations can be seen. A GFP negative peak, corresponding to the leukocytes, a bright GFP+ (high) peak corresponding to mature erythrocytes and a GFP+ low population that contains the erythroid progenitors. (C) The erythroid progenitors can be further discriminated using the gating strategy shown, similar to that described [17]. WKM was sorted by forward scatter (FSC), side scatter (SSC) and GFP, then contour plots of FSC vs. SSC were generated, and lymphocyte and precursor gates drawn. From each a GFP histogram was generated, and a GFP+ (low) gate drawn. The relative percent of GFP+ (low) cells was determined and used to track the erythroid progenitor populations. As shown in the box on the far right, these protocols allow accurate counts of adult erythroid cells at different developmental stages. (D) Shown are representative cytospin preparations of sorted cell types, as indicated. Cells were stained with Geimsa and May-Grünwald and photographed with a 100× oil objective. (i) peripheral erythrocytes, (ii) erythrocytes from WKM, (iii) myelo-monocytes, (iv) lymphocytes, (v) precursors, (vi) proerythroblasts, (vii) polychromatophilic erythroblasts.
Development of a model to study stress hematopoiesis in zebrafish
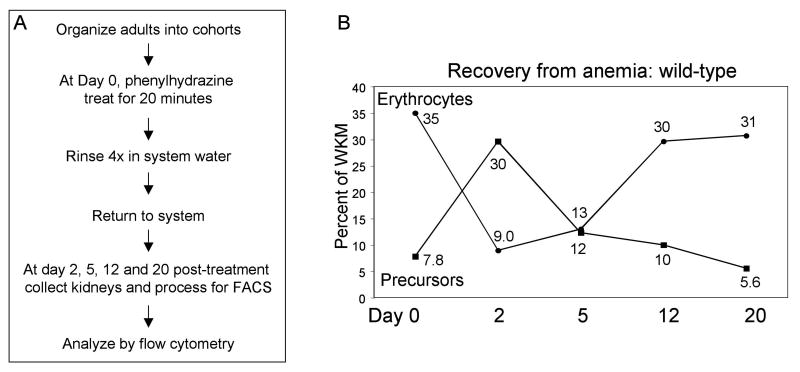
Phenylhydrazine treatment has been used to induce hemolytic anemia in mice, rats, Xenopus and other animals in order to study stress hematopoiesis [28-33]. We established a comparable model in zebrafish, inducing lysis of mature erythrocytes by treating adult fish with phenylhydrazine and defining a time-frame to study recovery (Fig. 2A). All fish survived the procedure, and anemia was induced. At day 2 the circulating blood isolated by cardiac puncture is visibly thin and the spleen is missing, while biliverdin is visible in the body cavity. The normal adult zebrafish spleen is comprised largely of erythroid cells. Phenylhadrazine treatment, resulting in extensive red cell lysis, therefore renders the spleen extremely hypocellular. By day 5, the spleen has recovered and is greatly enlarged compared to untreated fish. The kidney is relatively pale, and some biliverdin is still present in the body cavity. By day 12, the kidney is returning to normal color and the spleen of normal size, and by day 20 full recovery is seen with normal peripheral blood color, a normal kidney, and a normal spleen.
Fig. 2. A protocol for inducing hemolytic anemia in adult zebrafish.
(A) Phenylhydrazine treatment induces hemolytic anemia by lysis of erythrocytes. Gross visible signs of anemia can be seen in the fish at the time of sacrifice, as described in the text. Recovery of the anemia occurs over a 20 day time-course, documented visibly and by flow cytometry. (B) A group of wild-type fish (n=11) were treated with phenylhydrazine at day 0 and followed over a 20 day time course. Fish were sacrificed at days 2, 5, 12 and 20 following treatment and WKM prepared for flow cytometry. Data from matched sets of untreated fish was used to generate the numbers indicated by day 0. Erythrocytes and precursor populations were quantified at each time point. The line graph shows each as a percent of WKM (erythrocytes and precursors). As the erythrocyte count falls there is a reciprocal up-regulation of precursor production to compensate for the induced anemia.
Anemia and recovery was evaluated quantitatively, comparing by FACS cells isolated from WKM of untreated or treated wild-type adults (Fig. 2B). The mature erythrocyte count falls immediately following treatment. A gradual rise in erythrocyte count is seen over the recovery time course. The opposite trend is seen in the precursor population, which rises dramatically in the first two days following phenylhydrazine treatment. This stress-induced erythropoiesis is rapid and robust, with 100% of the fish recovering within three weeks of treatment.
Adult fish with a mutation in the smad5 gene are anemic
We next compared the steady state hematopoietic populations in WKM of fish that are mutant for the smad5 gene, a key mediator of the BMP signaling pathway. An allelic series of zebrafish mutants is available, including piggytail (pgy), caused by a base change in a conserved Asp (→ Glu) residue at position 447 [10], which confers a hypopmorphic allele, expressing a weakly functioning mutant Smad5 protein. The homozygous pgy fish are embryonic lethal with a dorsalized phenotype (C2-3) [11]. The somitabun (sbn) mutation is also homozygous embryonic lethal, but is maternal zygotic dominant and causes a more severe dorsalization (C5) phenotype. The sbn mutant is caused by a single base change at position 429 Thr (→ Ile); it has been proposed that this mutation interferes with the ability of Smad5 to interact with Smad4 [34]. Thus, the sbn mutant represents a significantly stronger allele.
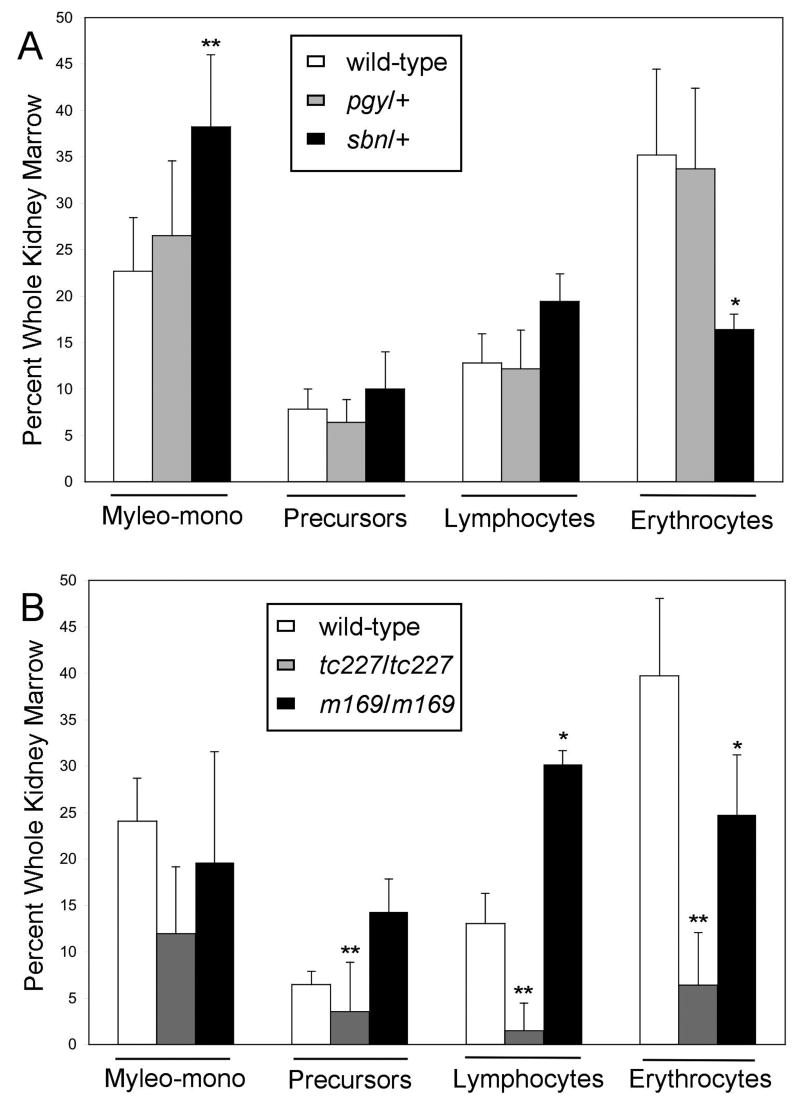
While both homozygous pgy and sbn embryos die by 3 days post-fertilization, the heterozygotes survive to adulthood and therefore the effect of Smad5 partial loss-of-function on definitive hematopoiesis can be determined. For this purpose the WKM derived from adult wild-type, pgy/+ heterozygotes, and sbn/+ heterozygotes were compared by FACS (Fig. 3A). No gross differences are seen at steady state in the WKM derived from pgy/+ adults, for any of the major characterized blood cell populations. In contrast, sbn/+ adults show a significant decrease in relative erythrocyte count (Fig. 3A). The fish carrying one mutant smad5 allele have less than half the normal representation of erythrocytes, 16% as compared to 32% for wild-type or pgy/+. The sbn/+ adults do not have a significant change in the lymphocyte or precursor population as a percent of whole kidney marrow, but there is a corresponding increase in the relative percentage of myelomonocytes. These data indicate that alteration of Smad5 function, as represented by the antimorphic sbn allele, leads to a significant defect in erythropoiesis in adult zebrafish. The WKM can be evaluated quantitatively only for “relative” cell numbers, since total cell numbers vary based on the kidney preparation. However, a defect in erythropoiesis was confirmed by counting erythrocytes in a defined volume of peripheral blood, which again showed a significant decrease in sbn/+ adults (3.56×106 cells/ml) compared to wild-type (4.03×106 cells/ml; P<0.003, n=4). We did not detect a significant change in the numbers of myeloid cells in the peripheral circulation, which were few (data not shown). Although the sbn/+ embryos survive to adulthood, the fish are anemic.
Fig. 3. Adult zebrafish with mutations in smad5 are anemic.
(A) Analysis of heterozygous adults. WKM was collected from adult wild-type, pgy/+ and sbn/+ fish. Samples were analyzed by flow cytometry for FSC and SSC; dead cells were gated out by PI staining. Four populations were resolved: erythrocytes, myelo-monocytes, lymphocytes and precursors. For each genotype, the mean percent of WKM was graphed for erythrocytes, lymphocytes, precursors and myelo-monocytes. P values were calculated by two-tailed student's T-test by comparing wild-type to smad5 mutants for each cell type. Sample numbers for each were: wild-type (n=11), pgy/+ (n=5), sbn/+ (n=5) *P<0.02, **P<0.001. (B) Homozygous mutants rescued from embryonic lethality to adulthood by injection of mRNA encoding Smad5. Two mutant alleles that could be rescued are tc227 (believed to be a null allele) and m169. Analysis was as described above in (A). Sample numbers for each were: wild-type (n=4), tc227/tc227 (n=5), m169/m169 (n=2) *P<0.07, **P<0.03. The rescue of m169/m169 proved very challenging, which precluded generation of sufficient numbers of fish to test rigorously statistical significance, while the trend toward anemia is evident. Meanwhile, the tc227/tc227 mutants represent a stronger allele and shows clear anemia and additional alterations not seen in other mutants, for example in lymphocyte numbers.
A unique advantage of the zebrafish model is that embryonic lethal alleles can sometimes be rescued to adulthood by injection at the one cell stage with mRNA encoding the wild-type gene product. While this was not possible for the sbn or pgy fish, we were able to evaluate hematopoiesis in rescued mutant adults carrying two other smad5 alleles: the captain hook (m169) mutant, which contains an insertion that generates a non-functional protein, and the tc227 allele, which is thought to be null [10]. Both m169/m169 and tc227/tc227 adult fish are anemic, showing that the phenotype is due to loss of smad5 function, and is not specific to the sbn/+ fish (Fig. 3B). In the case of tc227/tc227 fish, other lineages besides erythrocytes are affected, including lymphocytes. We note that smad5 transcripts are readily detected by qPCR of cDNA derived from each of these cell populations isolated from wild-type fish (data not shown).
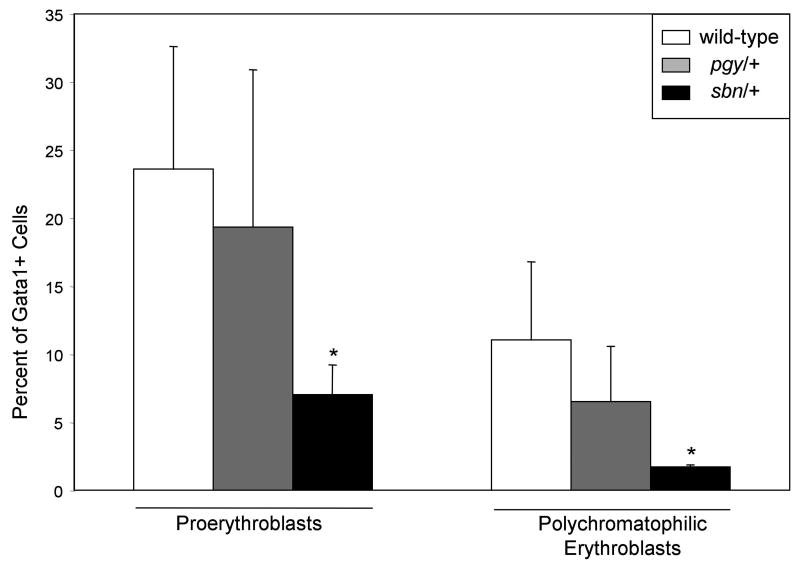
We focused further on the sbn/+ fish, to evaluate where the defect in erythropoiesis occurs. For this purpose, we crossed the gata1:gfp reporter onto the pgy and sbn mutant backgrounds. In the sbn/+ fish both the proerythroblasts and polychromatophilic erythroblast populations are decreased compared to wild-type or pgy/+ adults (Fig. 4). In the WKM of sbn/+ adults only 7% of the “precursor” gate are GPF(low) proerythroblasts, as compared to 24% and 19% for wild-type or pgy/+ fish, respectively. A similar relative decrease in polychromatophilic erythroblasts is seen in the sbn/+ adults, since less than 2% of the cells are GFP(low) in the “lymphocyte” gate, compared to 12% in wild-type fish. This decrease in the number of erythroid progenitors in sbn/+ fish is not due to an overall decrease in hematopoietic precursors, as this population is not changed significantly. The specific decrease in erythroid progenitors is likely the cause of the baseline anemia in sbn/+ fish, and demonstrates a requirement of normal smad5 function for definitive erythropoiesis in zebrafish.
Fig. 4. Sbn/+ heterozygous adults have decreased erythroid progenitors.
WKM was collected from adult wild-type, pgy/+ and sbn/+ fish on the gata1:gfp background. Samples were analyzed by flow cytometry for FSC, SSC and GFP; dead cells were gated out by PI staining. Two erythroid populations were quantified: proerythroblasts and polychromatophilic erythroblasts. For each genotype, the mean percent of progenitors within WKM was graphed for erythrocytes. For proerythroblasts and polychromatophilic erythroblasts the mean percent graphed, indicated by Gata1+ cells on the axis, refers to the percent GFP positive in the “precursor” or “lymphocyte” gate respectively, see Fig. 1 for details. P values were calculated by two-tailed student's T-test by comparing wild-type to smad5 mutants for each cell type. Sample numbers for each were: wild-type (n=8), pgy/+ (n=5), sbn/+ (n=3). *P<0.02.
Fish carrying a mutation in the smad5 gene have an altered response to stress anemia
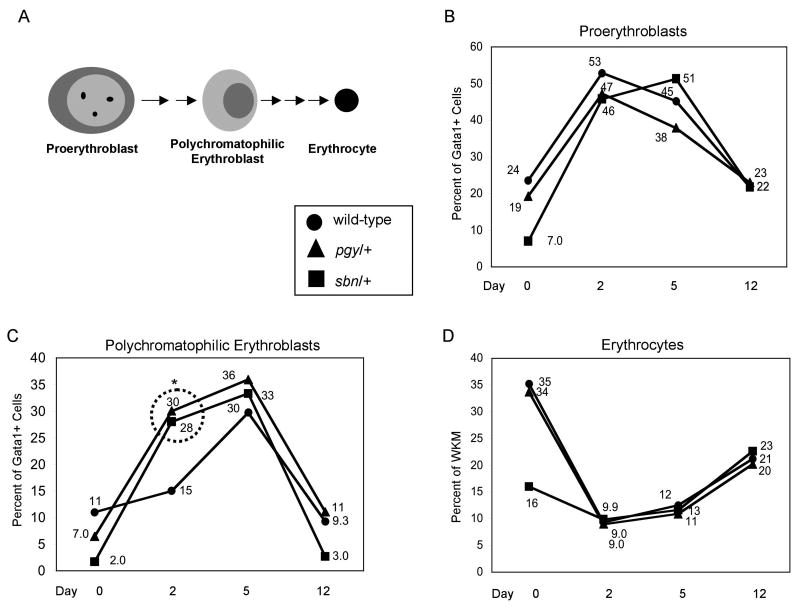
We next evaluated the relative ability of smad5 mutants to respond during recovery from hemolytic anemia. The sbn/+ and pgy/+ fish carrying the gata1:gfp reporter were compared to wild-type fish treated with phenylhydrazine. Fish were sacrificed on days 2, 5 and 12 post-treatment and erythropoiesis was analyzed by FACS (Fig. 5). Both sbn/+ and pgy/+ fish recover from treatment over the time course with full repopulation of the erythrocyte fraction. Proerythroblasts increased to ∼50% in all genotypes at 2 days post-treatment, including in the sbn/+ background that started with significantly fewer proerythroblasts (Fig. 5B). However, the percentage of polychromatophilic erythroblasts, a more mature progenitor cell type, are at abnormally high levels in both sbn and pgy heterozygotes at 2 days post-treatment, representing ∼30% of the gated cell population compared to ∼15% for wild-type fish (Fig. 5C). This accumulation of polychromatophilic erythroblasts does not however lead to an overproduction of mature erythrocytes (Fig. 5D). The aberrant pattern indicates that erythroid progenitors with a smad5 mutant allele advance precociously to the polychromatophilic stage, or are unable to progress efficiently past this stage at a normal rate.
Fig. 5. Pgy/+ and Sbn/+ heterozygous adults have an altered response to stress anemia.
(A) Scheme of erythroid development after commitment to the erythroid lineage. These three populations can be isolated using the gata1:gfp transgenic reporter line, as indicated in Fig. 1. (B-D) A cohort of wild-type, sbn/+ and pgy/+ adults carrying the gata1:gfp reporter transgene were treated with phenylhydrazine at day 0. On days 2, 5, and 12, adults were sacrificed, WKM prepared and samples analyzed by FACS. Dead cells were gated out using PI staining. The live cells were analyzed by forward scatter, side scatter, and GFP. Data from matched sets of untreated fish was used to generate the numbers indicated by day 0. The percent of each sub-population of erythroid progenitors was determined by gating on either the lymphoid or precursor fraction and then determining the number of GFP low positive cells in that fraction (see Fig. 1). The percentages were then graphed and P values were determined by two-tailed student's T-Test. For each time point, treated smad5 mutants were compared to age-matched sibling and treated wild-types. P values were as follows: polychromatophilic erythroblasts (day 2), for pgy/+ P<0.001, for sbn/+ P<0.02. For each time point, wild-type n=9, pgy/+ n=6 and sbn/+ n=3. As indicated in the boxed insert: ●=wild-type, ▲=pgy/+ ■=sbn/+. In B and C, the percent of Gata1+ cells refers to the “leukocyte” or “precursor” gate, respectively.
We investigated this question further by evaluating the kinetics caused by induced hemolysis through FACS analysis at an earlier time point following phenylhydrazine treatment. The experiment was repeated and whole kidney marrow was prepared from wild-type and pgy/+ on the gata1:gfp reporter background at one or two days post-treatment. In addition, the cells were co-stained with DAPI and Annexin V, in order to quantify relative levels of apoptosis in defined cell populations. Interestingly, the number of apoptotic proerythroblasts is similar at day 1 and day 2 in both wild-type and pgy/+ fish (approximately 45 and 32% each, on day 1 and day 2, respectively). However, the number of apoptotic polychromatophilic erythroblasts is markedly fewer in the pgy/+ fish on day 1 (25% in pgy/+ compared to 52% in wild-type), which provides a likely explanation for the overproduction of polychromatophilic erythroblasts in the pgy/+ fish at day 2 (data not shown). By day 2 the percentage of apoptotic polychromatophilic erythroblasts falls precipitously and is the same in both genotypes (2%, data not shown), although the percent of polychromatophilic erythroblasts in total WKM is much higher in the pgy/+ (Fig. 5C). Since ultimately there is not an overproduction of mature erythrocytes, it seems likely that the excessive polychromatophilic erythroblasts die in the pgy/+ fish at a later stage of development. This compensation allows for full recovery of the pgy/+ mutants despite abnormal developmental kinetics.
Expression of a dominant-negative BMP receptor isoform in Gata1+ cells
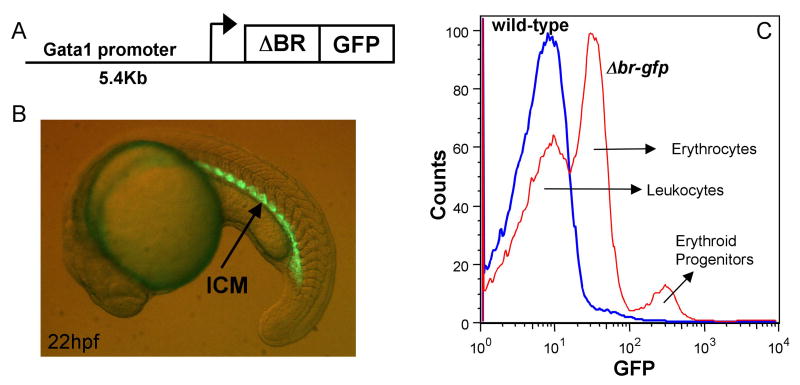
An alternative approach to study deregulated BMP signaling is by expression of a dominant-negative receptor. We showed previously that expression of a type II BMP receptor truncated to lack the kinase domain (ΔBR) is sufficient to blocking BMP signaling during gastrulation, and this inhibits erythropoiesis [9]. In contrast, expression of the same receptor in committed cells of the hemato-vascular system does not block erythropoiesis, and can even enhance it [24]. We therefore generated a transgenic line of fish expressing a ΔBR-GFP fusion isoform in Gata1+ cells, and confirmed that such embryos survive. The construct used for transgenesis is depicted in Fig. 6A. In embryos derived from the gata1:Δbr-gfp transgenic line, GFP expression is seen as expected beginning around 12 hpf in two bilateral stripes of the lateral mesoderm, and then in the ICM, the site of primitive erythropoiesis (Fig. 6B). These embryos appear similar to gata1:gfp transgenic fish, with GFP expression restricted to the early hematopoietic progenitors [20].
Fig. 6. A mutant receptor isoform is expressed in progenitors of a gata1:Δbr-gfp transgenic line.
(A) The diagram represents the construct used to generate the gata1:Δbr-gfp transgenic line. A 5.4 kb promoter sequence was cloned upstream of a fusion construct encoding a truncated BMP receptor with a C-terminal GFP tag. (B) A representative example of a gata1:Δbr-gfp embryo at 22 hpf viewed under fluorescence. The embryo shows GFP expression (arrow) in the intermediate cell mass (ICM), which recapitulates the endogenous gata1 expression pattern. GFP expression is significantly down-regulated as the cells differentiate (not shown). (C) WKM from homozygous adult gata1:Δbr-gfp fish was analyzed by FACS along with WKM from wild-type (non-transgenic) fish. Samples show three distinct patterns for GFP expression. An overlay histogram of GFP expression is shown and the corresponding cell types listed, wild-type (blue), and gata1:Δbr-gfp (red). Wild-type WKM cells have no GFP expression. The gata1:Δbr-gfp WKM shows a different profile of GFP expression in mature erythrocytes compared to gata1:gfp adults, because the fusion protein is down-regulated as erythroid cells differentiate. GFP is expressed at a low level in the progenitor cells of gata1:Δbr-gfp, while very low levels of GFP are expressed in the mature erythrocytes. These cell types were confirmed by sorting and cytospins (data not shown). Although not shown here (see Fig. 1B) WKM from gata1:gfp adults shows high GFP expression in mature erythrocytes and low GFP expression in the progenitors.
The ΔBR-GFP fusion protein is functional for blocking BMP signaling, since injection of RNA encoding the isoform is sufficient to dorsalize embryos (data not shown). Yet the transgenic embryos showed no obvious abnormal phenotype. In contrast to gata1:gfp fish, there is a marked decrease in GFP expression at approximately 22-24 hpf. This was seen in multiple independent lines. We suspect this phenomenon reflects a post-translational decline of the ΔBR-GFP fusion protein, perhaps mediated by receptor degradation in differentiating erythroid cells. This down-regulation of the mutant receptor might represent a normal process of receptor recycling during cell differentiation. This is consistent with previous data showing erythroid differentiation is not blocked by expression of the mutant receptor in the erythroid lineage [24], and fortuitously permitted us to establish lines of fish that can tolerate expression of the mutant receptor isoform in Gata1+ progenitors. Indeed, we found that GFP+ cells can be sorted from adult gata1:Δbr-gfp WKM, and these can be used to analyze comparable cell populations derived from gata1:gfp WKM (see Fig. 1C). The same populations can be isolated, although from the gata1:Δbr-gfp WKM they sort differently according to relative GFP expression levels, since in these samples the erythrocytes are present in a very low GFP population, below the erythroid progenitors (Fig. 6C).
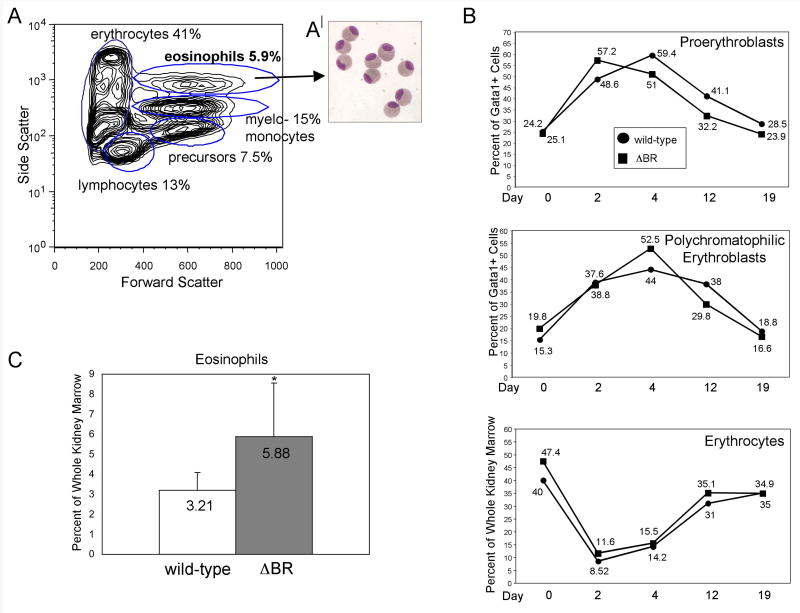
Steady state and stress erythropoiesis is not affected in gata1:Δbr-gfp adult zebrafish; however they display eosinophilia
Analysis of WKM from the gata1:Δbr-gfp fish showed that erythrocytes were present at normal levels (Fig. 7A). The fish were treated with phenylhydrazine and evaluated for recovery from hemolytic anemia (Fig. 7B). The treated fish were rendered severely anemic and all of the fish recovered to baseline during the time course analyzed. The gata1:gfp and gata1:Δbr-gfp fish recovered at similar rates with similar population profiles, indicating that expression of the mutant receptor isoform in erythroid progenitors does not alter erythroid differentiation. However, sorting of hematopoietic cells from WKM showed a consistent alteration of the myelomonocyte side scatter high population (Fig. 7A), representing eosinophils. This population is approximately 6% of total cells in the transgenic WKM, as compared to only 3% for a comparable population derived from WKM of wild-type or gata1:gfp fish (Fig. 7C, P<0.007). The cells were sorted from WKM of gata1:Δbr-gfp fish, centrifuged onto slides and stained, which confirmed they are eosinophils (Fig. 7A|), appearing identical to those isolated by Traver et al. [17] from the gata2:gfp transgenic line.
Fig. 7. The gata1:Δbr-gfp adults have a normal response to stress anemia, but increased eosinophil production in WKM.
(A) A contour plot from FSC vs. SSC analysis of gata1:Δbr-gfp adult WKM is shown. While the cell numbers of the four major sub-populations are relatively normal, a fifth side scatter high (very granular) population of cells can be discriminated in these fish, representing eosinophils. (B) Cohorts of wild-type (gata1:gfp) and gata1:Δbr-gfp adults were treated with phenylhydrazine at day 0. On days 2, 4, 12 and 19 adults were sacrificed and WKM prepared and analyzed by FACS. Dead cells were gated out using DAPI staining, and the live cells were analyzed by forward scatter, side scatter and GFP. The percent of each sub-population of erythroid progenitors was determined by gating on either the lymphoid or precursor fraction and then determining the number of GFP(low) positive in that fraction. No significant changes were seen when comparing wild-type and gata1:Δbr-gfp fish. For each time point n=4. As indicated in the insert: ●=wild-type ■=gata1:Δbr-gfp. (C) Comparison of WKM sorts with wild-type (gata1:gfp) shows a relative increase in the number of eosinophils in WKM of gata1:Δbr-gfp adults; for each n=16, P<0.007. (A′) This population of cells was collected, centrifuged onto slides and stained with Giemsa and May-Grünwald to confirm their identification as eosinophils.
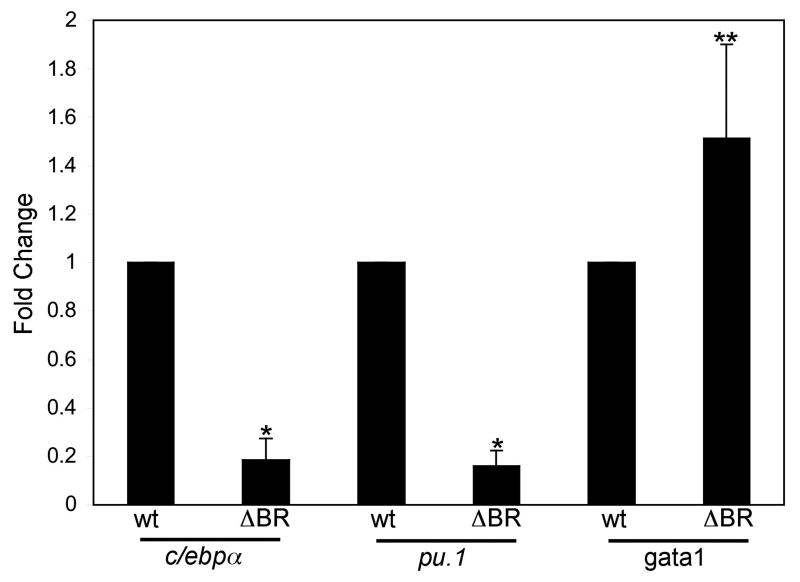
To gain insight into the cause of eosinophilia, we isolated comparable populations of wild-type and transgenic erythroid progenitors, identified by low expression of GFP, low side scatter and intermediate forward scatter as shown above (Fig. 1B). The corresponding population of erythroid progenitors was isolated from WKM of gata1:Δbr-gfp fish, avoiding the “very low” population that is comprised of erythrocytes (Fig. 6C). Purity was confirmed after sorting by cytospin histology (not shown). Total cDNA was synthesized from each sample and analyzed by quantitative real-time PCR to measure transcript levels for known key myelo-erythroid transcription factors. The relative expression levels were decreased significantly for both c/epbα (82% reduced) and pu.1 (84% reduced) in the samples derived from gata1:Δbr-gfp progenitors (Fig. 8). In contrast, relative gata1 transcript levels were enhanced by 51% in samples derived from the gata1:Δbr-gfp progenitors. The results indicate that expression of the mutant BMP receptor in an early Gata1+ progenitor population alters the expression of key transcription factors and corresponds with altered eosinophil output.
Fig. 8. QPCR analysis shows alterations in the expression levels for key regulatory genes in gata1:Δbr-gfp progenitors.
Gata1 (GFP+) progenitors were isolated from wild-type (gata1:gfp) adult WKM, and a corresponding population of Gata1+ progenitors was isolated from gata1:Δbr-gfp adult WKM by FACS, with DAPI-based exclusion of dead cells. Total RNA was isolated from these cells and cDNA synthesized. QPCR was performed for analyzing expression levels of RNA from c/ebpα, pu.1 and gata1. The data was processed using the 2-ΔΔCT method [26]. The median of each sample was normalized to wild-type (gata1:gfp) and the median average was graphed as fold change in RNA expression. Triplicates were performed in each of 4 independent experiments. P values were determined by T-test, * P<0.001, ** P<0.02.
Discussion
Mouse and fish genetics have shown the importance for BMP signaling and Smad5 in hematopoiesis, but these studies have been mostly limited to studying early development, since the defects are embryonic lethal. This limitation is particularly relevant to hematopoiesis, because the adult stage progenitors develop in a distinct temporal and spatial wave during development. There is information derived from cell culture studies. BMPs regulate the proliferation and differentiation of human hematopoietic stem cells isolated in vitro from umbilical cord blood [14]. Human embryonic stem cells treated with BMP4 form hematopoietic progenitors with an increased capacity for self-renewal compared to untreated cell [15]. Also, the Smad5 gene is required to block induction of globin mRNA and effectively inhibit hematopoietic progenitor growth in day 4 embryoid body cultures treated with TGFβ1 [6].
Two sets of studies have analyzed directly the role of SMAD5 in adult murine hematopoiesis. The flexed-tail mouse contains a spontaneously derived mutation in the Smad5 gene and the animals display a specific defect in the ability of a resident splenic population of erythropoietin responsive progenitors to mount an effective recovery from stress anemia [35]. The normal population of splenic stress-erythroid progenitors are responsive to BMP4, unlike bone marrow derived erythroid progenitors [35]. Second, a conditional mutation of Smad5 that depletes a floxed allele using Mx-Cre showed no significant changes in the adult murine hematopoietic system [36]. This latter study differs from results we report here studying smad5 mutations in adult zebrafish.
We find that Smad5 regulates erythropoiesis in adult zebrafish, demonstrated by the anemia and the decreased numbers of erythroid progenitors in the sbn/+ and the tc227/tc227 adult fish. There are several possible explanations for this difference compared with the mouse. First, hematopoiesis may be regulated differently in the zebrafish kidney marrow compared to mammalian bone marrow. Second, SMAD5 may be better compensated, for example by SMAD1, in the mouse compared to the zebrafish. We note that, in three independent experiments, we were unable to detect RNA encoding Smad1 in samples derived from proerythroblast or polychromatophilic erythroblasts, suggesting limited potential for compensation at these stages in zebrafish. In contrast, Smad1 was readily detected both in sorted precursor cells and in mature erythroid cells, and levels were found to be equivalent to those in samples from whole kidney marrow (data not shown)
Third, in our set of experiments the smad5 gene is mutant throughout the life of the animal, rather than being lost acutely as in the mouse studies. This might reflect developmental defects in the niche that subsequently impact hematopoiesis. The smad5 mutants likely function non-cell-autonomously, since transplantation studies [34] showed that smad5 mutant cells survive in a wild-type environment and can form blood. Indeed, in contrast to the conditional Smad5 knockout results, an earlier conditional knockout of BmprIa is sufficient to alter the adult bone marrow niche and deregulate HSC numbers [37].
We also found that Smad5 regulates the kinetics of recovery from hemolytic anemia, which is interesting in the context of altered hematopoiesis shown in the flexed-tail mouse. The mutant mice are anemic neonatally but recover in adulthood, except under conditions of stress hematopoiesis [35,38]. It is not known if stress hematopoiesis in fish is comparable to the program that occurs in mouse spleen in response to anemia. However, as in the mouse, the kinetics of erythroid recovery following stress anemia in zebrafish is dependent on Smad5 function. We note that pgy/+ fish do not show steady state defects in erythropoiesis, although (just like sbn/+ fish) they do show overproduction of polychromatophilic erythroblasts following phenylhydrazine treatment. We suspect that the difference is due to allele strength and that the two phenotypes have different threshold requirements for levels of Smad signaling. The sbn allele is stronger than the pgy allele, and has even been suggested to be dominant-negative. The pgy fish, harboring the weaker allele, are apparently able to compensate at the steady state level. However, the phenylhydrazine treatment is an acute insult, and thus may represent a lower threshold for generating the phenotypes described.
Finally, we found that the expression of a mutant BMP receptor in Gata1+ progenitors alters the normal numbers of eosinophils. Eosinophils were identified and isolated previously from adult WKM [18,39,40]. Our study suggests that decreased levels of BMP signaling in an early Gata1+ progenitor increases commitment toward the eosinophil fate. One possibility is that decreased levels of BMP signaling in the multipotent progenitor leads to changes in Gata1 levels. Even a modest increase in Gata1 could lead to subsequent decreases in Pu.1 and/or C/ebpα, and it is known that this network can control lineage output, as shown previously with respect to erythrocytes and macrophages [41-43]. The mutant BMP receptor provides a transient inhibition to BMP signaling in a narrow window of progenitor development. For technical reasons we have so far been unable to measure reliably changes in the levels of nuclear phospho-Smads in the defined sorted cell populations, although this would provide insight and is an important future goal. We speculate that there is no erythroid phenotype in the ΔBR-GFP fish because the mutant receptor is down-regulated post-translationally during erythroid development (which we confirmed by evaluating GFP expression). This down-regulation might reflect the normal process of receptor cycling and allow recovery of normal erythroid development. In contrast, the sbn mutant is an alteration of the downstream target, Smad5, and is also not restricted to hematopoietic cells. Overall, our study demonstrates feasibility of using the zebrafish model to probe molecular alterations that effect not only embryonic hematopoiesis, but also the adult definitive lineages.
Supplementary Material
Acknowledgments
We are indebted to David Traver (University of California, San Diego, CA) for providing the flow cytometry protocol prior to publication and for continued assistance in trouble-shooting, which made the study possible. We thank Robert Paulson (Pennsylvania State University, University Park, PA) for sharing the flexed-tail mouse data prior to publication, which inspired this study. We thank Barry Paw (Harvard Medical School, Boston, MA) for providing information regarding phenylhydrazine treatment. The authors are also very thankful to Kristie Gordon for assistance with flow cytometry, trouble-shooting and key observations. We thank William King for flow cytometry assistance, Nadia Severina for assistance with screening and genotyping and Spartak Kalinin for zebrafish care. We also thank members of the Evans Laboratory for critical analysis of the data and helpful suggestions. All authors contributed significantly to this work. L.J.M. designed the study, performed the experiments and helped write the manuscript. J.T. provided the embryonic-rescued adult homozygous mutant fish. M.C.M helped design the study and provided essential mutant strains. T.E. designed the study and wrote the manuscript. This work was supported by a grant to T.E. from the National Institutes of Health (HL056182) and M.C.M from the National Institutes of Health (GM56326). L.J.M. is supported by an MSTP training grant (T32 GM007288).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thisse C, Zon LI. Organogenesis--heart and blood formation from the zebrafish point of view. Science. 2002;295:457–462. doi: 10.1126/science.1063654. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein BM, Schier AF, Abdelilah S, Malicki J, Solnica-Krezel L, Stemple DL, Stainier DY, Zwartkruis F, Driever W, Fishman MC. Hematopoietic mutations in the zebrafish. Development. 1996;123:303–309. doi: 10.1242/dev.123.1.303. [DOI] [PubMed] [Google Scholar]
- 3.Lyons SE, Lawson ND, Lei L, Bennett PE, Weinstein BM, Liu PP. A nonsense mutation in zebrafish gata1 causes the bloodless phenotype in vlad tepes. Proc Natl Acad Sci U S A. 2002;99:5454–5459. doi: 10.1073/pnas.082695299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 5.Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Sun Y, Jiang F, Zhang S, Wu Y, Lan Y, Yang X, Mao N. Disruption of Smad5 gene leads to enhanced proliferation of high-proliferative potential precursors during embryonic hematopoiesis. Blood. 2003;101:124–133. doi: 10.1182/blood-2002-02-0398. [DOI] [PubMed] [Google Scholar]
- 7.Walters MJ, Wayman GA, Notis JC, Goodman RH, Soderling TR, Christian JL. Calmodulin-dependent protein kinase IV mediated antagonism of BMP signaling regulates lineage and survival of hematopoietic progenitors. Development. 2002;129:1455–1466. doi: 10.1242/dev.129.6.1455. [DOI] [PubMed] [Google Scholar]
- 8.Adelman CA, Chattopadhyay S, Bieker JJ. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free media. Development. 2002;129:539–549. doi: 10.1242/dev.129.2.539. [DOI] [PubMed] [Google Scholar]
- 9.Schmerer M, Evans T. Primitive erythropoiesis is regulated by Smad-dependent signaling in postgastrulation mesoderm. Blood. 2003;102:3196–3205. doi: 10.1182/blood-2003-04-1094. [DOI] [PubMed] [Google Scholar]
- 10.Kramer C, Mayr T, Nowak M, Schumacher J, Runke G, Bauer H, Wagner DS, Schmid B, Imai Y, Talbot WS, Mullins MC, Hammerschmidt M. Maternally supplied Smad5 is required for ventral specification in zebrafish embryos prior to zygotic Bmp signaling. Dev Biol. 2002;250:263–279. [PubMed] [Google Scholar]
- 11.Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Nusslein-Volhard C. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- 12.McReynolds LJ, Gupta S, Figueroa ME, Mullins MC, Evans T. Smad1 and Smad5 differentially regulate embryonic hematopoiesis. Blood. 2007;110:3881–3890. doi: 10.1182/blood-2007-04-085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadlon TJ, Lewis ID, D'Andrea RJ. BMP4: its role in development of the hematopoietic system and potential as a hematopoietic growth factor. Stem Cells. 2004;22:457–474. doi: 10.1634/stemcells.22-4-457. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick JE. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 1999;189:1139–1148. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 16.Traver D. Cellular dissection of zebrafish hematopoiesis. Methods Cell Biol. 2004;76:127–149. doi: 10.1016/s0091-679x(04)76008-2. [DOI] [PubMed] [Google Scholar]
- 17.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 18.de Jong JL, Zon LI. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu Rev Genet. 2005;39:481–501. doi: 10.1146/annurev.genet.39.073003.095931. [DOI] [PubMed] [Google Scholar]
- 19.Galloway JL, Zon LI. Ontogeny of hematopoiesis: examining the emergence of hematopoietic cells in the vertebrate embryo. Curr Top Dev Biol. 2003;53:139–158. doi: 10.1016/s0070-2153(03)53004-6. [DOI] [PubMed] [Google Scholar]
- 20.Long Q, Meng A, Wang H, Jessen JR, Farrell MJ, Lin S. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124:4105–4111. doi: 10.1242/dev.124.20.4105. [DOI] [PubMed] [Google Scholar]
- 21.Westerfield M. The Zebrafish Book. Eugene: Eugene; 2000. [Google Scholar]
- 22.Wang H, Long Q, Marty SD, Sassa S, Lin S. A zebrafish model for hepatoerythropoietic porphyria. Nat Genet. 1998;20:239–243. doi: 10.1038/3041. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Evans T. BMP-like signals are required after the midblastula transition for blood cell development. Dev Genet. 1996;18:267–278. doi: 10.1002/(SICI)1520-6408(1996)18:3<267::AID-DVG7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Zhu H, Zon LI, Evans T. BMP signaling restricts hemato-vascular development from lateral mesoderm during somitogenesis. Development. 2006;133:2177–2187. doi: 10.1242/dev.02386. [DOI] [PubMed] [Google Scholar]
- 25.Meng A, Jessen Jason R, Lin Shuo. The Zebrafish- Genetics and Genomics: Transgenesis. San Diego: San Diego; 1999. [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Sawyer SJ, Gerstner KA, Callard GV. Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: gene specific tissue distribution, sex differences, developmental programming, and estrogen regulation. Gen Comp Endocrinol. 2006;147:108–117. doi: 10.1016/j.ygcen.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Criswell KA, Sulkanen AP, Hochbaum AF, Bleavins MR. Effects of phenylhydrazine or phlebotomy on peripheral blood, bone marrow and erythropoietin in Wistar rats. J Appl Toxicol. 2000;20:25–34. doi: 10.1002/(sici)1099-1263(200001/02)20:1<25::aid-jat624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Hodges VM, Winter PC, Lappin TR. Erythroblasts from friend virus infected- and phenylhydrazine-treated mice accurately model erythroid differentiation. Br J Haematol. 1999;106:325–334. doi: 10.1046/j.1365-2141.1999.01535.x. [DOI] [PubMed] [Google Scholar]
- 30.DeSimone J, Biel SI, Heller P. Stimulation of fetal hemoglobin synthesis in baboons by hemolysis and hypoxia. Proc Natl Acad Sci U S A. 1978;75:2937–2940. doi: 10.1073/pnas.75.6.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chegini N, Aleporou V, Bell G, Hilder VA, Maclean N. Production and fate of erythroid cells in anaemic Xenopus laevis. J Cell Sci. 1979;35:403–415. doi: 10.1242/jcs.35.1.403. [DOI] [PubMed] [Google Scholar]
- 32.Huebers HA, Eng M, Graham TC, Deeg HJ, Storb R, Finch CA. Erythropoietic reserve in marrow-transplanted dogs. Exp Hematol. 1986;14:97–100. [PubMed] [Google Scholar]
- 33.McMullin MF, Lappin TR, Elder GE, Taylor T, Bridges JM. Serum erythropoietic activity in acute anemia--an animal model. Biochem Med Metab Biol. 1989;41:30–35. doi: 10.1016/0885-4505(89)90005-4. [DOI] [PubMed] [Google Scholar]
- 34.Hild M, Dick A, Rauch GJ, Meier A, Bouwmeester T, Haffter P, Hammerschmidt M. The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development. 1999;126:2149–2159. doi: 10.1242/dev.126.10.2149. [DOI] [PubMed] [Google Scholar]
- 35.Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105:2741–2748. doi: 10.1182/blood-2004-02-0703. [DOI] [PubMed] [Google Scholar]
- 36.Singbrant S, Moody JL, Blank U, Karlsson G, Umans L, Zwijsen A, Karlsson S. Smad5 is dispensable for adult murine hematopoiesis. Blood. 2006;108:3707–3712. doi: 10.1182/blood-2006-02-003384. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 38.Gregory CJ, McCulloch EA, Till JE. The cellular basis for the defect in haemopoiesis in flexed-tailed mice. III. Restriction of the defect to erythropoietic progenitors capable of transient colony formation in vivo. Br J Haematol. 1975;30:401–410. doi: 10.1111/j.1365-2141.1975.tb01854.x. [DOI] [PubMed] [Google Scholar]
- 39.Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, Langenau DM, Delahaye-Brown A, Zon LI, Fleming MD, Look AT. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- 40.Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood. 2001;98:3087–3096. doi: 10.1182/blood.v98.10.3087. [DOI] [PubMed] [Google Scholar]
- 41.McNagny K, Graf T. Making eosinophils through subtle shifts in transcription factor expression. J Exp Med. 2002;195:F43–47. doi: 10.1084/jem.20020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNagny KM, Sieweke MH, Doderlein G, Graf T, Nerlov C. Regulation of eosinophil-specific gene expression by a C/EBP-Ets complex and GATA-1. Embo J. 1998;17:3669–3680. doi: 10.1093/emboj/17.13.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nerlov C, McNagny KM, Doderlein G, Kowenz-Leutz E, Graf T. Distinct C/EBP functions are required for eosinophil lineage commitment and maturation. Genes Dev. 1998;12:2413–2423. doi: 10.1101/gad.12.15.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.