Abstract
Glomerular injury is often characterized by the effacement of podocytes, loss of slit diaphragms, and proteinuria. Renal ischemia or the loss of blood flow to the kidneys has been widely associated with tubular and endothelial injury but rarely has been shown to induce podocyte damage and disruption of the slit diaphragm. In this study, we have used an in vivo rat ischemic model to demonstrate that renal ischemia induces podocyte effacement with loss of slit diaphragm and proteinuria. Biochemical analysis of the ischemic glomerulus shows that ischemia induces rapid loss of interaction between slit diaphragm junctional proteins Neph1 and ZO-1. To further understand the effect of ischemia on molecular interactions between slit diaphragm proteins, a cell culture model was employed to study the binding between Neph1 and ZO-1. Under physiologic conditions, Neph1 co-localized with ZO-1 at cell-cell contacts in cultured human podocytes. Induction of injury by ATP depletion resulted in rapid loss of Neph1 and ZO-1 binding and redistribution of Neph1 and ZO-1 proteins from cell membrane to the cytoplasm. Recovery resulted in increased Neph1 tyrosine phosphorylation, restoring Neph1 and ZO-1 binding and their localization at the cell membrane. We further demonstrate that tyrosine phosphorylation of Neph1 mediated by Fyn results in significantly increased Neph1 and ZO-1 binding, suggesting a critical role for Neph1 tyrosine phosphorylation in reorganizing the Neph1-ZO-1 complex. This study documents that renal ischemia induces dynamic changes in the molecular interactions between slit diaphragm proteins, leading to podocyte damage and proteinuria.
Glomerular diseases of the kidney are the major cause of end stage renal disease worldwide. Diseases, including diabetes, hypertension, and ischemia, are associated with glomerular dysfunction that leads to proteinuria and loss of kidney function. Renal ischemia-reperfusion injury is the major cause of acute renal failure as observed in the native kidneys and after renal transplantation with a more than 50% mortality rate (1, 2). There is no specific treatment for such a devastating disease, primarily due to lack of understanding of the pathophysiology and the mechanism of progression of this disease. Identification of major cellular pathways affected by ischemia-reperfusion is an active area of research and has revealed many molecular targets for therapeutic intervention (3–5).
Glomerular podocytes contribute to the kidney filtration barrier and have a footlike appearance of numerous interdigitating processes that arise from their cell bodies and completely cover the glomerular basement membrane. The interdigitation of foot processes is linked via 40 nm wide porous slits commonly known as slit diaphragms (6). These are specialized cell junctions that constitute an important component of the glomerular filtration barrier, restricting the passage of proteins and solutes into urine. Increasing evidence now suggests that injuries to the glomerulus leads to podocyte effacement and loss of slit diaphragm structure/function, resulting in proteinuria. Although a significant amount of work has been done to study the effects of renal ischemia on kidney tubules and endothelium (2), relatively less is known about the effects of ischemia on glomerular podocytes. Moreover, ischemia-induced changes in the molecular organization of slit diaphragm proteins have never been studied.
Recent efforts to understand the mechanisms underlying the loss of slit diaphragm have led to the identification of a number of proteins that are specifically localized at the foot process in podocytes. It is believed that the slit diaphragm is formed by homophilic and heterophilic interactions between the extracellular domains of various podocyte-specific proteins, including Nephrin, Neph1, Fat1, and Fat2 (6). Proteins such as ZO-1, CD2AP, Nck, and Grb2 are thought to connect the slit diaphragm membrane proteins to the actin microfilaments (7, 8). In addition to their ability to provide structural integrity to the slit diaphragm, recent reports suggest that these proteins also participate in the important signaling events that help maintain the physiologic glomerular filtration function (8–10). The present study provides evidence that the ischemic injury induces dynamic changes in the protein complexes at the slit diaphragm with a corresponding loss of actin cytoskeleton and proteinuria.
Neph1, a member of the immunoglobulin superfamily, is structurally related to nephrin (11) and consists of five extracellular immunoglobulin-like repeats, followed by a transmembrane domain and a cytoplasmic domain of ∼198–235 amino acids (12). In the glomerulus, Nephrin and Neph1 are expressed in podocytes and are co-localized at the insertion site of the slit diaphragm (13, 14). Unlike Nephrin, which is primarily localized in the kidney, Neph1 is also found in other tissues, including the brain, heart, and liver (11). Genetic analysis of the NEPH1 gene suggests that it belongs to a family of three closely related proteins (Neph1, Neph2, and Neph3) that bind to the carboxyl-terminal domain of podocin. Although little characterization of Neph1 has been published, deletion of mouse Neph1 results in a podocyte effacement phenotype with proteinuria and early postnatal death similar to Nephrin (11). It is widely believed that the extracellular domains of Neph1 and Nephrin form a complex that is located at the foot process and together provide a structural framework for the slit diaphragm. Interaction of Neph1 and Nephrin at the intracellular level with various actin-associated proteins, including CD2AP, ZO-1, CASK, IQGAP1, β-arrestin, Nck, Grb2, α-actinin 4, and Synaptopodin, suggests their participation in transducing signals that induce actin polymerization (6, 15, 16). Indeed, engagement of the extracellular domains of either Nephrin or Neph1 results in Fyn-mediated tyrosine phosphorylation of these proteins on specific tyrosine residues and recruitment of various cytoskeleton proteins, including Nck and Grb2 (8, 17, 18). Whereas Nck recruitment to Nephrin was necessary for actin polymerization, Grb2 recruitment to Neph1 was required for actin polymerization (8, 17). Interestingly, it was found that Nephrin and Neph1 act cooperatively to induce actin reorganization in a cell culture model system (17).
Apart from its interaction with adaptor protein Grb2, through a conserved Src homology 2 binding site, the cytoplasmic tail of Neph1 interacts with the PDZ1 domain of ZO-1 (9, 19). ZO-1 is a 225-kDa multidomain, membrane-associated adaptor protein of the MAGUK protein family (20). ZO-1 plays a role by sealing tight junctions and organizing the components of the tight junction and linking them to the cortical actin cytoskeleton (20–22). ZO-1 is localized at the cytoplasmic face of intercellular junctions and associates with proteins such as ZO-2, F-actin, α-actinin 4, α- and β-catenins, and occludins (19, 21). Therefore, ZO-1 may function in organizing signal transduction and transmembrane protein complexes through its association with Neph1 at the slit diaphragm. Studies from various epithelial cell lines suggest that alterations in ZO-1 expression, localization, and phosphorylation are associated with increased paracellular permeability (23–25). Similar changes in ZO-1 expression and localization were recently reported in a diabetic nephropathy mouse model, where it was also associated with proteinuria and loss of glomerular function (25). Various studies now suggest that the foot processes of podocytes respond to glomerular injury in a dynamic fashion by regulating their associated protein complexes, thus resulting in reorganization of the actin cytoskeleton (6, 15, 26–28). Therefore, it is likely that glomerular injury affects the interactions of and distribution of ZO-1, resulting in loss of slit diaphragm structure. Since interaction of ZO-1 with Neph1 links Neph1 to the cytoskeleton, we wanted to examine the possibility that glomerular injury leads to the dissociation of this complex.
In this study, we demonstrate that renal ischemia induces significant podocyte effacement with a corresponding loss of interaction between slit diaphragm proteins Neph1 and ZO-1. The recovery from injury induces Neph1 tyrosine phosphorylation and restores this interaction. Our results further suggest a critical role for Neph1 tyrosine phosphorylation mediated by Fyn in the assembly of Neph1 and ZO-1 complex during renal ischemia.
MATERIALS AND METHODS
Antibodies and Reagents—Polyclonal purified antibody to Neph1 was described previously (13). Other antibodies, including ZO-1 (Zymed Laboratories Inc.), Grb2 (Cell Signaling), anti-phosphotyrosine mouse monoclonal antibody (P-Tyr-100; Cell Signaling; catalog number 9411), and Fyn antibodies were obtained commercially (Cell Signaling; catalog number 4023). The cell transfection reagent Fugene was purchased from Roche Applied Science. All chemical reagents, including PP2 and SU6656, were obtained commercially from Sigma and Calbiochem. Purified active His-Fyn was obtained from Upstate Biotechnology, Inc.
Plasmids—Mammalian expression plasmid encoding FLAG-Neph1 (13, 17) and various Src family kinases were previously described (17, 29). A plasmid encoding GST-Neph1CD fusion protein has been described previously (17). The plasmid encoding Myc-ZO-1 was a gift from Dr. Meng Jer Lee (University of Louisville). Recombinant Glutathione S Transferase2 fusion proteins were prepared and purified from bacterial lysates, as described previously (17). Tyrosine-phosphorylated GST-Neph1CD was expressed in and purified from TKB1 cells (Stratagene), as described previously (17). Mammalian plasmids encoding mouse Fyn and Fyn-kd (K295M) were described previously. Mammalian expression plasmids encoding Neph1 point mutations (Y637F, Y638F, Y637F/Y638F, Y716F, Y719F, and Y716F/Y719F) were prepared commercially (by Top Gene Technologies, Inc.) in pECFPN1 vector (Invitrogen) using standard methods. Restriction digestion and DNA sequencing were used to validate all construct sequences.
Cell Culture—HEK293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen) and 200 units/ml penicillin and streptomycin (Roche Applied Science). The transfections were performed using Fugene-6 (Roche Applied Science) according to the manufacturer's protocols. The human podocyte cell line was graciously provided by Dr. Moin Saleem and was cultured in RPMI 1640-based medium, as described previously (30). The cells were grown to confluence at 33 °C before thermoswitching to 37 °C (30). Cellular ATP depletion was accomplished as previously reported (31, 32). Before depletion, cells were washed three times with PBS. Depleted medium (Dulbecco's modified Eagle's medium without glucose, pyruvate, or amino acids) was added to control plates, and depleted medium containing 0.1 μm antimycin A was added to the experimental plates. The cells were returned to the incubator for the indicated ATP depletion periods. The cells were lysed in radioimmune precipitation buffer (phosphate-buffered saline (PBS) containing 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 100 mm potassium iodide).
Subcellular Fractionation—All procedures were carried out at 4 °C. Podocyte cells in 150-mm flasks were washed three times with PBS, and 1 ml of hypotonic buffer (10 mm Tris-HCl, pH 7.4, 1 mm MgCl2) containing protease inhibitors (Roche Applied Science) was added to each flask. Cells were collected by scraping with a scraper and lysed by using a Dounce homogenizer. The lysate was spun at 14,000 rpm for 3 s in a tabletop microcentrifuge, and the pellet containing nuclei was discarded. Cytosolic and membrane fractions were separated by ultracentrifugation for 60 min at 100,000 × g at 4 °C in a Beckman TLA100.3 rotor. The supernatant was collected, and the pellet was washed twice with PBS. Both fractions were reconstituted in SDS-PAGE sample buffer and analyzed by Western blotting.
Animals and Acute Renal Ischemia—Male Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN). All experiments were conducted in accordance with NIH Guidelines (57) and approved by the Institutional Animal Care and Use Committee. Male rats weighing 250–300 g were anesthetized intraperitoneally with an injection of pentobarbital (60 mg/kg). They were then placed on a homeothermic table to maintain a core temperature of 37 °C. A midline incision was made, and the renal pedicles were isolated. Bilateral ischemia was induced by clamping the renal pedicles for 45 min with microserrefines. After removal of the microserrefines, reperfusion was observed visually. Two milliliters of prewarmed saline was instilled into the abdominal cavity prior to closure of the abdominal incision. Animals were allowed to recover on the homeothermic pad to maintain body temperature until the righting reflex was restored. Reperfusion time varied from 0 min to 4 h. The serum creatinine concentration was determined from the blood samples collected from control and ischemic rats using the Creatinine Analyzer 2 (Beckman). The urine albumin/creatinine ratio was determined from urine of control and ischemic rats obtained in metabolic cages by a direct competitive enzyme-linked immunosorbent assay for urine albumin (Nephrat II; Exocell Inc.) and the Jaffe reaction for urine creatinine (Creatinine Companion; Exocell).
Glomerular Isolation—Rat glomeruli were isolated by graded sieving, as described previously (17). The average purity of the rat glomerular preparations was 80–90%. Glomeruli were resuspended at a concentration of 10,000 glomeruli/ml of extraction buffer.
Pull-down—Wherever indicated, GST-Neph1CD recombinant protein was expressed and purified from TKB1 Escherichia coli (Stratagene) to induce tyrosine phosphorylation. Purified GST fusion proteins were incubated with immunoprecipitated ZO-1 on protein G-agarose beads. After washing with PBS containing 0.1% Tween 20, 1 mm sodium orthovanadate, and 1 mm sodium fluoride, protein complexes were eluted with SDS-PAGE sample buffer. Eluate was resolved by SDS-polyacrylamide gel electrophoresis prior to immunoblotting with the indicated antibodies.
Immunoprecipitation and Immunoblotting—Immunoprecipitation and immunoblotting experiments were performed using the procedures described previously (17, 29). For coimmunoprecipitation experiments of endogenous Neph1 and ZO-1, glomeruli were isolated from kidneys of male Sprague-Dawley rats (200–250 g) by graded sieving, as described previously (17), and lysed by incubation in radioimmune precipitation buffer. Detergent-insoluble material was removed by centrifugation (10,000 × g for 2 min).
Indirect Immunofluorescence Microscopy—The cells were grown on coverslips and fixed in 4% paraformaldehyde, followed by permeabilization with 0.1% SDS. Antibodies were diluted in 3% bovine serum albumin in PBS, and primary antibodies were incubated for 2 h at 37 °C followed by PBS washes and secondary antibody incubation for 1 h at 37 °C. Following PBS washes, the coverslips were mounted using GelMount. All experiments were conducted using a PerkinElmer Life Sciences Ultraview confocal microscope system mounted on a Nikon TE 2000U inverted microscope, using Nikon ×100, numerical aperture 1.4 oil immersion planapochromatic objectives (Melville, NY). The system is equipped with an Ixon air-cooled EMCCD 12-bit camera (Andor). Image volumes were collected by collecting a sequential vertical series of images, each 0.2 μm apart. Cells were labeled for ZO-1 (Alexa 488), F-actin (phalloidin), and Neph-1 (Cy5) using the 488-, 568-, and 633-nm laser lines of the argon and helium-neon lasers. Chromatic aberration between the green and far red channel was calculated using fluorescent beads to be ∼0.2 μm. Image analysis was conducted using Image J software. For Pearson's correlation coefficient (Rr) determination, single image planes were selected. After background subtraction, the ZO-1 image was thresholded and used to create a binary mask for analysis of the Rr. Regions of interest were selected to distinguish junctional areas and cytosol. The integrated mean intensity of the same regions of interest was calculated on the original image planes after image subtraction. Representative images from a minimum of three experiments are shown, and analysis was performed on at least eight image fields for each time point.
Electron Microscopy—Preparation of samples for electron microscopy was performed by standard methods (17). An infrarenal aortic cannula (PE 240 tubing) was introduced, and both kidneys were fixed by perfusion with a 4% paraformaldehyde solution at 120 mm Hg pressure for 5 min (∼50 ml/min). The kidneys were then diced and stored in 4% paraformaldehyde. Selected samples containing glomeruli were thin-sectioned and examined by scanning and transmission electron microscopy. At least 10 glomeruli from each kidney were examined before representative images were chosen.
RESULTS
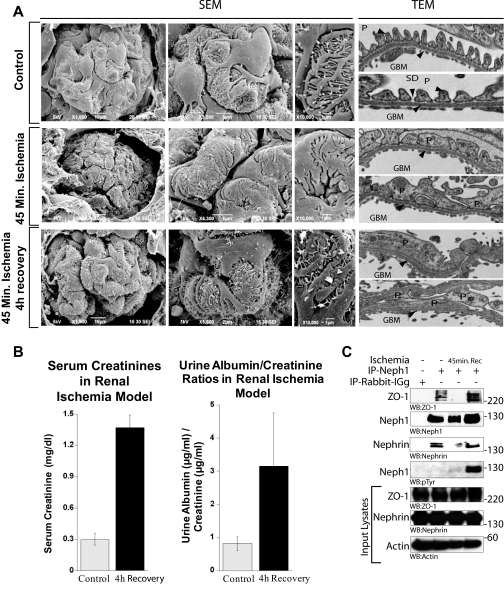
Ischemic Injury to Rat Kidney Induces Podocyte Effacement and Loss of Slit Diaphragm—The effect of ischemia on tubular cell damage has been studied extensively; however, ischemia-induced loss of glomerular filtration barrier slit diaphragm has rarely been addressed. To investigate the changes induced by ischemia, we used an in vivo rat model that uses bilateral clamping of the renal arteries (33). The kidney sections were analyzed for structural changes in the glomerulus and podocytes by scanning electron microscopy and transmission electron microscopy (Fig. 1A). Both scanning electron microscopy and transmission electron microscopy pictures showed podocytes exhibiting dramatic morphological changes, including gradual effacement in response to ischemia (Fig. 1A). After 45 min of ischemia, podocytes appeared swollen with narrowed filtration slits (Fig. 1A). After 4 h of recovery, loss of the morphological integrity of podocytes and their processes along with the loss of slit diaphragms was still observed (Fig. 1A). In addition, an increase in microvillus formation (Fig. 1A) was apparent, which indicates proteinuric conditions. Indeed, the analysis of urine from ischemic rats showed a significant increase in protein excretion postischemia (Fig. 1B).
FIGURE 1.
Ischemic injury to rat kidney induces podocyte effacement. A, scanning and transmission electron micrographs of kidneys from untreated and ischemic rats at 0 and 4 h after 45 min of ischemia. Foot process structure was lost after ischemia and ischemia, followed by 4 h of recovery. After 45 min of ischemia, glomerular foot processes appear swollen with decreased filtration slits, and by 4 h of recovery, podocyte effacement became prominent with numerous microvilli. SEM, scanning electron micrographs; TEM, transmission electron micrographs; P, podocytes; GBM, glomerular basement membrane; SD, slit diaphragm. B, serum creatinine concentration and urine albumin/creatinine ratios of control and ischemic rats evaluated at 4 h of recovery (p < 0.04) suggest increased proteinuria compared with control. Injury to rat kidney by ischemia induces dissociation of Neph1 from ZO-1 and podocyte effacement. C, rat glomerular lysate was obtained after 45 min of ischemia and ischemia followed by recovery for 4 h. Neph1 was immunoprecipitated (IP) from these and control untreated glomerular lysate using Neph1 antibody and immunoblotted with ZO-1, Neph1, Nephrin, and Tyr(P) antibodies. Equivalent amounts of Neph1, ZO-1, and Nephrin in the tissue lysates were examined using their respective antibodies. This experiment was performed at least five times with similar results.
Ischemic Injury to Rat Glomerulus Results in Loss of Neph1 Interaction with ZO-1 and Nephrin—We wanted to further determine if ischemia induces changes in the molecular interactions between slit diaphragm proteins that are critical for the maintenance of integrity of the slit diaphragm junction (6, 17, 34). Therefore, isolated glomeruli of rats subjected to 45 min of ischemia were analyzed for Neph1 interaction with ZO-1 and nephrin by coimmunoprecipitation experiments. As shown in Fig. 1C, interaction of Neph1 with ZO-1 and nephrin was significantly reduced after 45 min of ischemia. 4 h of reperfusion restored this interaction and was associated with enhanced Neph1 tyrosine phosphorylation (Fig. 1C). Together, these results provide evidence for a dynamic interaction between slit diaphragm proteins Neph1, ZO-1, and nephrin that is significantly altered by glomerular injury but, importantly, has the capacity to recover following reperfusion. It is to be noted that although interaction between slit diaphragm proteins was restored, recovery of glomerular function (proteinuria) and podocyte morphology was not observed (Fig. 1A). This suggests that the dynamics of recovery for molecular interaction is different than the recovery at the functional and morphological level. This prompted us to further investigate the effect of ischemia on the molecular interactions of slit diaphragm proteins and hypothesize that ischemia-induced changes in the binding of slit diaphragm proteins can either be used as markers for the glomerular injury or possibly as targets for developing therapies against renal ischemia.
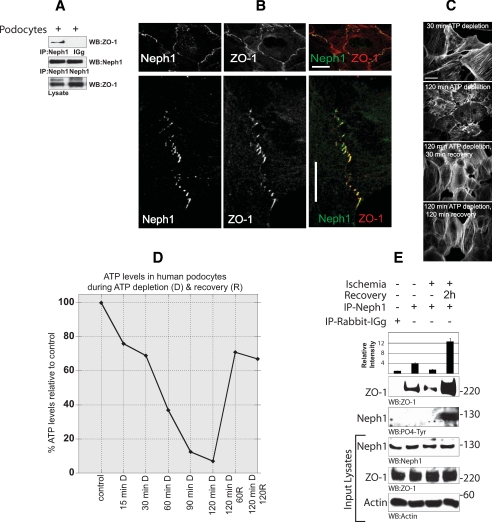
Neph1 and ZO-1 Are Colocalized at the Cell-Cell Junction in Cultured Podocytes—Fig. 1C suggests that ischemia affects multiple Neph1 interactions, including its binding with ZO-1 and nephrin. Although Neph1 tyrosine phosphorylation has been shown to play a critical role in regulating Neph1 and Nephrin interaction (17), it is not clear how Neph1 binding with ZO-1 is regulated. Since ZO-1 is a tight junction protein that interacts with Neph1 and has been shown in multiple systems to regulate junction formation, we sought to determine the regulation of this interaction as a model system to study the effect of ischemia on the molecular interactions of slit diaphragm proteins. Therefore, a human podocyte cell line was employed where these proteins are endogenously expressed (30). A coimmunoprecipitation assay from this cell line indicated that interaction between endogenous Neph1 and ZO-1 can be detected in these cells (Fig. 2A). To further determine the subcellular localization of these proteins in this cell line, the cells were grown to confluence and immunostained with Neph1 and ZO-1 antibodies, followed by confocal microscopic analysis. The results presented in Fig. 2B support the conclusion that Neph1 and ZO-1 are primarily localized at the cell-cell junctions. The colocalization of these proteins is shown in yellow (Fig. 2B). Numerous studies have established the importance of ZO-1 as a tight junction organizing protein (20, 21, 35). The observation that Neph1 is also localized in this region and interacts with ZO-1 provides primary evidence that this interaction may play a role in organizing the Neph1 complex at the slit diaphragm junctions in podocytes.
FIGURE 2.
Neph1 and ZO-1 are localized at the cell-cell junction in cultured podocytes. A, lysate obtained from the cultured human podocyte cell line was immunoprecipitated (IP) with Neph1 antibody and immunoblotted with Neph1 and ZO-1 antibodies. B, localization of Neph1 and ZO-1 in the podocyte cell line was examined by staining the cells with Neph1 and ZO-1 antibodies. Immunofluorescence analysis was done using confocal microscopy. Colocalization of Neph1 and ZO-1 at the membrane junctions appears yellow on merged images. Ischemia results in dissociation of Neph1 and ZO-1. C and D, podocyte cells were subjected to ATP depletion by antimycin A for the indicated times. Following ATP depletion, the medium was replaced with fresh growth medium, and the cells were grown for an additional 30 and 120 min. At each time point, the cells were stained with actin (C), and relative ATP levels were determined using the Promega Enliten ATP assay system (D). E, the cells were subjected to injury by treatment with ischemia for 120 min. Following injury, the medium in the cells was replaced with fresh growth medium, and the cells were grown for an additional 2 h. The cells were lysed and immunoprecipitated with Neph1 antibody. The presence of ZO-1 in the immune complex was examined by immunoblotting with ZO-1 antibody. Relative binding of ZO-1 with Neph1 during ischemia and recovery is also presented in the form of a bar diagram, where data are presented as a mean of three independent experiments.
Treatment of Podocytes with ATP Depletion Results in Loss of Neph1 and ZO-1 Interaction—To further determine the effect of ischemia on Neph1 and ZO-1 interaction in podocyte cells, the effect of ATP depletion was studied in the cultured human podocyte cell line. As shown in Fig. 2C, actin organization was significantly affected when the cells were depleted of ATP. It is to be noted that the ATP levels during this time dropped more than 90% (Fig. 2D). Interestingly, this effect was rapidly reversed when the cells were allowed to recover by culturing them in fresh growth medium (Fig. 2, C and D). To analyze the effect of ATP depletion on the binding of Neph1 and ZO-1, a coimmunoprecipitation experiment was performed from these cells. Endogenous Neph1 was immunoprecipitated, and the immune complexes were analyzed for the presence of ZO-1 and Neph1 tyrosine phosphorylation before and after the injury. Treatment of these cells with ischemia resulted in a significant reduction in Neph1 and ZO-1 binding (Fig. 2E). Notably, the interaction between Neph1 and ZO-1 was restored to normal levels upon recovery from these injuries, suggesting that these effects are reversible (Fig. 2E). It has been previously suggested that the Neph1 tyrosine phosphorylation is induced during junction formation (17). In accordance, we find that the recovery from ischemia also results in Neph1 tyrosine phosphorylation (Fig. 2E), suggesting that these events mimic reformation of junctions under in vivo conditions. In fact, disruption of normal actin organization in diverse cell types is an important element of ischemia-induced acute renal injury and recovery in proximal tubule cells (32, 33, 36–38). Collectively, these data provide strong evidence that glomerular injuries that affect actin cytoskeletal organization also regulate the interaction between Neph1 and ZO-1.
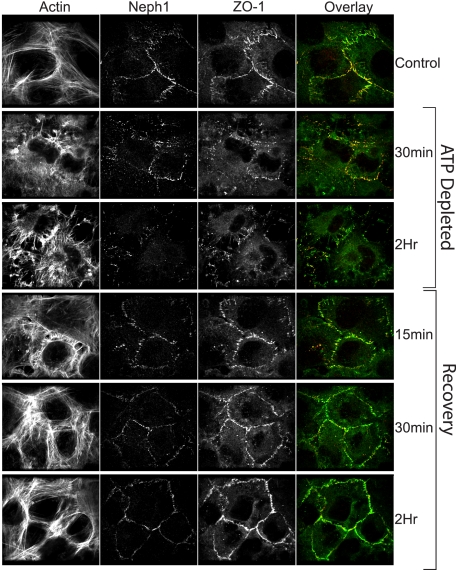
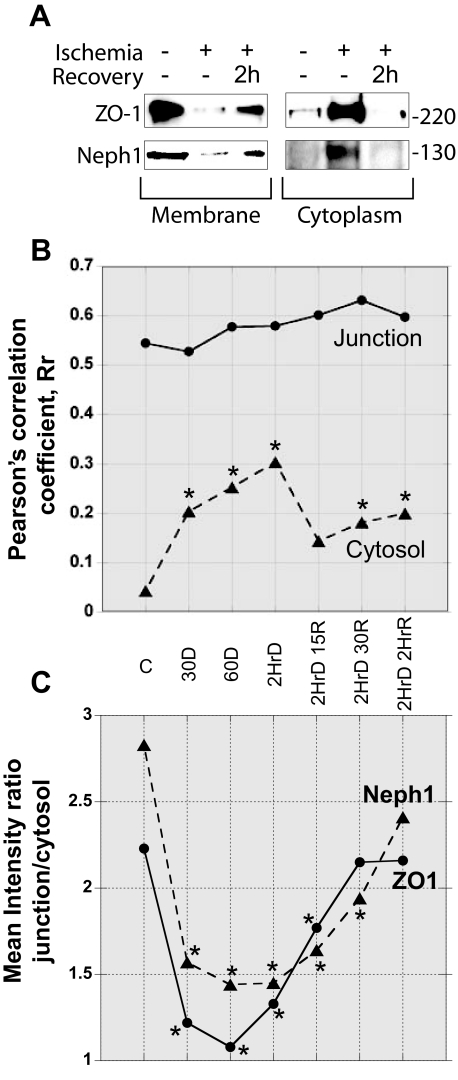
Injury to Podocytes Results in Redistribution of Neph1 and ZO-1 from the Surface Membrane—To further evaluate the dynamic interaction between Neph1 and ZO-1, we performed immunofluorescence analysis of cultured podocytes under physiological and nonphysiological conditions. In Fig. 3, representative single confocal planes (black and white images) and one overlay of ZO-1 (green) and Neph1 (red) from six time points (control, 30 min, and 2 h of ATP depletion and 2 h of ATP depletion followed by repletion for 15 min, 30 min, or 2 h) are presented. Sequential collection of fluorophore channels (Alexa 488 for ZO-1, Texas Red phalloidin for F-actin, and Cy5 for Neph1) ensured that there was no spectral overlap. Notably, a strong labeling of both ZO-1 and Neph1 at the cell periphery in control was observed, with significant reorganization taking place following ATP depletion that results in an apparent decrease of these proteins at the cell periphery. Recovery following ATP repletion was rapid, with F-actin, ZO-1, and Neph1 staining showing significant recovery within 15 min. To further confirm these findings, subcellular distribution of Neph1 and ZO-1 was studied in response to ischemia. In correlation with the imaging results (Fig. 3), subcellular fractionation of cells following ischemia and recovery shows that Neph1 and ZO-1 are primarily localized in the membrane fraction, and following ischemia, they accumulate in the cytoplasm, and recovery leads to their enrichment in the membrane fraction (Fig. 4A). To better define the changes taking place and the interaction between Neph1 and ZO-1, we calculated the correlation between Neph1 and ZO-1 and measured the total integrated intensity for these proteins. For these analyses, the junction was defined as the location at the cell periphery containing ZO-1. In Fig. 4B, Pearson's correlation coefficient, Rr, was calculated for both the junction and cytosol during the injury/recovery. Each time point was compared with the control using an unpaired Student's t test with a probability of <0.05 represented by an asterisk. Although there was no significant change at the junction, a significant increase in the cytosolic Rr occurred rapidly following ATP depletion. Interestingly, this remained altered even after 2 h of ATP repletion. Given the well defined aggregation of actin and actin-associated proteins that occurs with ATP depletion, the apparent lack of Rr change at the junction may be misleading. To address this issue, we calculated the mean integrated intensity in the same regions for Neph1 and ZO-1 and present the data as the ratio of mean junction intensity/mean cytosol intensity for each protein (Fig. 4C). In this case, note the dramatic and significant decrease in this ratio for both ZO-1 and Neph1, supporting movement away from the junction area and the equally rapid recovery with ATP repletion. Further analysis of the changes taking place will be aided by similar calculations and live cell analysis.
FIGURE 3.
Ischemia/reperfusion induces a significant shift in the localization of Neph1 and ZO-1. Podocyte cells were grown on coverslips and subjected to ATP depletion for 30 min or 2 h. The recovery followed a 2-h injury and was performed by replacing the medium with fresh growth medium and growing the cells for the indicated times. At each time point, the cells were stained with Neph1 and ZO-1 antibodies to determine the localization of these proteins. Immunofluorescence analysis was performed using confocal microscopy. To determine the extent of Neph1 and ZO-1 co-localization, each of the figures was analyzed using Image J software, as described under “Materials and Methods.”
FIGURE 4.
Analysis of subcellular fractionation and immunofluorescent data supports changes in localization of ZO-1 and Neph1 during ischemia/reperfusion. A, podocyte cells were grown and subjected to ischemia, followed by recovery. The cells were fractionated to isolate cytoplasmic and membrane fractions, and the amounts of Neph1 and ZO-1 in each fraction were analyzed by Western blotting. B, Pearson's correlation coefficient, Rr, was calculated to address the interaction between ZO-1 and Neph1 control (c), ischemic, and recovery time points. Note the increase in Rr in the cytosol during ATP repletion. C, mean integrated intensities of ZO-1 and Neph1 were calculated in the same junction and cytosol regions used to calculate the Rr. The ratio of junction/cytosol for each protein is shown. An unpaired Student's t test compared each time point to control. Experiments were run a minimum of three times, with each data point representing the analysis of more than eight cell fields.
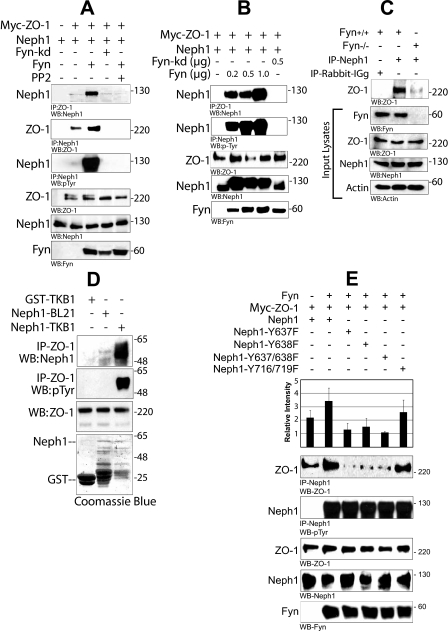
Interaction between Neph1 and ZO-1 Is Dependent on the Catalytic Activity of Fyn—It was recently demonstrated that the binding of Neph1 with Grb2 and Nephrin is regulated by Fyn-dependent tyrosine phosphorylation of Neph1 (17). Since recovery from ischemia induced tyrosine phosphorylation of Neph1, we sought to determine the effect of Fyn-dependent tyrosine phosphorylation of Neph1 on the interaction between Neph1 and ZO-1. Therefore, interaction between Neph1 and ZO-1 was determined by co-expressing Neph1 and ZO-1 proteins in the presence of either Fyn wild type or catalytically inactive Fyn (Fyn-kd). Co-immunoprecipitation of either Neph1 or ZO-1 from these cell lysates suggested a significant increase in the binding between Neph1 and ZO-1 in the presence of Fyn (Fig. 5A). Notably, this increase in the interaction between Neph1 and ZO-1 was lost in the presence of catalytically inactive Fyn (Fyn-kd) or when the cells were treated with Src family kinase inhibitor PP2 (Fig. 5A). To further confirm this observation, the interaction between Neph1 and ZO-1 was examined by co-expressing FLAG-Neph1 and Myc-ZO-1 in the presence of increasing amounts of Fyn in HEK-293 cells (Fig. 5B). Here increased concentration of Fyn resulted in a significant increase in Neph1 immunoprecipitated with ZO-1. This observation was further extended by analyzing the Neph1 and ZO-1 interaction in mice where Fyn was depleted by gene targeting (Fig. 5C). Neph1 was immunoprecipitated from the glomerular lysate obtained from wild type mice and Fyn null mice (Fyn-/-), and the immune complexes were analyzed for the presence of ZO-1. As shown in Fig. 5C, the amount of ZO-1 immunoprecipitated with Neph1 was significantly higher in wild type mice compared with Fyn null mice. Collectively, these results suggest that the catalytic activity of Fyn significantly enhances the association between Neph1 and ZO-1.
FIGURE 5.
Fyn increases the interaction between Neph1 and ZO-1 by directly phosphorylating Neph1. A, HEK293 cells were transfected with plasmids encoding FLAG-Neph1, Myc-ZO-1, and Fyn or Fyn-kd (catalytically inactive). ZO-1 or Neph1 was immunoprecipitated from the cell lysate, and immune complexes were evaluated for binding of Neph1 to ZO-1 and phosphorylation of Neph1. The experiment was repeated five times with similar results. B, plasmids encoding Myc-ZO-1 and FLAG-Neph1 were co-transfected with either Fyn-kd or increasing amounts of Fyn plasmids in HEK-293 cells. ZO-1 and Neph1 were immunoprecipitated from the cell lysate and immunoblotted with Neph1 and Tyr(P) (phosphotyrosine) antibodies, respectively. C, isolated glomeruli obtained from Fyn null mice or wild type mice (with the same genetic background sv129 strain) were lysed and immunoprecipitated (IP) with Neph1 antibody and immunoblotted with ZO-1. This experiment was repeated twice with similar results. D, phosphorylation of Neph1 increases its interaction with ZO-1. ZO-1 was immunoprecipitated from the HEK293 cell lysate and incubated with either GST alone or phosphorylated GST-Neph1 cytoplasmic domain expressed in tyrosine kinase expressing E. coli TKB1 or E. coli BL21. Binding of Neph1 to ZO-1 was analyzed by immunoblotting with Neph1 and phosphotyrosine antibodies. E, phosphorylation of Neph1 on sites 637 and 638 is required for its increased interaction with ZO-1. Plasmids encoding various indicated Neph1 mutants were co-transfected with Myc-ZO-1 and Fyn in HEK-293 cells. Neph1 was immunoprecipitated from the cell lysate, and the immune complexes were analyzed for the binding of ZO-1 and Neph1 phosphorylation by ZO-1 and Tyr(P) antibodies, respectively. Data from four independent experiments were analyzed, and the results presented are means plus S.E. for these experiments.
Phosphorylation of Neph1 by Fyn Is Required for Increased Interaction between Neph1 and ZO-1—To further establish a direct effect of Neph1 tyrosine phosphorylation on the binding between Neph1 and ZO-1, the binding ability of bacterially produced tyrosine phosphorylated cytoplasmic domain of Neph1 (GST-Neph1CD) with ZO-1 was assessed. Therefore, a coimmunoprecipitation experiment was performed, where GST-Neph1CD prepared in either BL21 (nonphosphorylated) or TKB1 (tyrosine-phosphorylated) cells was mixed with immunoprecipitated Myc-ZO-1. Notably, binding of tyrosine-phosphorylated Neph1 was severalfold higher when compared with the nonphosphorylated Neph1 (Fig. 5D). This further suggested that the tyrosine phosphorylation of Neph1 increases its affinity for ZO-1. In our previous report, we mapped Fyn-dependent tyrosine phosphorylation sites in Neph1. To further extend this observation, a coimmunoprecipitation experiment was performed using various Neph1 mutants. The results from this analysis showed that the individual or combined point mutations on phosphorylation sites Tyr637 and Tyr638 in Neph1 resulted in a loss of Fyn-dependent increased interaction between Neph1 and ZO-1 (Fig. 5E), whereas mutations on other Fyn phosphorylation sites Tyr716 and Tyr719 in Neph1 did not significantly affect Neph1 and ZO-1 binding (Fig. 5E). Collectively, these results establish that the Neph1 tyrosine phosphorylation by Fyn enhances the interaction between Neph1 and ZO-1. Based on these results, we speculate that the reassembly of Neph1 and ZO-1 complex during recovery from ischemia is induced by Fyn-dependent Neph1 tyrosine phosphorylation.
DISCUSSION
Podocyte injury is a common event observed in a number of glomerular diseases, including diabetic nephropathy, focal segmental glomerulosclerosis, minimal change disease, congenital nephrotic syndrome, and other disorders leading to end stage renal disease (9, 27, 35, 39, 40). Injury to podocytes results in loss of foot processes commonly referred to as “effacement.” Effacement is believed to be initiated by changes in the podocyte actin cytoskeleton that also determine the shape and architecture of podocytes (27, 41). Proteins that regulate the actin cytoskeleton are therefore important to the normal functioning of podocytes. This further implies that changes that alter the functioning of such proteins may lead to loss of podocyte function. Morphological alterations in podocytes have been best studied by electron microscopy, where scanning electron microscopy (scanning electron microscopy) has generally been used to document effacement in podocytes, and transmission electron microscopy is used to determine the fusion of foot process and loss of slit diaphragm (10, 17, 42, 43). It is well established that ischemia induces proximal tubule cell and renal damage; however, ischemia-induced podocyte alterations are less well defined. Moreover, the molecular events that induce podocyte effacement during ischemia are unknown. The rapid change in podocyte morphology and renal function following ischemia reported in this study suggests that ischemia also induces similar morphological changes in podocytes as have been observed for other established models of renal injury (24, 41, 44).
Under experimental conditions, foot processes can behave as dynamic structures to support the repair mechanisms during recovery from glomerular injury (15, 27). However, under most clinical conditions, the podocyte effacement is associated with loss of slit diaphragm, leading to proteinuria and loss of kidney function (27, 40, 41). In this study, 45 min of ischemia induced a gradual loss of renal function and podocyte structure. Although 4 h of reperfusion restored Neph1 and ZO-1 interaction (Fig. 1C), the recovery of podocyte structure was minimal. It is possible that a complete recovery at the morphological level can be achieved through input from various molecular interactions and pathways that are affected by ischemia. Several studies have documented the effects of renal ischemia on actin cytoskeleton reorganization that is mediated by RhoGTPases (2). Moreover, since Neph1 interacts with other proteins, including Grb2, Nephrin, and polarity proteins Par3, Par6, and atypical protein kinase C (45), it is possible that ischemia affects multiple Neph1 interactions that may participate in the recovery process. Whether the recovery from ischemia is dependent on a synergistic effect of these interactions and pathways will be a subject of future investigation.
Podocyte differentiation and damage in glomerular development and diseases has been the primary focus of many recent studies. With the identification of various proteins localized at the slit diaphragm, it has been suggested that the interactions organized by these proteins are critical for the slit diaphragm to function as a filtration barrier. Biochemical analysis of the slit diaphragm suggests that it contains features of both tight and adherens junctions. Its similarity to the tight junction is marked by the presence of ZO-1, which was the first slit diaphragm protein identified (46), and like the tight junction, the maintenance of this polar slit diaphragm serves as a signaling platform and regulatory barrier that limits the trafficking of solutes and proteins across the membranes (22, 26, 35, 47). Therefore, it is likely that the proteins localized at the slit diaphragm also function in a manner similar to tight junction proteins to maintain the integrity of these junctions. The fact that the Neph1 and ZO-1 complex is localized at cell-cell junctions in podocytes and responds dynamically to the physiological changes in cells provides preliminary evidence for this hypothesis. Based on our results, we further hypothesize that Neph1 and ZO-1 interaction is an important event that determines the stability of slit diaphragm junction under basal conditions. Induction events, including glomerular injury by ischemia, lead to the dissociation of Neph1 and ZO-1 complex and loss of slit diaphragm junction. Determination of whether loss of Neph1 and ZO-1 binding induces loss of slit diaphragm junction will require further investigation. Recovery from injury results in Neph1 tyrosine phosphorylation-induced reorganization of the Neph1 complex, which stimulates reformation of junctions (Fig. 6). This conclusion is supported in part by the biochemical studies demonstrating a dynamic interaction between Neph1 and ZO-1, which is dependent on Neph1 tyrosine phosphorylation by Fyn. Overall, the observation that glomerular injury events lead to podocyte effacement and proteinuria with a simultaneous loss of interaction between Neph1 and ZO-1 suggest a critical role for this interaction in determining podocyte structure integrity.
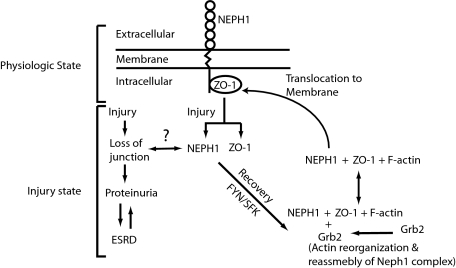
FIGURE 6.
A schematic representation of the dynamic interaction between Neph1 and ZO-1. The model summarizes the hypothesis suggesting that under basal conditions, Neph1 and ZO-1 exist as a complex. Injury by ischemia induces dissociation of Neph1 and ZO-1 and their movement to cytoplasm with a corresponding loss of slit diaphragm junction. Recovery events that follow induce Neph1 tyrosine phosphorylation in an Src family kinase-dependent manner and lead to reorganization of Neph1 and ZO-1 complex and subsequent localization of this complex to the cell membrane.
Several studies have now established ZO-1 as a tight junction protein that serves as a scaffold and defines the tight junction integrity (20, 22, 35). One of the major functions of ZO-1 is to regulate the function of transmembrane proteins by coordinating their assembly with actin at cell junctions (22, 48, 49). Although Neph1 phosphorylation induces actin reorganization (17), it is not clear how the Neph1 complex with actin is organized. Moreover, the molecular mechanisms by which foot process and its junctions are assembled are also poorly defined. In addition to inducing actin reorganization by regulating the interaction of Neph1 with Grb2 and Nephrin (17), we now show that Neph1 tyrosine phosphorylation also induces the formation of a signaling complex with ZO-1. In contrast to the previous study (17), where injury and recovery events were not defined, the current study utilizes an established rat ischemic model and cell culture model to demonstrate that recovery from ischemia induces significant Neph1 tyrosine phosphorylation and reformation of the Neph1 and ZO-1 complex. These results provide further evidence that in response to ischemia, Neph1 organizes a signaling module containing ZO-1. Therefore, in addition to other Neph1 complexes, the Neph1 and ZO-1 complex also links the Neph1 signaling module to the actin cytoskeleton and is regulated by Neph1 tyrosine phosphorylation. It is likely that the Neph1 binding to ZO-1 and Grb2 are components of the same signaling module that enables efficient actin cytoskeletal reorganization in podocytes in response to ischemia. How these different interactions of Neph1 result in actin cytoskeleton reorganization will be the subject of future investigation. Interestingly, Neph1 and ZO-1 interaction is regulated by the Fyn-dependent tyrosine phosphorylation sites Tyr637 and Tyr638 that also regulate Neph1 and Grb2 interaction (17, 50). This result is not surprising, since these mutations have been shown to induce loss of function in Neph1 by inhibiting its ability induce actin reorganization (17). Based on these results, we speculate that phosphorylation of Neph1 on Tyr637 and Tyr638 residues induces structural changes in Neph1 that lead to its increased affinity for ZO-1. However, a detailed structural analysis of the phosphorylated and nonphosphorylated Neph1 will provide more insight into the phosphorylation associated structural changes in Neph1.
It is widely accepted that ischemia induces loss of tight junctions, thereby disrupting the permeability barrier of the polarized epithelium, with recovery from ischemia requiring reestablishment of the junction (21, 32, 42). In addition, ischemic injury also affects the polarization of membrane proteins and results in significant changes in the actin cytoskeleton (21, 51). Using the cell culture and animal models, we were able to study changes in the molecular interactions of Neph1 during injury and recovery. Treatment of podocytes with ATP depletion induced redistribution of Neph1 and ZO-1 to the intracellular compartments. Intracellular redistribution of ZO-1 from podocyte membrane has been documented under various conditions of injury, including the PAN cell culture model (52), the ischemic cell culture model (53), and the in vivo model of diabetic nephropathy (25). The translocation of ZO-1 from plasma membrane to the cytoplasm is frequently associated with the structural and functional disruption of tight junctions (25, 54, 55). In this study, increased intracellular distribution of Neph1 and ZO-1 following injury was associated with a decrease in Neph1 and ZO-1 binding (Figs. 3 and 4). This suggests that Neph1 and ZO-1 may localize to different subcellular compartments under these conditions. Based on these results, we speculate that injury induces disruption and redistribution of the Neph1-ZO-1 complex from the cell junctions that may lead to an increase in paracellular permeability. Interestingly, recovery in these models was associated with increased Neph1 phosphorylation and restoration of Neph1 and ZO-1 interaction and their redistribution at the cell membrane (Figs. 3 and 4). Indeed, tyrosine phosphorylation-dependent assembly of tight junction proteins, including occludin and ZO-1, has previously been reported in an ATP depletion model using Madin-Darby canine kidney cells (56). Since unphosphorylated Neph1 also interacts with ZO-1, it is not clear why Neph1 phosphorylation would increase its association with ZO-1. Based on the established function of ZO-1, we speculate that reformation of cell junction requires increased localization of ZO-1 at the cell membrane, which is achieved through an enhanced interaction between phosphorylated Neph1 and ZO-1. We further speculate that increased binding between Neph1 and ZO-1 during recovery establishes this complex at the membrane and aids in reestablishing cell junctions. Since Nephrin and Grb2 have been shown to regulate actin reorganization through their interaction with phosphorylated Neph1 (17), it is likely that junction formation also requires a cooperative effort of Neph1 interaction with ZO-1, Grb2, and Nephrin. Our previous results (17) and the results presented in Fig. 1C further support this conclusion. In addition, a study by Liu et al. (34) suggests that interaction of Neph1 with Nephrin stabilizes the binding between Neph1 and ZO-1, further implicating the role of other Neph1 interactions in regulating the Neph1 and ZO-1 complex. During the preparation of this manuscript, a new study was published that describes interaction of Neph1 with polarity proteins Par3, Par6, and aPKC (45). This further suggests that input from additional signaling pathways may be critical in maintaining the integrity of slit diaphragm. How these different Neph1 interactions participate in the junction formation in response to cellular injury will be a subject of future investigation.
Overall, this study provides novel insight into the molecular mechanisms that are affected by renal ischemia. The results from this study suggest that in addition to morphological changes in podocyte structure and function, ischemia also induces loss of slit diaphragm integrity and disassembly of slit diaphragm proteins Neph1 and ZO-1. Based on our results, we hypothesize that Neph1 tyrosine phosphorylation is induced during the “recovery” from renal injury and leads to the assembly of the Neph1-dependent complex that is required for actin cytoskeleton reorganization. It remains to be seen whether these interactions are critical for the junction formation during recovery from ischemia. Future studies aimed at selective inhibition of these intracellular signaling events and their effect on podocyte function during injury will provide further insight into their significance in renal injury.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health, NIDDK, Grant K01 DK072047-03 (to D. N.) and Grants RO1 (DK069408) and P-50 (DK061594) (to B. M.). This work was also supported by a Research Support Funds Grant from Indiana University (to D. N.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
Footnotes
The abbreviations used are: GST, glutathione S-transferase; PBS, phosphate-buffered saline.
References
- 1.Molitoris, B. A., Melnikov, V. Y., Okusa, M. D., and Himmelfarb, J. (2008) Nat. Clin. Pract. Nephrol. 4 154-165 [DOI] [PubMed] [Google Scholar]
- 2.Molitoris, B. A., and Sutton, T. A. (2004) Kidney Int. 66 496-499 [DOI] [PubMed] [Google Scholar]
- 3.Kelly, K. J., and Molitoris, B. A. (2000) Semin Nephrol. 20 4-19 [PubMed] [Google Scholar]
- 4.Molitoris, B. A. (2003) J. Am. Soc. Nephrol. 14 265-267 [DOI] [PubMed] [Google Scholar]
- 5.Parikh, C. R., Edelstein, C. L., Devarajan, P., and Cantley, L. (2007) J. Investig. Med. 55 333-340 [DOI] [PubMed] [Google Scholar]
- 6.Tryggvason, K., Pikkarainen, T., and Patrakka, J. (2006) Cell 125 221-224 [DOI] [PubMed] [Google Scholar]
- 7.Liu, X. L., Kilpelainen, P., Hellman, U., Sun, Y., Wartiovaara, J., Morgunova, E., Pikkarainen, T., Yan, K., Jonsson, A. P., and Tryggvason, K. (2005) FEBS J. 272 228-243 [DOI] [PubMed] [Google Scholar]
- 8.Verma, R., Kovari, I., Soofi, A., Nihalani, D., Patrie, K., and Holzman, L. B. (2006) J. Clin. Invest. 116 1346-1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnstone, D. B., and Holzman, L. B. (2006) Nat. Clin. Pract. Nephrol. 2 271-282 [DOI] [PubMed] [Google Scholar]
- 10.Tryggvason, K., Patrakka, J., and Wartiovaara, J. (2006) N. Engl. J. Med. 354 1387-1401 [DOI] [PubMed] [Google Scholar]
- 11.Donoviel, D. B., Freed, D. D., Vogel, H., Potter, D. G., Hawkins, E., Barrish, J. P., Mathur, B. N., Turner, C. A., Geske, R., Montgomery, C. A., Starbuck, M., Brandt, M., Gupta, A., Ramirez-Solis, R., Zambrowicz, B. P., and Powell, D. R. (2001) Mol. Cell Biol. 21 4829-4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellin, L., Huber, T. B., Gerke, P., Quack, I., Pavenstadt, H., and Walz, G. (2003) FASEB J. 17 115-117 [DOI] [PubMed] [Google Scholar]
- 13.Barletta, G. M., Kovari, I. A., Verma, R. K., Kerjaschki, D., and Holzman, L. B. (2003) J. Biol. Chem. 278 19266-19271 [DOI] [PubMed] [Google Scholar]
- 14.Gerke, P., Huber, T. B., Sellin, L., Benzing, T., and Walz, G. (2003) J. Am. Soc. Nephrol. 14 918-926 [DOI] [PubMed] [Google Scholar]
- 15.Patari-Sampo, A., Ihalmo, P., and Holthofer, H. (2006) Ann. Med. 38 483-492 [DOI] [PubMed] [Google Scholar]
- 16.Patrakka, J., and Tryggvason, K. (2007) Trends Mol. Med. 13 396-403 [DOI] [PubMed] [Google Scholar]
- 17.Garg, P., Verma, R., Nihalani, D., Johnstone, D. B., and Holzman, L. B. (2007) Mol. Cell Biol. 27 8698-8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma, R., Wharram, B., Kovari, I., Kunkel, R., Nihalani, D., Wary, K. K., Wiggins, R. C., Killen, P., and Holzman, L. B. (2003) J. Biol. Chem. 278 20716-20723 [DOI] [PubMed] [Google Scholar]
- 19.Huber, T. B., Schmidts, M., Gerke, P., Schermer, B., Zahn, A., Hartleben, B., Sellin, L., Walz, G., and Benzing, T. (2003) J. Biol. Chem. 278 13417-13421 [DOI] [PubMed] [Google Scholar]
- 20.Fanning, A. S., Jameson, B. J., Jesaitis, L. A., and Anderson, J. M. (1998) J. Biol. Chem. 273 29745-29753 [DOI] [PubMed] [Google Scholar]
- 21.Denker, B. M., and Nigam, S. K. (1998) Am. J. Physiol. 274 F1-F9 [DOI] [PubMed] [Google Scholar]
- 22.Hartsock, A., and Nelson, W. J. (2008) Biochim. Biophys. Acta 1778 660-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurihara, H., Anderson, J. M., and Farquhar, M. G. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 7075-7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurihara, H., Anderson, J. M., Kerjaschki, D., and Farquhar, M. G. (1992) Am. J. Pathol. 141 805-816 [PMC free article] [PubMed] [Google Scholar]
- 25.Rincon-Choles, H., Vasylyeva, T. L., Pergola, P. E., Bhandari, B., Bhandari, K., Zhang, J. H., Wang, W., Gorin, Y., Barnes, J. L., and Abboud, H. E. (2006) Diabetes 55 894-900 [DOI] [PubMed] [Google Scholar]
- 26.Benzing, T. (2004) J. Am. Soc. Nephrol. 15 1382-1391 [DOI] [PubMed] [Google Scholar]
- 27.Shankland, S. J. (2006) Kidney Int. 69 2131-2147 [DOI] [PubMed] [Google Scholar]
- 28.Wang, L., Fields, T. A., Pazmino, K., Dai, Q., Burchette, J. L., Howell, D. N., Coffman, T. M., and Spurney, R. F. (2005) J. Am. Soc. Nephrol. 16 3611-3622 [DOI] [PubMed] [Google Scholar]
- 29.Nihalani, D., Wong, H., Verma, R., and Holzman, L. B. (2007) Mol. Cell Biol. [DOI] [PMC free article] [PubMed]
- 30.Saleem, M. A., Ni, L., Witherden, I., Tryggvason, K., Ruotsalainen, V., Mundel, P., and Mathieson, P. W. (2002) Am. J. Pathol. 161 1459-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkinson, S. J., Hosford, M. A., and Molitoris, B. A. (2004) J. Biol. Chem. 279 5194-5199 [DOI] [PubMed] [Google Scholar]
- 32.Bacallao, R., Garfinkel, A., Monke, S., Zampighi, G., and Mandel, L. J. (1994) J. Cell Sci. 107 3301-3313 [DOI] [PubMed] [Google Scholar]
- 33.Ashworth, S. L., Wean, S. E., Campos, S. B., Temm-Grove, C. J., Southgate, E. L., Vrhovski, B., Gunning, P., Weinberger, R. P., and Molitoris, B. A. (2004) Am. J. Physiol. 286 F988-F996 [DOI] [PubMed] [Google Scholar]
- 34.Liu, G., Kaw, B., Kurfis, J., Rahmanuddin, S., Kanwar, Y. S., and Chugh, S. S. (2003) J. Clin. Invest. 112 209-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, D. B., Huang, E., and Ward, H. J. (2006) Am. J. Physiol. 290 F20-F34 [DOI] [PubMed] [Google Scholar]
- 36.Barnes, J. L., Osgood, R. W., Reineck, H. J., and Stein, J. H. (1981) Lab. Invest. 45 378-386 [PubMed] [Google Scholar]
- 37.Kwon, O., Phillips, C. L., and Molitoris, B. A. (2002) Am. J. Physiol. Renal Physiol. 282 F1012-1019 [DOI] [PubMed] [Google Scholar]
- 38.Mandel, L. J., Doctor, R. B., and Bacallao, R. (1994) J. Cell Sci. 107 3315-3324 [DOI] [PubMed] [Google Scholar]
- 39.Obeidova, H., Merta, M., Reiterova, J., Maixnerova, D., Stekrova, J., Rysava, R., and Tesar, V. (2006) Prague Med. Rep. 107 5-16 [PubMed] [Google Scholar]
- 40.Pagtalunan, M. E., Miller, P. L., Jumping-Eagle, S., Nelson, R. G., Myers, B. D., Rennke, H. G., Coplon, N. S., Sun, L., and Meyer, T. W. (1997) J. Clin. Invest. 99 342-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf, G., and Ziyadeh, F. N. (2007) Nephron. Physiol. 106 26-31 [DOI] [PubMed] [Google Scholar]
- 42.Andrews, P. (1988) J. Electron. Microsc. Tech. 9 115-144 [DOI] [PubMed] [Google Scholar]
- 43.Yin, M., Currin, R. T., Peng, X. X., Mekeel, H. E., Schoonhoven, R., and Lemasters, J. J. (2002) Ren. Fail. 24 147-163 [DOI] [PubMed] [Google Scholar]
- 44.Lowenborg, E. K., Jaremko, G., and Berg, U. B. (2000) Nephrol. Dial. Transplant. 15 1547-1555 [DOI] [PubMed] [Google Scholar]
- 45.Hartleben, B., Schweizer, H., Lubben, P., Bartram, M. P., Moller, C. C., Herr, R., Wei, C., Neumann-Haefelin, E., Schermer, B., Zentgraf, H., Kerjaschki, D., Reiser, J., Walz, G., Benzing, T., and Huber, T. B. (2008) J. Biol. Chem. 283 23033-23038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnabel, E., Anderson, J. M., and Farquhar, M. G. (1990) J. Cell Biol. 111 1255-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiser, J., Kriz, W., Kretzler, M., and Mundel, P. (2000) J. Am. Soc. Nephrol. 11 1-8 [DOI] [PubMed] [Google Scholar]
- 48.Fanning, A. S., Little, B. P., Rahner, C., Utepbergenov, D., Walther, Z., and Anderson, J. M. (2007) Mol. Biol. Cell 18 721-731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee, J. F., Zeng, Q., Ozaki, H., Wang, L., Hand, A. R., Hla, T., Wang, E., and Lee, M. J. (2006) J. Biol. Chem. 281 29190-29200 [DOI] [PubMed] [Google Scholar]
- 50.Harita, Y., Kurihara, H., Kosako, H., Tezuka, T., Sekine, T., Igarashi, T., and Hattori, S. (2008) J. Biol. Chem. 283 9177-9186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutton, T. A., and Molitoris, B. A. (1998) Semin. Nephrol. 18 490-497 [PubMed] [Google Scholar]
- 52.Rico, M., Mukherjee, A., Konieczkowski, M., Bruggeman, L. A., Miller, R. T., Khan, S., Schelling, J. R., and Sedor, J. R. (2005) Am. J. Physiol. 289 F431-F441 [DOI] [PubMed] [Google Scholar]
- 53.Ye, J., Tsukamoto, T., Sun, A., and Nigam, S. K. (1999) Am. J. Physiol. 277 F524-F532 [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Mariscal, L., Betanzos, A., and Avila-Flores, A. (2000) Semin. Cell Dev. Biol. 11 315-324 [DOI] [PubMed] [Google Scholar]
- 55.Rao, R. K., Basuroy, S., Rao, V. U., Karnaky, K. J., Jr., and Gupta, A. (2002) Biochem. J. 368 471-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsukamoto, T., and Nigam, S. K. (1999) Am. J. Physiol. 276 F737-F750 [DOI] [PubMed] [Google Scholar]
- 57.National Institutes of Health (1996) The Guide for the Care and Use of Laboratory Animals, National Academy Press, Washington, DC
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.