Abstract
Inositol 1,4,5-trisphosphate (IP3) receptors form tetrameric channels in endoplasmic reticulum membranes of mammalian cells and mediate IP3-induced calcium mobilization. In response to various extracellular stimuli that persistently elevate IP3 levels, IP3 receptors are also ubiquitinated and then degraded by the proteasome. Here, for endogenous type 1 IP3 receptor (IP3R1) activated by endogenous signaling pathways and processed by endogenous enzymes, we sought to determine the sites of ubiquitination and the composition of attached ubiquitin conjugates. Our findings are (i) that at least 11 of the 167 lysines in IP3R1 can be ubiquitinated and that these are clustered in the regulatory domain and are found in surface regions, (ii) that at least ∼40% of the IP3R1-associated ubiquitin is monoubiquitin, (iii) that both Lys48 and Lys63 linkages are abundant in attached ubiquitin chains, and (iv) that Lys63 linkages accumulate most rapidly. Additionally, we find that not all IP3R1 subunits in a tetramer are ubiquitinated and that nontetrameric IP3R1 complexes form as degradation proceeds, suggesting that ubiquitinated subunits may be selectively extracted and degraded. Overall, these data show that endogenous IP3R1 is tagged with an array of ubiquitin conjugates at multiple sites and that both IP3R1 ubiquitination and degradation are highly complex processes.
Inositol 1,4,5-trisphosphate (IP)34 receptors (IP3Rs) are ∼300-kDa endoplasmic reticulum (ER) membrane proteins that tetramerize to form Ca2+ channels that are gated by the co-agonists IP3 and Ca2+ and that govern Ca2+ release from the ER (1–3). There are three homologous mammalian IP3R types (termed IP3R1, IP3R2, and IP3R3) that can form either homo- or heterotetrameric channels. Each can be divided into an N-terminal ligand-binding domain, a large central regulatory domain that contains several modulatory sites, and a C-terminal channel domain that contains six membrane-spanning helices and the channel pore (see Fig. 1D). IP3R1, which is 2749 amino acids in length, is the most widely expressed and best studied of the three types (see Fig. 1D).
FIGURE 1.
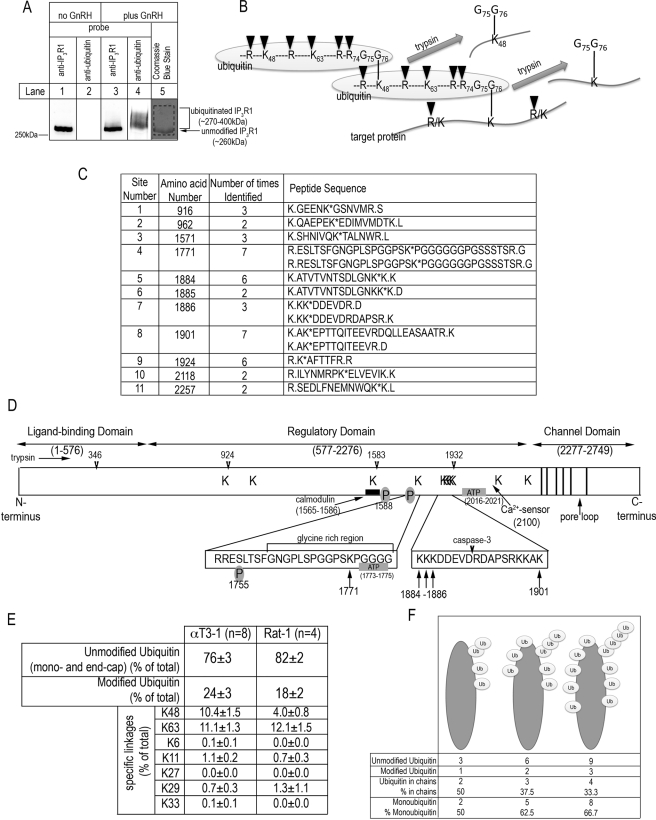
Identification of IP3R1 ubiquitination sites and ubiquitin chain linkages by mass spectrometry. A, αT3-1 cells were incubated without or with 100 nm GnRH for 7 min. The cell lysates were then prepared and incubated with anti-IP3R1 to immunoprecipitate IP3R1. The samples were then electrophoresed by 5% SDS-PAGE and either stained with Coomassie Blue (lane 5) or transferred to nitrocellulose and probed with anti-IP3R1 or anti-ubiquitin (lanes 1–4). The bracket indicates ubiquitinated IP3R1 migrating at ∼270–400 kDa, the arrow indicates unmodified IP3R1 migrating at ∼260 kDa, and the dashed box in lane 5 indicates the region excised for mass spectrometry. B, a schematic representation of the trypsinization of a ubiquitinated substrate. Cleavage of ubiquitin and the target protein at the lysine (K) or arginine (R) residues indicated (black arrowheads) generates a target protein-derived signature peptide with an added mass of 114.04 Da (from the covalently linked Gly75-Gly76 motif of ubiquitin) and a missed cleavage at the modified lysine. Comparison of mass spectra with data bases allows for the identification of ubiquitination sites in substrate proteins. Application of the same logic to peptides derived from ubiquitin allows for the determination of ubiquitin chain linkages; in the example shown, a linkage via Lys48 yields a signature peptide. C, listed are the 11 ubiquitin-conjugated lysines found in IP3R1 and the peptide sequences in which they were identified. The periods indicate the trypsin cleavage sites, and the asterisks indicate the ubiquitinated lysines. The data shown are from eight independent analyses, and lysines were defined as ubiquitination sites only if they were identified in two or more of the independent analyses. Seven additional lysines, Lys720, Lys1544, Lys1951, Lys2079, Lys2650, Lys2700, and Lys2719, were each identified only once and thus have not been listed. D, a schematic representation of IP3R1, with ubiquitination sites indicated by K in the main diagram or by arrows in the expanded regions. The three domains of IP3R1 are the ligand-binding domain, the regulatory domain, and the channel domain, which contains six membrane-spanning helices (indicated by vertical lines) and the pore loop (between helices 5 and 6). Mild trypsinization of IP3R1 generates five fragments by virtue of cleavage at amino acids 346, 924, 1583, and 1932 (indicated by arrowheads). A glycine-rich region, a caspase-3 cleavage site, ATP- and calmodulin-binding sites, the Ca2+ sensor, sites of protein kinase A phosphorylation, and a coiled-coil region are all highlighted. E, αT3-1 and Rat-1 cells were incubated with 100 nm GnRH for 7 min or 10 nm endothelin-1 for 10 min, respectively, were prepared as in Fig. 1A (lane 5), and were subjected to ubiquitin-AQUA analysis. The values presented are percentages of total ubiquitin (modified plus unmodified) associated with IP3R1, from at least four independent experiments (means ± S.E.). Unmodified ubiquitin refers to either a single ubiquitin moiety (monoubiquitin) or the terminal ubiquitin in a chain (end cap ubiquitin). Modified ubiquitin refers to ubiquitin residues that are ubiquitinated. For αT3-1 and Rat-1 cells, total ubiquitin after stimulus was 1812 ± 473 and 290 ± 92 fmol, respectively. F, depicted for an individual IP3R1 subunit are models of how ubiquitin could be attached at maximal ubiquitination. In the examples given, IP3R1 subunits are tagged with 4, 8, or 12 ubiquitin moieties at a 3:1 ratio of unmodified to modified ubiquitin, and in each case the number and percentage of ubiquitin moieties involved in chains or present as monoubiquitin is tabulated.
In response to activation of certain G protein-coupled receptors that persistently elevate IP3, IP3Rs are degraded via the ubiquitin-proteasome pathway (4–7). This phenomenon, termed “IP3R down-regulation,” which has been described for all three IP3R types in various cell types and tissues (8, 9), appears to protect cells from the deleterious effect of prolonged Ca2+ mobilization (6). The ubiquitin-proteasome pathway is used by cells to maintain homeostasis by degrading both key proteins involved in important cellular processes (e.g. those that govern transcription and cell cycle transitions) and by allowing for ER-associated degradation (ERAD), a mechanism that accounts for the disposal of aberrant or unrequired ER proteins (e.g. misfolded proteins or unassembled subunits of multimeric protein complexes) (10). ERAD is currently being intensely studied and appears to rely on several key proteins, including Ubc7 and Hrd1 (10–12), which ubiquitinate substrates, and p97, an AAA-ATPase that aids in the removal of substrates from the ER (10, 13). It appears that IP3Rs are processed by ERAD, because both p97 and Ubc7 are involved in their degradation (14, 15).
Ubiquitination refers to the process of conjugating ubiquitin, a 76-amino acid protein, to a target protein through the concerted action of E1, E2, and E3 enzymes (16). The E1 ubiquitin-activating enzyme first activates ubiquitin by forming a thiolester bond with the C-terminal glycine of ubiquitin. The activated ubiquitin moiety is then transferred to a ubiquitin-conjugating enzyme (E2 or Ubc), again through a thiolester bond. A ubiquitin-protein ligase (E3) then recognizes the target protein and recruits a charged cognate E2 to its vicinity. Ubiquitin is then conjugated to the target protein via a covalent linkage between the C-terminal glycine of ubiquitin and an ε-amino group of a lysine residue. Remarkably, ubiquitin contains seven lysines (Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, and Lys63), which can also be selected for ubiquitination, allowing for the formation of ubiquitin chains. Thus, targeted proteins can be tagged with either monoubiquitin or ubiquitin chains. Although our knowledge of the abundance and role of the various possible chain linkages is far from complete, it is clear that ubiquitin chains formed through Lys48 linkages signal for recognition and degradation by the proteasome, whereas Lys63-linked chains appear to be involved in diverse cellular processes, such as DNA damage response pathways, signal transduction through the NF-κB pathway, and protein trafficking (17, 18). Ubiquitin is removed from targeted proteins by deubiquitinating enzymes (DUBs), which serve to reverse the consequences of ubiquitination and/or to recycle ubiquitin (19, 20).
Little is known about how IP3Rs are processed by ERAD, including which of the 167 lysines in IP3R1 are targeted for ubiquitin conjugation. Unlike most other post-translational modifications, there is no consensus sequence for ubiquitination, and it remains unclear how lysines are selected (21, 22). Further, although it is clear that IP3Rs shift to much higher Mr values upon stimulation and thus are modified by many ubiquitin moieties (6, 7), it is not known whether IP3Rs are multiply monoubiquitinated or modified by ubiquitin chains, and if so, through what linkages. Recent advances in mass spectrometry now allow for the determination of both of the lysines occupied by ubiquitin (23) and the composition and quantity of ubiquitin chains associated with substrates (24). The latter relies on a novel technique termed absolute quantification (AQUA) (25) that uses isotopically labeled internal standard peptides to quantitate peptides generated by tryptic digestion. To date, several ubiquitin-AQUA studies have been performed on ubiquitinated substrates and have yielded some interesting results; e.g. that ∼40% of the ubiquitin associated with overexpressed epidermal growth factor receptor contains Lys63 linkages (26) and that purified cyclin B1 is first multiply monoubiquitinated and then chains are added at those ubiquitin moieties via Lys48, Lys63, and Lys11 linkages (27). However, a caveat with these studies is that the substrates analyzed were either overexpressed or ubiquitinated in vitro and may not accurately reflect the situation in vivo. Nevertheless, these mass spectrometric techniques provide a powerful tool for identifying sites of ubiquitin conjugation and ubiquitin chain linkages.
In the present study, we sought to define the molecular details of IP3R1 ubiquitination by first determining the sites at which IP3R1 is ubiquitinated and then the composition of attached conjugates. For this work, we focused on αT3-1 mouse pituitary gonadotropes, in which gonadotropin-releasing hormone (GnRH) induces a robust stimulation of endogenous IP3R1 ubiquitination and degradation (7), and on Rat-1 fibroblasts, in which endothelin-1 has a similar effect (14). Mass spectrometric analysis of ubiquitinated IP3R1 identified 11 lysines that can be ubiquitinated and revealed that IP3R1 is conjugated with both monoubiquitin and Lys48- and Lys63-linked ubiquitin chains. We also show that not all IP3R1 subunits in a tetramer are ubiquitinated and provide evidence that individual subunits in a tetramer can be selectively degraded. Taken together, this indicates that IP3R1 ubiquitination and degradation are highly complex events.
EXPERIMENTAL PROCEDURES
Materials—αT3-1 cells and Rat-1 fibroblasts were obtained and maintained as previously described (7, 14). The antibodies used were: anti-IP3R1, a rabbit polyclonal antibody raised against amino acids 2731–2749 of IP3R1 (7, 8); NT1, a rabbit polyclonal antibody raised against amino acids 326–341 of IP3R1 (a kind gift from Dr. Suresh Joseph, Thomas Jefferson University, Philadelphia, PA); and anti-ubiquitin (clone FK2), which was purchased from Biomol. GnRH, endothelin-1, and N-ethylmaleimide were purchased from Sigma. Bortezomib was a kind gift from Millennium Pharmaceuticals Inc. Reagents for immunoprecipitation, electrophoresis, and immunoblotting were obtained as previously described (7).
Immunoprecipitation for Mass Spectrometric Analysis—αT3-1 and Rat-1 cells were grown to confluence in 15-cm-diameter dishes, and 24 h prior to harvest, Rat-1 cells were cultured in serum-free medium. After stimulation, the cells were harvested by adding 3–6 ml/dish of ice-cold lysis buffer (50 mm Tris, 150 mm NaCl, 1% Triton X-100, 1 mm EDTA, 0.2 mm phenylmethylsulfonyl fluoride, 10 μm pepstatin, 0.2 μm soybean trypsin inhibitor, pH 8.0). The lysates were then incubated with 2.5 mm N-ethylmaleimide for 1 min to inhibit DUBs, quenched by adding 5 mm dithiothreitol, incubated at 4°C for 30 min with occasional mixing, and cleared by centrifugation (16,000 × g for 10 min at 4°C). Supernatants were then incubated overnight at 4 °C with protein A-Sepharose CL-4B beads and anti-IP3R1. Immune complexes were then isolated by centrifugation (1000 × g for 1 min at 4°C), washed three to five times with 1 ml of ice-cold lysis buffer, resuspended in gel loading buffer, boiled for 5 min, subjected to SDS-PAGE, and either Coomassie Blue-stained (10% acetic acid, 45% ethanol, 2.5 g/liter Brilliant Blue R) followed by destaining (10% acetic acid, 45% ethanol) or transferred to nitrocellulose and immunoblotted and analyzed as previously described (7).
Ubiquitin Site Identification and AQUA Analysis—Regions corresponding to unmodified and/or ubiquitinated IP3R1 were excised from gels, destained (50 mm ammonium bicarbonate, 50% acetonitrile), and then incubated overnight at 37 °C with 20 μg/ml trypsin (Promega) diluted in 50 mm ammonium bicarbonate, 0.005% n-dodecyl β-d-maltoside. Following digestion, ubiquitin and/or IP3R1 isotope-labeled internal standard AQUA peptides were added to samples, and then peptides were extracted from the gel regions twice using 50% acetonitrile, 5% formic acid and dried in a Speedvac. Ubiquitin-AQUA peptides include diglycine signature peptides corresponding to the seven ubiquitin chain linkages, as well as a battery of unbranched tryptic peptides from ubiquitin as previously described (27, 28). The IP3R1-AQUA peptides LEELGDQR (amino acids 538–545) and LQDIVSALEDR (amino acids 1603–1613) were generated by Cell Signaling Technologies (Danvers, MA). Extracted peptides were desalted with Stage-Tip as described (29), dried, resuspended with 5% formic acid, 0.01% H2O2, and injected onto a 100-μm-internal diameter fused silica column pulled to a tip and packed with Magic C18 material (Michrom Bioresources, Auburn, CA). Peptides injected onto the column were separated using a linear gradient of buffer B (acetonitrile, 0.125% formic acid) in buffer A (3% acetonitrile, 0.125% formic acid), and eluting peptides were analyzed using either an LTQ-FT or LTQ-Orbitrap mass spectrometer (Thermo, San Jose, CA) operating in data-dependent MS/MS (tandem mass spectrometry) mode. During each duty cycle, the mass spectrometer collected a high resolution precursor ion scan, followed by MS/MS on the 10 most abundant precursor ions. For IP3R1 ubiquitination site identification, MS/MS spectra were searched using Sequest with a variable mass addition of 114.0429 Da on lysines to match the diglycine signature, and spectral matches corresponding to IP3R1 ubiquitination sites were validated based on the mass accuracy of the precursor ion and manual inspection of the MS/MS spectra. For AQUA analysis, the ion-extracted chromatogram was drawn with 10 ppm mass accuracy, and cell-derived IP3R1 and ubiquitin peptides were quantified from the ratio of the area under the curve for these peptides versus that for ubiquitin- or IP3R1-derived AQUA peptides. Throughout the AQUA analysis, conditions were optimized to avoid differences in the reference peptide recovery as described (25, 27). This allowed for a <50% coefficient of variation for quantitation of total ubiquitin amount using the four ubiquitin-AQUA peptides, and a <20% coefficient of variation for quantitation of IP3R1 amount using the two IP3R1-AQUA peptides.
Immunoprecipitation from Microsomal Preparations—For immunoprecipitations involving anti-ubiquitin, we first prepared microsomes to remove free ubiquitin. Control or stimulated cells in 15-cm-diameter dishes were rinsed with ice-cold 155 mm NaCl, 10 mm HEPES, 1 mm EDTA, pH 7.4, were detached with 10 ml of 10 mm Tris, 1 mm EGTA, 0.2 mm phenylmethylsulfonyl fluoride, 10 μm pepstatin, 0.2 μm soybean trypsin inhibitor, pH 7.4), and were processed in a Dounce homogenizer (20 strokes in the presence of 2.5 mm N-ethylmaleimide and two additional strokes after the addition of 5 mm dithiothreitol), and the microsomes were isolated by centrifugation (38,000 × g for 10 min at 4°C). They were then solubilized by incubation in 5 ml of lysis buffer for 30 min at 4 °C and then centrifuged (16,000 × g for 10 min at 4°C), and supernatants were incubated overnight at 4 °C with either anti-IP3R1 or anti-ubiquitin and protein A-Sepharose CL-4B beads. Immune complexes were then processed for immunoblotting as previously described (7).
Blue Native PAGE—All of the reagents for blue native PAGE were obtained from Invitrogen, and the methods used are described in the Invitrogen NativePAGE™ Novex® Bis-Tris gel system user manual. Briefly, αT3-1 cells in six-well Falcon plates were harvested with 200 μl of ice-cold lysis buffer and after 30 min at 4 °C were centrifuged (16,000 × g for 10 min). 75 μl of supernatants were mixed with 25 μl of 4 × sample buffer and 5 μl of 5% G-250 sample additive, and 20 μg of protein for each sample was loaded into wells of a 3–12% NativePAGE™ Novex® Bis-Tris gel and electrophoresed for 1 h at 150 V in dark cathode buffer and then for 45 min at 250 V in light cathode buffer. The gel was then washed for 10 min in blotting buffer (7) and transferred to polyvinylidene difluoride membrane, which was activated by washing in methanol. After transfer, the membrane was washed in 40% methanol, 10% acetic acid for 15 min, rinsed in water, and processed for immunoblotting as previously described (7). Duplicate aliquots of supernatants were also subjected to regular SDS-PAGE and processed for immunoblotting as described (7).
RESULTS
At Least 11 of the 167 Lysines in IP3R1 Can Be Ubiquitinated—We have previously demonstrated that exposure of αT3-1 cells to GnRH results in the ubiquitination and degradation of IP3R1 (7), which quantitative immunoblotting (8) reveals, represents ∼99% of the IP3R complement in these cells (data not shown). To further our understanding of the mechanisms of IP3R ubiquitination, we initially sought to identify the sites of ubiquitin conjugation by subjecting ubiquitinated IP3R1 to mass spectrometry. Samples were prepared from αT3-1 cells treated with 100 nm GnRH for 7 min, which causes maximal IP3R1 ubiquitination (7). In the absence of GnRH, immunopurified IP3R1 migrates as a single band at ∼260 kDa (Fig. 1A, lane 1) and does not exhibit ubiquitin immunoreactivity (lane 2). In contrast, after exposure to GnRH, immunopurified IP3R1 is now strongly recognized by anti-ubiquitin, with immunoreactivity beginning at ∼270 kDa and extending upward (lane 4), consistent with the addition of multiple ubiquitin moieties; the reason that IP3R1 immunoreactivity is only partially shifted upward (lane 3) is that only ∼10% of IP3R1 is ubiquitinated under these conditions (7). For mass spectrometry, immunopurified IP3R1s were excised from analogous Coomassie Blue-stained gels (lane 5) and subjected to in-gel trypsin digestion, which cleaves ubiquitin between Arg74 and Gly75 and leaves diglycine motifs (Gly75-Gly76) covalently attached to modified lysines (Fig. 1B) (23). This and missed cleavages that result from the modification of lysines generates signature peptides that allows for identification of lysines as ubiquitin conjugation sites. Using this method, 11 lysines were identified as sites of ubiquitination: Lys916, Lys962, Lys1571, Lys1771, Lys1884, Lys1885, Lys1886, Lys1901, Lys1924, Lys2118, and Lys2257 (Fig. 1C). In the eight independent analyses performed, peptides were detected that covered ∼64% of the IP3R1 sequence and included 128 of the 167 lysines in IP3R1 (supplemental Fig. S1). These detectable lysines were distributed randomly throughout IP3R1, indicating that there was no bias for or against observing ubiquitination of lysines in a particular region. Clearly, however, given that 39 lysines were not covered, it remains a possibility that there are ubiquitination sites additional to the 11 described in Fig. 1C. As a control, we also analyzed IP3R1 immunoprecipitated from unstimulated cells, and as expected, almost no ubiquitination sites were identified (Lys962 only was identified in two of four experiments; data not shown).
How the 11 ubiquitination sites are located within the IP3R1 primary sequence is depicted in Fig. 1D; as yet, high resolution structural data for full-length IP3R1 have not been defined (3), so these sites cannot yet be mapped in three dimensions. However, all of the ubiquitination sites are located within the regulatory domain and are within or adjacent to binding sites for modifiers (1) or regions predicted to be surface-exposed loops on the basis of their susceptibility to mild trypsinization (Fig. 1D) (30, 31). Specifically, four sites, Lys916, Lys962, Lys1571, and Lys1924 are located near these trypsin cleavage sites, Lys1884–1886 and Lys1901 flank a caspase-3 cleavage site (Asp1891), and Lys1771 is located between a cAMP-dependent protein kinase phosphorylation site (position 1755) and an ATP-binding site (positions 1773–1775) and is in a glycine-rich region thought to form a flexible connection between the N-terminal cytosolic region of IP3R1 and the region encompassing the channel domain (1, 3). Additionally, Lys1571 is adjacent to a phosphorylation site (position 1588) and within a calmodulin-binding site (positions 1565–1586), and Lys2118 and Lys2257 are near a critical Ca2+-binding site, Glu2100 (1). Overall, these data show that the ubiquitination sites in IP3R1 are clustered in the regulatory domain and are found at sites likely to be surface-exposed.
Monoubiquitin and Lys48- and Lys63-linked Ubiquitin Chains Are the Predominant Modifications on IP3R1—To quantitate and characterize the attached ubiquitin conjugates, we used ubiquitin-AQUA (23, 25). This method relies on the fact that digestion of unmodified ubiquitin, either a single ubiquitin residue attached to the substrate (monoubiquitin) or the last ubiquitin of a chain (end cap ubiquitin), produces a series of peptides with unmodified lysine residues, whereas digestion of a ubiquitinated ubiquitin (i.e. that found in a chain) generates peptides containing the characteristic diglycine motif associated with the modified lysine (Fig. 1B). This revealed that in the absence of stimulus, the gel region encompassing IP3R1 contained 148 ± 34 fmol of total ubiquitin (i.e. unmodified plus modified ubiquitin), which increased dramatically to 1812 ± 473 fmol after exposure to GnRH, and remarkably, that 76 ± 3% of IP3R1-associated ubiquitin under stimulated conditions is unmodified, with the bulk of the remainder containing Lys48 and Lys63 linkages (10.4 and 11.1%, respectively) (Fig. 1E). To determine whether IP3R1 is similarly affected in another cell type, we also examined IP3R1 from endothelin-1-stimulated Rat-1 cells. Again, most of the ubiquitin associated with IP3R1 was unmodified (82 ± 2%), with Lys63 and Lys48 linkages making up the bulk of the remainder (Fig. 1E). In both cell types, other possible linkages were either undetectable or present only in very small amounts (Fig. 1E). Overall, these data show that in stimulated αT3-1 cells, only ∼25% of ubiquitin is ubiquitinated and, thus, that a maximum of ∼50% of total ubiquitin is involved in chains (Fig. 1F). Conversely, and remarkably, it can be concluded that the predominant modification on IP3R1 is monoubiquitin, which, at minimum (if diubiquitin is assumed to be the longest chain), comprises ∼50% of total IP3R1-associated ubiquitin (Fig. 1F). This is the theoretical minimum, however, and the real value could be higher, because if longer chains are attached, the proportion of monoubiquitin increases (Fig. 1F).
Kinetics and Stoichiometry of IP3R1 Ubiquitination—It was remarkable to find that ubiquitinated IP3R1 was modified so strongly by monoubiquitin and that only ∼10% of attached ubiquitin was Lys48-linked (Fig. 1E). Our expectation was that ubiquitin would be attached predominantly via Lys48-linked chains, because the current consensus is that it is Lys48-linked chains that direct substrates to the proteasome (17, 18, 32), and the only known consequence of IP3R ubiquitination is to evoke IP3R degradation (6).
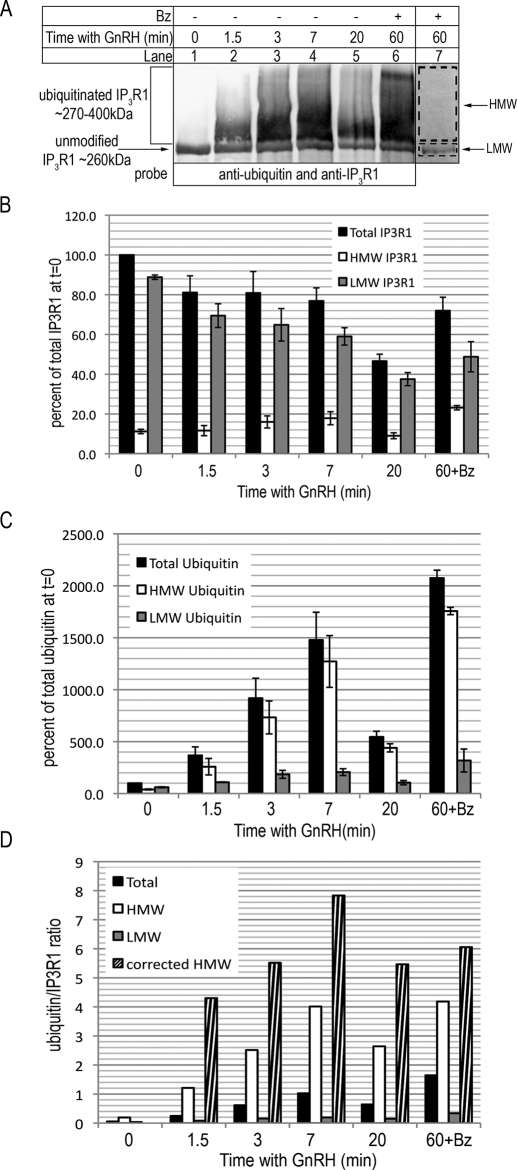
To extend this work we first examined the kinetics of IP3R1 ubiquitination and also, by quantitating IP3R1 abundance, its stoichiometry. For these studies, immunopurified IP3R1 was separated on 4% gels to allow for clear separation between unmodified IP3R1 and the smear of ubiquitinated IP3R1, which was most readily visualized when nitrocellulose was probed simultaneously with anti-IP3R1 and anti-ubiquitin (Fig. 2A). Ubiquitin immunoreactivity was evident 1.5 min after GnRH addition, peaked at 7 min, and then declined, at least in part because of IP3R1 degradation, as evidenced by a marked decrease in the unmodified IP3R1 band at 20 min (lanes 2–5). Consistent with the role of the proteasome in IP3R degradation, bortezomib both inhibited IP3R1 degradation and caused the accumulation of ubiquitinated species (lane 6). For mass spectrometry, regions corresponding to unmodified IP3R1 (low molecular weight (LMW)) and ubiquitinated IP3R1 (high molecular weight (HMW)) were excised from Coomassie Blue-stained gels (lane 7) and analyzed for ubiquitin and IP3R1 content using AQUA (Fig. 2, B and C). Total IP3R1 levels (the sum of that present in the HMW and LMW regions) was reduced by GnRH treatment, to ∼47% of control levels after 20 min (Fig. 2B). This decline was inhibited by pretreatment with bortezomib and was largely accounted for by a decline in the IP3R1 content of the LMW region, in which the vast majority of IP3R1 was found (Fig. 2B). Conversely, total ubiquitin increased dramatically after GnRH treatment, peaking at ∼1500% of control levels after 7 min and at ∼2000% of control levels in the presence of bortezomib plus GnRH, and this was largely accounted for by an increase in the ubiquitin content of the HMW region (Fig. 2C). Importantly, these increases in ubiquitin content paralleled an increase in the IP3R1 content of the HMW region, confirming that ubiquitinated IP3R1 was present in the HMW region. Additionally, after 20 min of exposure to GnRH, both the IP3R1 and ubiquitin content of the HMW region declined markedly, consistent with ongoing degradation of ubiquitinated IP3R1 (Fig. 2, B and C). Finally, it should be noted that the mass spectrometry and immunoblotting data concur very well (Fig. 2, A–C), indicating that the mass spectrometric methods accurately quantitate the proteins extracted from gels.
FIGURE 2.
Kinetics and stoichiometry of IP3R1 ubiquitination. A, αT3-1 cells were incubated with 100 nm GnRH for 0, 1.5, 3, 7, and 20 min, or preincubated with 1 μm bortezomib (Bz) for 60 min and then treated with 100 nm GnRH for 60 min. The cell lysates were then prepared and incubated with anti-IP3R1 to immunoprecipitate IP3R1, which was then electrophoresed by 4% SDS-PAGE, and either transferred to nitrocellulose and probed simultaneously for anti-ubiquitin and anti-IP3R1 (lanes 1–6) or stained with Coomassie Blue (lane 7; only data from the bortezomib/GnRH incubation condition are shown). The arrow and bracket indicate unmodified and ubiquitinated IP3R1, respectively, and the dashed boxes in lane 7 delineate the HMW and LMW regions excised for mass spectrometry. B and C, mass spectrometric quantification of IP3R1 and ubiquitin levels. The data shown are the IP3R1 and ubiquitin levels in the HMW and LMW regions and total ubiquitin (the sum of that present in both HMW and LMW regions). In each independent experiment, the data were normalized to the amount of total IP3R1 or ubiquitin at t = 0, and normalized values were then combined and graphed (mean ± S.E., n = 3). Total ubiquitin and total IP3R1 levels at t = 0 were 176 ± 36 and 3436 ± 872 fmol, respectively. D, the total, HMW, and LMW ubiquitin/IP3R1 ratios were determined from the fmol values of IP3R1 and ubiquitin at each time point. Corrected HMW ratios were obtained from corrected fmol values of ubiquitin and IP3R1. For ubiquitin, these were determined by subtracting the fmol value present at t = 0 from the fmol values at each time point. For IP3R1, the values were determined by subtracting 11% of the total amount of IP3R1 at each time point from that present in the HMW regions, which accounts for the incomplete separation of unmodified IP3R1 from ubiquitinated IP3R1.
The stoichiometry of ubiquitination was calculated from the molar ratio of ubiquitin and IP3R1 in each gel region (Fig. 2D). The total ubiquitin/IP3R1 ratio was ∼0.05 in unstimulated cells and ∼1 under conditions of maximal ubiquitination. This information is of limited value, however, because only a small proportion of IP3R1 (∼10%) is found in ubiquitinated form in stimulated cells (7), and both ubiquitinated and unmodified IP3R1 are included in this calculation. Much more meaningful are data from the HMW region, which should contain only ubiquitinated IP3R1. In this region, the ubiquitin/IP3R1 ratio was ∼0.2 in unstimulated cells and ∼4 at maximal stimulation, indicating that, on average, a maximally ubiquitinated IP3R1 is coupled to ∼4 molecules of ubiquitin. This is likely to be an underestimate, however, because it appears that separation of unmodified receptor from ubiquitinated IP3R1 was not as complete as anticipated and that some unmodified IP3R1 migrates in the HMW region. This is evident from the fact that, in unstimulated cells, 11 ± 3% of total IP3R1 was found in the HMW region (Fig. 2B); why this “tailing” occurs is not clear, but it is certainly not due to IP3R1 ubiquitination because the ubiquitin/IP3R1 ratio in the HMW region under unstimulated conditions was very low (∼0.2). Correcting for this aberration increases the ubiquitin/IP3R1 ratio to ∼8 at peak ubiquitination (Fig. 1D). Overall, these data show that ubiquitin is rapidly attached to activated IP3R1 in a manner that precedes IP3R1 degradation and that under conditions of maximal stimulation, an average of ∼8 ubiquitin moieties are attached to those receptors modified. However, it is important to note that this is the average value for species present in the HMW region and that the likelihood is that some IP3R1s will be tagged with >8 ubiquitin moieties, and some <8.
Next we examined, using AQUA analysis, the time dependence of ubiquitin chain formation on IP3R1 and, again, focused on the HMW region, because this region contains the vast majority of ubiquitin (Fig. 2C). Consistent with the data in Fig. 1E, the GnRH-induced increase in ubiquitin content is predominately accounted for by unmodified ubiquitin, which at each time point comprised ∼70% of total ubiquitin (Fig. 3, A and B). Thus, monoubiquitin plus end cap ubiquitin are the predominant species, and it can be deduced, as in Fig. 1F, that at each time point, a minimum of ∼40% of total ubiquitin associated with IP3R1 is monoubiquitin.
FIGURE 3.
Kinetics of ubiquitin chain formation. A, the HMW regions defined in Fig. 2A were subjected to AQUA analysis to quantitate ubiquitin chain linkages. The values tabulated are fmol total ubiquitin (modified plus unmodified), total, modified, or unmodified ubiquitin expressed as percentages of the increase in total ubiquitin 7 min after GnRH addition, and unmodified ubiquitin expressed as a percentage of total ubiquitin at each time point. Other linkages is the sum of Lys11, Lys29, Lys33, Lys27, and Lys6 linkages. The percentage of GnRH-induced increase was calculated by first subtracting the fmol ubiquitin at t = 0 from the fmol ubiquitin present at each time point. These values were then normalized to fmol ubiquitin at t = 7, and normalized values were combined. B–D, graphs of selected data. All of the data shown are the means ± S.E. from three independent experiments.
The increase in modified ubiquitin is predominately accounted for by increases in Lys48- and Lys63-linked ubiquitin, with only a minimal contribution from other linkages (Lys11, Lys29, Lys33, Lys27, and Lys6) (Fig. 3, A and C). Interestingly, Lys63-linked ubiquitin initially accumulates on IP3R1 at approximately twice the rate of Lys48-linked ubiquitin, such that at early time points (1.5 and 3 min) Lys63 linkages are predominant (Fig. 3, A and C). Thereafter, Lys48- and Lys63-linked ubiquitin accumulate to equal levels and then decline in parallel (Fig. 3, A and C). In contrast, in the presence of bortezomib plus GnRH, levels of Lys48-linked ubiquitin exceed Lys63-linked ubiquitin (Fig. 3, A and D). Overall, these data show that Lys48- and Lys63-linked ubiquitin chains are the predominant forms associated with IP3R1 and that the accumulation of these chains is subject to differential regulation.
Individual IP3R1s in a Tetramer Are Ubiquitinated and Degraded—We next sought to establish whether or not all IP3R1s in a tetramer are ubiquitinated. To do this, we immunoprecipitated microsomal proteins from control and GnRH-stimulated αT3-1 cells with anti-ubiquitin and probed for IP3R1 (Fig. 4A); we reasoned that if both ubiquitinated and unmodified IP3R1s were isolated by anti-ubiquitin, it would show that the latter was co-immunoprecipitating with the former and that not all IP3R1 subunits in a tetramer are ubiquitinated. This turned out to be the case, because anti-ubiquitin isolated IP3R1 immunoreactivity from stimulated αT3-1 cells only that migrated as a smear beginning at ∼260 kDa (lanes 3 and 4); this smear co-migrated with both ubiquitinated IP3R1 migrating at ∼270–400 kDa (lane 6) and with unmodified IP3R1 migrating at ∼ 260 kDa (lanes 1 and 2). Thus, unmodified IP3R1 co-immunoprecipitates with ubiquitinated IP3R1, indicating that not all IP3Rs in a tetramer are ubiquitinated.
FIGURE 4.
Individual IP3Rs in a tetramer are ubiquitinated and degraded. A, αT3-1 cells were incubated with 100 nm GnRH for 7 min, and microsomes were prepared, lysed, and incubated with anti-IP3R1 to immunoprecipitate IP3R1 or with anti-ubiquitin to immunoprecipitate ubiquitinated proteins. Immunoprecipitated proteins were then electrophoresed by 4% SDS-PAGE, transferred to nitrocellulose, and probed with either anti-IP3R1 or anti-ubiquitin. The bracket indicates ubiquitinated IP3R1 at ∼270–400 kDa and the arrow indicates unmodified IP3R1 at ∼260kDa. B, αT3-1 cells were incubated with 100 nm GnRH for 0,7, 20, or 60 min. The cell lysates were then electrophoresed by blue native PAGE and probed with anti-IP3R1. The arrows indicate the migration positions of tetrameric IP3R1 at ∼1.1 MDa and immunoreactivity that corresponds in size to trimeric and dimeric IP3R1 complexes. Also shown is the percentage of total IP3R1 immunoreactivity at each time point that migrated in the trimer/dimer region (mean, n = 2). C, 7% SDS-PAGE of the same samples, probed with anti-IP3R1 (lanes 1–4) and NT1 (lanes 5–8), which recognize the C and N termini of IP3R1, respectively.
We next wondered whether this might lead to the degradation of individual subunits in a tetramer and the appearance of nontetrameric IP3R1s in stimulated cells. To assess this possibility, we subjected lysates from control and stimulated cells to nondenaturing blue native PAGE, which allows for the separation of proteins while still in complexes (Fig. 4B). Under these conditions, IP3R1 immunoreactivity in unstimulated cells migrated primarily at ∼1.1 MDa, consistent with IP3R1 existing as tetramers (lane 1). GnRH caused a reduction in the ∼1.1 MDa immunoreactivity, consistent with the induction of IP3R1 degradation (lanes 2–4), and remarkably, the appearance of a substantial amount of immunoreactivity at ∼ 600 and ∼800 kDa, perhaps because of the formation of dimeric and trimeric IP3R1 complexes (lanes 2–4). The appearance of this immunoreactivity at ∼600 and ∼800 kDa was strongest after 7–20 min exposure to GnRH (lane 2 and 3) and declined thereafter (lane 4) and was not due to IP3R1 cleavage, because GnRH did not induce the formation of <260-kDa IP3R1 fragments when samples subjected to denaturing SDS-PAGE were probed with antibodies against either the C terminus (Fig. 4C, lanes 1–4) or N terminus of IP3R1 (Fig. 4C, lanes 5–8). Taken together, these data show that not all IP3R1s in a tetramer are ubiquitinated and suggest that as IP3R1 degradation proceeds, trimeric and dimeric IP3R1 complexes form.
DISCUSSION
In summary, the data presented represent the first comprehensive analysis of the ubiquitination of an endogenous substrate by endogenous enzymes and reveals a far more complex scenario than originally anticipated. Specifically we show that at least 11 of the 167 lysines in IP3R1 are sites of ubiquitination, that these sites are occupied primarily by monoubiquitin, that both Lys48- and Lys63-linked ubiquitin chains are added, that Lys63-linked chains accumulate most rapidly, and that not all subunits in an IP3R1 tetramer are ubiquitinated.
Typically, post-translational modification sites are selected by adjacent sequence information (e.g. NX(S/T) for N-linked glycosylation), but there is no known consensus sequence for ubiquitination (21). There is, however, growing evidence that selection of lysines for ubiquitination may rely on structural features rather than sequence, because in a systematic analysis of 135 ubiquitination sites in proteins from Saccharomyces cerevisiae, it was found that ubiquitin was preferentially added to lysines in surface-exposed loops, as compared with other structural features like α-helices and β-sheets (21). Additionally, for human epidermal growth factor receptor overexpressed in porcine aortic endothelial cells, six ubiquitination sites were identified in the kinase domain, and all of these were located on exposed surfaces (26). For IP3R1, it also appears that the ubiquitination sites are located in exposed regions, because many are found adjacent to sites cleaved by mild trypsinization or are near binding sites for modifiers of IP3R1 (Fig. 1D). At present, only the structure of the IP3R1 ligand-binding domain, in which we were unable to detect any ubiquitination sites, has been solved (33). As more of the structure is solved, it will be fascinating to see how the ubiquitination sites we identified are arranged in three dimensions. Finally, all of the ubiquitinated lysines we identified in mouse IP3R1 are completely conserved in IP3R1 from other mammalian species (rat and human), but there is only partial conservation of these lysines in IP3R2 and IP3R3 (data not shown). Nevertheless, it is likely that IP3R1, IP3R2, and IP3R3 are ubiquitinated in similar regions, because the three receptor types are 60–70% identical at the amino acid level (34) and are likely to adopt similar conformations, and IP3R2 and IP3R3 do have lysine residues in regions corresponding to those ubiquitinated in IP3R1.
Interestingly, our data differ considerably from a previous immunoprecipitation/immunoblotting-based analysis of the sites of ubiquitination in IP3R1. In that study (35), examining rat IP3R1 overexpressed in CHO-K1 cells, it was concluded that the region C-terminal to Asp1891 was monoubiquitinated and that the region N-terminal to Asp820 was polyubiquitinated. Our data, in contrast, did not consistently detect any ubiquitination sites N-terminal to Asp820 and found multiple sites C-terminal of Asp1891. This discrepancy is unlikely to be due to mouse and rat IP3R1 being different (they are 98% identical at the amino acid level (34)) or that we were unable to assess the ubiquitination status of every lysine in IP3R1 (the 39 lysines we were unable to assess were spread evenly throughout IP3R1). Rather, it is likely due to the fact that we analyzed endogenous IP3R1, whereas Bhanumathy et al. (35) analyzed overexpressed exogenous receptors. Our previous work has indicated that endogenous and exogenous IP3Rs are ubiquitinated with different characteristics, with highly overexpressed exogenous receptors being ubiquitinated even in the absence of stimulus, and with stimulus-dependent ubiquitination occurring only at very low levels of expression (7). Why highly overexpressed IP3R1s are ubiquitinated differently than endogenous IP3R1 is currently unknown but could result from misfolding or atypical interactions with the endogenous ubiquitination machinery.
During the course of this work, it was shown that iodoacetamide-based alkylation methods can cause 2-acetaminoacetamidation of lysines, a modification with an atomic composition (C4H6N2O2) and mass addition (114.0429 Da) identical to that seen with diglycine conjugation and that this can result in the false identification of lysines as ubiquitination sites (36). One of the experiments performed in this study (the data from which was later omitted) employed iodoacetamide and contained evidence for iodoacetamide-derived lysine modifications identical to those described (36), apparently because of incomplete removal of iodoacetamide prior to trypsin digestion. In that experiment, 48 lysines in IP3R1 were found to have a mass addition of 114.0429 Da, and of those, 10 were found at the C termini of peptides. Because trypsin cannot cleave adjacent to ubiquitin-modified lysines (37), these lysine modifications must result from the effects of iodoacetamide. Furthermore, extracted ion chromatograms generated for the Lys48 and Lys63 linkage-derived diglycine signature peptides revealed peak doublets for each, with one member of the doublet corresponding to the bona fide diglycine-modified signature peptide and the other corresponding to a 2-acetamidoacetamide-modified lysine. Additionally, we noticed that some lysines were modified with a single acetamide group (C2H3NO) as evidenced by a mass increase of 57.021 Da. Thus, if iodoacetamide is employed as an alkylating agent, we recommend monitoring for these three indicators of possible iodoacetamide-induced artifacts to ensure accurate identification of ubiquitination sites.
Our AQUA analysis of ubiquitinated IP3R1 yielded some intriguing results. First, IP3R1 is predominately tagged with monoubiquitin (∼40–50% of total ubiquitin, if diubiquitin chains are the longest present, but >50% if longer chains are attached). This is remarkable, because the consensus view has been that tetraubiquitin chains linked though Lys48 is the minimal requirement for efficient proteasomal degradation (32). However, it is becoming apparent that the proteasome may recognize different ubiquitin conjugates, including monoubiquitin, through interactions with selective ubiquitin-binding domains found in proteasomal subunits (17). Indeed, recently, a novel ubiquitin-binding domain, called Pru for pleckstrin-like receptor for ubiquitin, that preferentially interacts with monoubiquitin and diubiquitin chains was identified in Rpn13, a proteasomal subunit (38, 39). Thus, the monoubiquitin and short ubiquitin chains that are most likely to be attached to IP3R1, could be acting as signals for proteasomal degradation.
Second, we found that Lys63 and Lys48 linkages were the predominant ubiquitin chain linkages associated with IP3R1. The presence of Lys63 linkages was a surprise, because these typically play a regulatory role in processes like the DNA damage response pathway and NF-κB signaling, rather than in mediating proteasomal degradation (17, 18). This raises the possibility that some of the ubiquitin conjugates (i.e. the Lys63 linkages) on IP3R1 may have functions other than signaling for proteasomal degradation. However, Lys63-linked ubiquitin chains have also been shown to mediate proteasomal degradation in vitro (27, 40), so it remains a possibility that the Lys63 linkages on IP3R1 also act as a degradation signal. It is important to note that the techniques used herein cannot define whether chains longer than diubiquitin are present and if so, whether chains of three or more ubiquitin moieties are homogeneous, i.e. contain all the same linkages (either Lys48 or Lys63), or are mixed linkage chains, i.e. contain both Lys48 and Lys63 linkages. With regard to the generation of Lys48 and Lys63 linkages, this could be accomplished by two E2/E3 pairs, each adding a specific linkage, or by a single E2/E3 pair that synthesizes both linkages. Recent in vitro analyses of ubiquitin chain formation by various purified E2s and E3s demonstrated that either scenario could explain ubiquitin chain formation on IP3R1 (27, 39). Specifically, analysis of ubiquitin chain formation on cyclin B1 by APC, an E3, showed that, when paired with the E2s, UbcH10, or Ubc4, ubiquitin chains were formed via Lys48, Lys63, and Lys11 linkages (27). On the other hand, when the purified ligases, E6AP and Nedd4, were paired with UbcH5, the human homologue of Ubc4, homogenous Lys48- and Lys63-linked chains, respectively, were synthesized (41). At present, we cannot distinguish between these possibilities for IP3R1 ubiquitination, because the relevant E3 or E3s have yet to be identified. We do know, however, that Ubc7 is an E2 that mediates IP3R1 ubiquitination (15). Interestingly, there are several studies that show in vitro that Ubc7 catalyzes the formation Lys48 linkages, and there is currently no evidence that this E2 can form Lys63 linkages (42, 43). Thus, it is likely that an additional E2 and multiple E2/E3 pairs mediate the ubiquitination of IP3R1.
Third, we found that Lys63-linked ubiquitin chains accumulate more rapidly on IP3R1 than Lys48-linked ubiquitin chains, and in the presence of bortezomib, Lys48 linkages become predominant. These findings likely result from interplay between the addition of ubiquitin by ligases, the removal of ubiquitin by DUBs, and IP3R1 degradation by the proteasome. For example, the more rapid accumulation of Lys63 linkages may be accounted for by more efficient synthesis of Lys63 linkages, whether catalyzed by one or multiple E2/E3 pairs, and the marked slowing of Lys63-linked chain accumulation after 3 min could be due to an increase in Lys63-specific DUB activity that occurs as ubiquitin builds up on IP3R1. An interesting alternative is that Lys63-linked ubiquitin chains on IP3R1 may be edited in a manner similar to that described for A20. This protein, a potent inhibitor of the NF-κB pathway, contains both ligase and DUB activities, and for both RIP1 and IRAK1, A20 first cleaves Lys63-linked ubiquitin chains and then catalyzes the formation of Lys48 linkages, which results in proteasomal degradation of RIP1 and IRAK1 (28, 44). Further, the selective accumulation of Lys48 linkages on IP3R1 after proteasome inhibition with bortezomib may be due to preferential cleavage of Lys63 linkages by DUBs; several studies show that certain DUBs preferentially cleave Lys63-linked ubiquitin (19, 20), including a recent in vitro demonstration that ataxin-3 binds both homogenous Lys48- and Lys63-linked chains but preferentially cleaves Lys63 linkages (45). Further exploration of these possibilities will require the identification and characterization of the DUBs that interact with and mediate the deubiquitination of IP3R1.
Finally, because the mass spectrometry used herein assesses the ubiquitination of a large population of IP3R1 subunits and yields information that is the average of ubiquitination in that population, it is not yet possible to define which sites are ubiquitinated in an individual subunit or whether an individual subunit is modified with only one kind of ubiquitin conjugate (monoubiquitin, Lys48-linked chains, or Lys63-linked chains) or a mixture. It is clear, however, that ubiquitinated IP3R1s are modified with an average of ∼8 ubiquitin moieties and that at least ∼40% of these are monoubiquitin. This leads to the prediction that, on average, IP3R1s are modified at 6 lysines. That these lysines are often within or near sites involved in IP3R1 regulation raises the intriguing possibility that ubiquitination could, in addition to mediating IP3R1 degradation, modulate the activity of IP3R1.
With regard to the degradation of IP3R1, it was intriguing to find that not all subunits in an IP3R1 tetramer are ubiquitinated and that nontetrameric associations of IP3R1 appear to be formed during the degradation process. This raises the possibility that ubiquitinated IP3R1 subunits are selectively removed from tetramers, leaving behind trimers or dimers. How the ubiquitinated subunits are segregated and removed from the ER membrane likely involves p97, which helps remove ubiquitinated substrates from the ER through its ATPase activity (13, 14, 46) and which is required for IP3R1 ERAD (14). Because the degradation of IP3Rs by ERAD serves to protect cells from excessive Ca2+ mobilization (6), and it is highly unlikely that the nontetrameric IP3R1 complexes will be active as channels, the selective degradation of individual IP3R subunits may be the most efficient way of disabling channels without degrading the entire tetramer. It will now be intriguing to determine whether these putative nontetrameric IP3R1 complexes retetramerize or are subject to further degradation.
In summary, upon activation, tetrameric IP3R1 channels open and allow Ca2+ to be released from the ER, and this activation also triggers their recognition for ERAD (47). IP3R1s are then ubiquitinated at, on average, ∼6 lysines/subunit and have, on average, ∼8 ubiquitin moieties attached per subunit. Ubiquitination is likely accomplished by multiple E2/E3 pairs that catalyze the formation of monoubiquitin and Lys48- and Lys63-linked ubiquitin chains, on one or more, but not all, subunits in a tetramer. Degradation of the ubiquitinated subunits then appears to lead to the formation of trimeric and dimeric IP3R1 complexes. Overall, our data show that IP3R1 ubiquitination is far more complex than originally anticipated and suggest that the composition of ubiquitin conjugates is regulated by interplay between ligases, DUBs, and the proteasome. To better understand the mechanisms controlling IP3R1 processing by the ubiquitin-proteasome pathway, it will now be necessary to define the ubiquitin ligases and DUBs involved, and the manner in which IP3R1s are removed from the ER membrane.
Supplementary Material
Acknowledgments
We thank Maggie Pearce and Yuan Wang for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants DK49194 (to R. J. H. W.) and GM67945 (to S. P. G.). This work was also supported by American Heart Association Grant 0815754D (to D. A. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: IP3, inositol 1,4,5-trisphosphate; IP3R, inositol 1,4,5-trisphosphate receptor; ER, endoplasmic reticulum; ERAD, ER-associated degradation; DUB, deubiquitinating enzyme; AQUA, absolute quantification; GnRH, gonadotropin-releasing hormone, MS/MS, tandem mass spectrometry; E1, ubiquitin-activating enzyme; E2, ubiquitin conjugating enzyme; E3, ubiquitin-protein isopeptide ligase; LMW, low molecular weight; HMW, high molecular weight.
References
- 1.Foskett, J. K., White, C., Cheung, K. H., and Mak, D. O. D. (2007) Physiol. Rev. 87 593–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikoshiba, K. (2007) J. Neurochem. 102 1426–1446 [DOI] [PubMed] [Google Scholar]
- 3.Taylor, C., da Fonseca, P. A., and Morris, E. P. (2004) Trends Biochem. Sci. 29 210–219 [DOI] [PubMed] [Google Scholar]
- 4.Bokkala, S., and Joseph, S. K. (1997) J. Biol. Chem. 272 12454–12461 [DOI] [PubMed] [Google Scholar]
- 5.Oberdorf, J., Webster, J. M., Zhu, C. C., Luo, S. G., and Wojcikiewicz, R. J. H. (1999) Biochem. J. 339 453–461 [PMC free article] [PubMed] [Google Scholar]
- 6.Wojcikiewicz, R. J. H. (2004) Trends Pharmacol. Sci. 25 35–41 [DOI] [PubMed] [Google Scholar]
- 7.Wojcikiewicz, R. J. H., Xu, Q., Webster, J. M., Alzayady, K., and Gao, C. (2003) J. Biol. Chem. 278 940–947 [DOI] [PubMed] [Google Scholar]
- 8.Wojcikiewicz, R. J. H. (1995) J. Biol. Chem. 270 11678–11683 [DOI] [PubMed] [Google Scholar]
- 9.Wojcikiewicz, R. J. H., Ernst, S. A., and Yule, D. (1999) Gastroenterology 116 1194–1201 [DOI] [PubMed] [Google Scholar]
- 10.Meusser, B., Hirsch, C., Jarosch, E., and Sommer, T. (2005) Nat. Cell Biol. 7 766–772 [DOI] [PubMed] [Google Scholar]
- 11.Carvalho, P., Goder, V., and Rapoport, T. A. (2006) Cell 126 361–373 [DOI] [PubMed] [Google Scholar]
- 12.Tiwari, S., and Weissman, A. M. (2001) J. Biol. Chem. 276 16193–16200 [DOI] [PubMed] [Google Scholar]
- 13.Ye, Y., Meyer, H. H., and Rapoport, T. A. (2001) Nature 414 652–656 [DOI] [PubMed] [Google Scholar]
- 14.Alzayady, K. J., Panning, M. M., Kelley, G. G., and Wojcikiewicz, R. J. H. (2005) J. Biol. Chem. 280 34530–34537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster, J. M., Tiwari, S., Weissman, A. M., and Wojcikiewicz, R. J. H. (2003) J. Biol. Chem. 278 38238–38246 [DOI] [PubMed] [Google Scholar]
- 16.Fang, S., and Weissman, A. M. (2004) Cell. Mol. Life Sci. 61 1546–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda, F., and Dikic, I. (2008) EMBO Rep. 9 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickart, C. M., and Fushman, D. (2004) Curr. Opin. Chem. Biol. 8 610–616 [DOI] [PubMed] [Google Scholar]
- 19.Love, K., Catic, A., Schlieker, C., and Ploegh, H. L. (2007) Nat. Chem. Biol. 3 697–705 [DOI] [PubMed] [Google Scholar]
- 20.Ventii, K. H., and Wilkinson, K. D. (2008) Biochem. J. 414 161–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catic, A., Collins, C., Church, G. M., and Ploegh, H. L. (2004) Bioinformatics 20 3302–3307 [DOI] [PubMed] [Google Scholar]
- 22.Passmore, L. A., and Barford, D. (2004) Biochem. J. 379 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng, J., Schwartz, D., Elias, J. E., Thoreen, C. C., Cheng, D., Marsischky, G., Roelofs, J., Finley, D., and Gygi, S. P. (2003) Nat. Biotech. 21 921–926 [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick, D. S., Denison, C., and Gygi, S. P. (2005) Nat. Cell Biol. 7 750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkpatrick, D. S., Gerber, S. A., and Gygi, S. P. (2005) Methods 35 265–273 [DOI] [PubMed] [Google Scholar]
- 26.Huang, F., Kirkpatrick, D., Jiang, X., Gygi, S., and Sorkin, A. (2006) Mol. Cell 21 737–748 [DOI] [PubMed] [Google Scholar]
- 27.Kirkpatrick, D. S., Hathaway, N. A., Hanna, J., Elsasser, S., Rush, J., Finley, D., King, R. W., and Gygi, S. P. (2006) Nat. Cell Biol. 8 700–710 [DOI] [PubMed] [Google Scholar]
- 28.Newton, K., Matsumoto, M. L., Wertz, I. E., Kirkpatrick, D. S., Lill, J. R., Tan, J., Dugger, D., Gordon, N., Sidhu, S. S., Fellouse, F. A., Komuves, L., French, D. M., Ferrando, R. E., Lam, C., Compaan, D., Yu, C., Bosanac, I., Hymowitz, S. G., Kelley, R. F., and Dixit, V. M. (2008) Cell 134 668–678 [DOI] [PubMed] [Google Scholar]
- 29.Rappsilber, J., Mann, M., and Ishihama, Y. (2007) Nat. Protocols 2 1896–1906 [DOI] [PubMed] [Google Scholar]
- 30.Maes, K., Missiaen, L., Parys, J. B., De Smet, P., Sienaert, I., Waelkens, E., Callewaert, G., and De Smedt, H. (2001) J. Biol. Chem. 276 3492–3497 [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa, F., Iwasaki, H., Michikawa, T., Furuichi, T., and Mikoshiba, K. (1999) J. Biol. Chem. 274 316–327 [DOI] [PubMed] [Google Scholar]
- 32.Thrower, J. S., Hoffman, L., Rechsteiner, M., and Pickart, C. M. (2000) EMBO J. 19 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosanac, I., Alattia, J. R., Mal, T. K., Chan, J., Talarico, S., Tong, F. K., Tong, K. I., Yoshikawa, F., Furuichi, T., Iwai, M., Michikawa, T., Mikoshiba, K., and Ikura, M. (2002) Nature 420 696–700 [DOI] [PubMed] [Google Scholar]
- 34.Patel, S., Joseph, S. K., and Thomas, A. P. (1999) Cell Calcium 25 247–264 [DOI] [PubMed] [Google Scholar]
- 35.Bhanumathy, C. D., Nakao, S. K., and Joseph, S. K. (2006) J. Biol. Chem. 281 3722–3730 [DOI] [PubMed] [Google Scholar]
- 36.Nielsen, M. L., Vermeulen, M., Bonaldi, T., Cox, J., Moroder, L., and Mann, M. (2008) Nat. Methods 5 459–460 [DOI] [PubMed] [Google Scholar]
- 37.Kirkpatrick, D. S., Haas, W., and Gygi, S. P. (2008) in Protocols for the Bioanalytical Discovery of Post-translational Modifications (Lill, J., Sandoval, W., and Pham, V., eds) pp. 103–137, Transworld Research Network, Kerala, India
- 38.Husnjak, K., Elsasser, S., Zhang, N., Chen, X., Randles, L., Shi, Y., Hofmann, K., Walters, K., Finley, D., and Dikic, I. (2008) Nature 453 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiner, P., Chen, X., Husnjak, K., Randles, L., Zhang, N., Elsasser, S., Finley, D., Dikic, I., Walter, K., and Groll, M. (2008) Nature 453 548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann, R. M., and Pickart, C. M. (2001) J. Biol. Chem. 276 27936–27943 [DOI] [PubMed] [Google Scholar]
- 41.Kim, H. T., Kim, K. P., Lledias, F., Kisselev, A. F., Scaglione, K. M., Skowyra, D., Gygi, S. P., and Goldberg, A. L. (2007) J. Biol. Chem. 282 17375–17386 [DOI] [PubMed] [Google Scholar]
- 42.Li, W., Tu, D., Brunger, A. T., and Ye, Y. (2007) Nature 446 333–337 [DOI] [PubMed] [Google Scholar]
- 43.Ravid, T., and Hochstrasser, M. (2007) Nat. Cell Biol. 9 422–427 [DOI] [PubMed] [Google Scholar]
- 44.Wertz, I. E., O'Rourke, K. M., Zhou, H., Eby, M., Aravind, L., Seshagiri, S., Wu, P., Wiesmann, C., Baker, R., Boone, D. L., Ma, A., Koonin, E. V., and Dixit, V. M. (2004) Nature 430 694–699 [DOI] [PubMed] [Google Scholar]
- 45.Winborn, B. J., Travis, S. M., Todi, S. V., Scaglione, K. M., Xu, P., Williams, A. J., Cohen, R. E., Peng, J., and Paulson, H. L. (2008) J. Biol. Chem. 283 26436–26443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi, T., Tanaka, K., Inoue, K., and Kakizuka, A. (2002) J. Biol. Chem. 277 47358–47365 [DOI] [PubMed] [Google Scholar]
- 47.Alzayady, K. J., and Wojcikiewicz, R. J. H. (2005) Biochem. J. 392 601–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.