Abstract
Corticotropin-releasing factor receptor 1 (CRFR1) mediates the physiological actions of corticotropin-releasing factor in the anterior pituitary gland and the central nervous system. Using chemical cross-linking we have previously reported that residue 16 of sauvagine (SVG) is in a close proximity to the second extracellular loop of CRFR1. Here we introduced p-benzoylphenylalanine (Bpa) at position 17 of a sauvagine analog, [Tyr0, Gln1, Bpa17]SVG, to covalently label CRFR1 and characterize the cross-linking site. Using a combination of receptor mutagenesis, peptide mapping, and N-terminal sequencing, we identified His117 within the first transmembrane domain (TM1) of CRFR1 as the cross-linking site for Bpa17 of 125I-[Tyr0, Gln1, Bpa17]SVG. These data indicate that, within the SVG-CRFR1 complex, residue 17 of the ligand lies withina9Å distance from residue 117 of the TM1 of CRFR1. The molecular proximity between residue 17 of the ligand and TM1 of CRFR1 described here and between residue 16 of the ligand and the CRFR1 second extracellular loop described previously provides useful molecular constraints for modeling ligand-receptor interaction in mammalian cells expressing CRFR1.
Corticotropin-releasing factor (CRF),2 the main regulator of the hypothalamic pituitary adrenal axis (1), binds to specific G protein-coupled receptors CRFR1 (2, 3) and CRFR2 (4–7) cloned from several mammalian and nonmammalian species. A third CRF receptor, CRFR3, was found only in fish (8). Several CRF-like peptides were shown to interact with the CRF receptors with high affinity: sauvagine (SVG) characterized from the frog skin (9), urotensin I from the urophysis of fish (10), and urocortin I (UCN1) from the mammalian brain (11). Later, UCN2 and UCN3 (or stresscopins) (12–14) where characterized as CRFR2-specific ligands with little or no affinity to CRFR1.
The CRF receptors, which belong to group B of the G protein-coupled receptor superfamily, have six conserved cysteine residues and several N-linked glycosylations in their N-terminal extracellular domains. The extracellular cysteine residues form disulfide bridges that stabilize the structure of the N terminus (15, 16). The N-linked glycosylation moieties are important for processing through the Golgi system and cell surface expression (17). Activation of the cloned CRF receptors stimulates adenylate cyclase (4–7), phospholipase C (18), and mitogen-activated protein kinases (MAPKs) (19, 20).
Chemical and/or photoaffinity cross-linking have been extensively used for characterization of ligand-receptor interaction within group B of the G protein-coupled receptor superfamily (21–25), including the CRF receptors (26, 27). Recently, the photosensitive amino acid analog p-benzoylphenylalanine (Bpa) was used to substitute several residues within the UCN1 sequence, and photoaffinity cross-linking was used to map the interaction site; the data showed that the very N-terminal ligand residues (1–11) that are responsible for receptor activation are in a close proximity to the second extracellular loop within the juxtamembrane (J-) domain of CRFR1 (26). These data contradict the proposed model derived from the interaction of the free receptor N terminus with the ligand (28–31). We have previously used an oxidation-resistant SVG analog, [Tyr0, Gln1, Leu17]SVG (YQL-SVG), which binds to CRFR1 and CRFR2 with high affinity and which cross-links to the CRF receptors with a high efficiency (32), to map the disuccinimidyl suberate-cross-linked residues, and identified covalent cross-linking between Lys16 of SVG and Lys257 of CRFR1 in the second extracellular loop (27). In this paper we have used a Bpa-substituted SVG analog, [Tyr0, Gln1, Bpa17]SVG (YQB-SVG), which has high affinity for CRFR1, and mapped the Bpa17 cross-linking site to His117 of CRFR1 and thus established that position 17 of the radioligand lies within9Åof position 117 located in the first transmembrane-spanning domain (TM1) of CRFR1.
EXPERIMENTAL PROCEDURES
Materials—Unless indicated, all of the chemicals were purchased from Sigma. Na125I was purchased from PerkinElmer Life Sciences. The peptides, hCRF(1–41) (CRF), [Tyr0, Gln1, Leu17]SVG (YQL-SVG), [Tyr0, Gln1, Bpa17]SVG (YQB-SVG), and [Gln1, Bpa17, Tyr23]SVG (QBY-SVG) were synthesized in the Massachusetts General Hospital Biopolymer Facility, HPLC-purified, and analyzed by mass spectroscopy, N-terminal sequencing, and acid hydrolysis. Tissue culture media were from the Massachusetts General Hospital Media Facilities (Boston, MA). Centricon 30 centrifugal filters were purchased from Millipore (Bedford, MA). The molecular weight markers were purchased from Amersham Biosciences Pharmacia Life Science.
Cell Culture—COS-7 cells were transiently transfected with the murine (m) CRFR1 and the various CRFR1 mutants at 90% confluency using the DEAE dextran method as previously reported (27). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 200 units/ml penicillin, and 20 μg/ml streptomycin sulfate. The cells were cultured at 37 °C in a humidified atmosphere in 95% air, 5% CO2.
Site-directed Mutagenesis—Site-directed mutagenesis was performed as described previously (17, 33). Single and multiple mutations were introduced into CRFR1 cloned in the pcDNA1 plasmid. All of the mutations were confirmed by DNA sequencing.
Measurement of Receptor Expression on the Cell Surface—Nine of the ten c-myc epitope tag sequences (QKLISEEDL) were introduced within the N-terminal domain of mCRFR1 between Glu31 and Ser32 (16). Stably transfected LLCPK-1 cells (90–95% confluent) and transiently transfected COS-7 cells in 24-well plates (72 h after transfection) were rinsed with phosphate-buffered saline (PBS) containing 5% heat-inactivated fetal bovine serum and incubated with the 9E10 monoclonal antibody (16) at 1:1000 dilution. The cells were incubated for 2 h at room temperature, rinsed with PBS, and incubated for 2 h more with 125I-labeled sheep anti-mouse immunoglobulin G (200,000 cpm/well) diluted in PBS containing 5% fetal bovine serum. The supernatant was removed, and the cells were washed and lysed with 1 n NaOH. The cell lysates were collected and counted in a Micromedic γ-counter.
Radioligand Binding—The SVG analogs were radiolabeled, HPLC-purified, and used for binding assays as described previously (16). COS-7 cells were transiently transfected with mCRFR1 or mCRFR1 mutants in 10-cm tissue culture plates as described above. Two days after transfection the cells were trypsinized and plated into 24-well plates at a density of 200,000 cells/well. The binding assay was performed 2 days after plating when the cells reached 90–95% confluency. The cells were rinsed with a Tris-based binding buffer (50 mm Tris-HCl, pH 7.6, 100 mm NaCl, 2 mm CaCl2, 5 mm KCl, 5% heat-inactivated horse serum, 0.5% heat-inactivated fetal bovine serum). 125I-YQLS (100,000 cpm/well), prepared and purified as previously described (32), was added in the presence of increasing concentrations of the competing peptide for 2–4 h at room temperature. The cells were then rinsed (three times) with binding buffer and lysed with 1 n NaOH (0.25 ml × 3). The cell lysates were collected, and the radioactivity was counted in an automated γ-counter (Micromedic Systems, Horsham, PA).
Photo-cross-linking of 125I-YQB-SVG to CRFR1 and CRFR1 Mutants—Cells expressing wild type or mutant receptors were plated in 24-well plates and allowed to reach 90–95% confluency. The cells were then rinsed with PBS. 125I-YQB-SVG (1,000,000 cpm/well) was added for 2–4 h at room temperature. The cells were rinsed twice with ice-cold PBS and placed on an ice and exposed to UV light source (Blak Ray long-wave lamp, 366 nm, 7 milliwatts/cm2; UV Products, San Gabriel, CA) at a distance of ∼5 cm for 15 min while being covered with ice-cold PBS. The covering buffer was drained, and the cells were rinsed with PBS, lysed with SDS sample buffer, and analyzed on a 5–20% SDS-PAGE. After electrophoresis the gels were dried and autoradiographed using x-ray films or phosphorimaging screen.
cAMP Stimulation Assay—COS-7 cells were plated in 24-well plates and were allowed to reach 90–95% confluency. The cells were chilled on ice and rinsed with ice-cold PBS. The cells were then challenged with YQLS at different concentrations in Dulbecco's modified Eagle's medium containing 2 mm 3-isobutyl-1-methylxanthene, 1 mg/ml bovine serum albumin, and 35 mm HEPES, pH 7.4, at 37 °C for 15 min. The media were then removed, and the cells were rapidly frozen on dry ice. Intracellular cAMP was extracted by thawing the cells in 1 ml of 50 mm HCl, and cAMP was then measured using a specific radioimmunoassay (34).
CNBr Peptide Cleavage—After autoradiography, the radioactive protein bands were cut, and the proteins were electro-eluted in 1× SDS running buffer (25 mm of Tris base, 192 mm of glycine, and 0.1% of SDS). The eluate was concentrated using a Centricon 30 unit (Millipore). CNBr (100 mm) was added to the sample and dissolved in 70% formic acid at room temperature for 24 h. CNBr and formic acid were removed by lyophilization (three times). The proteins were analyzed on a 5–20% gradient SDS gel or a Tricine/SDS-PAGE (12% acrylamide) and autoradiographed using x-ray films or a phosphorimaging screen as previously described (27).
Sequencing of the CNBr-cleaved 125I-YQB-SVG Cross-linked Receptor Fragments—To determine the cross-linking site, we developed a radiosequencing method using Edman degradation of the CNBr-cleaved 125I-labeled photo-cross-linked receptor fragments as described previously (35). To facilitate N-terminal sequencing of the CNBr-cleaved receptor fragments, we used a receptor mutant bearing Met substitution at position 114; this places the potential cross-linking sites, determined from receptor mutagenesis and peptide mapping, closer to the N terminus of the cleaved fragment. Because the sequencing cycles release N-terminal residues from both the receptor fragments and the ligand, we synthesized 125I-[Gln1, Bpa17, Tyr23]SVG (125I-QBY-SVG), in which the 125I-Tyr is C-terminal to Bpa17. The radioactivity of the 125I-QBY-SVG remains covalently attached to the cross-linked fragment during Edman degradation. The Met114 mutant was expressed in 70–90% confluent COS-7 cells in a 10-cm tissue culture dish and incubated with ∼2,000,000 cpm of the ligand; binding and photo-cross-linking was performed as above. As predicted, CNBr cleavage of the 125I-QBY-SVG cross-linked Met114 mutant resulted in a sharp labeled band of ∼16 kDa on the Tricine-SDS-PAGE. The 125I-labeled receptor fragment was identified by autoradiography of the wet gel for 1 h and was then cut from the gel. The 125I-labeled CNBr-cleaved peptide was eluted from the gel fragment (typically ∼10,000–20,000 cpm are recovered), desalted on a Sephadex G-25 column, lyophilized, and reconstituted in 50 μl of double distilled H2O. The sample (equivalent to ∼10,000 cpm) was sequenced on a pulsed liquid-gas phase sequencer (model 477A; PE Applied Biosystems) in the peptide core facility of the Endocrine Unit (Ashok Khatri, Endocrine Unit, Massachusetts General Hospital). In brief, the purified CNBr fragment was covalently coupled to an aryl-amine-derived polyvinylidene difluoride membrane, which was placed in the sequencer. After each sequencing cycle, the membrane was rinsed with methanol, followed by heptane/ethyl acetate (1:1, v/v), to ensure full recovery of the 125I-labeled residues from the membrane. At each cycle, the released radioactivity was collected and counted in an automated γ-counter (Micromedic Systems, Horsham, PA). Approximately 60% of total radioactivity submitted for sequencing was recovered during 10 sequencing cycles.
Molecular Modeling—The structure of sauvagine was generated based on homology with the structures of urocortin-1 recently determined by NMR methods (36). The C terminus of sauvagine was then superimposed onto the structure of the C terminus of astressin while bound to the N terminus of the CRFR2 (37). The high sequence identify of both the ligands (astressin, sauvagine) and receptors (CRFR1 and CRFR2) generates favorable interacts as noted in the NMR analysis. The N terminus of sauvagine was modeled into the central portion of the TM-helical bundle of the receptor, with the far N terminus interacting with the third extracellular loop, based on multiple contact points generated by photoaffinity cross-linking for similar receptor systems including those for parathyroid hormone (PTH) (25, 38) and secretin (39).
RESULTS
We first examined ligand binding properties and cAMP stimulation potency of [Tyr0, Gln1, Bpa17]SVG (YQB-SVG) in LLCPK1 cells stably expressing CRFR1 (∼200,000 receptor copies/cell). The apparent Kd for YQB-SVG binding to LLCPK1 cell membranes stably expressing CRFR1 was 8 ± 2 nm, whereas that for the parent peptide, YQL-SVG, was 3 ± 1 nm (Fig. 1A). YQB-SVG and YQL-SVG increased cAMP accumulation with a similar EC50 of 3 nm to a similar maximum level (Fig. 1A). We then examined the cross-linking specificity of 125I-YQB-SVG to cell membranes prepared from LLCPK1 cells stably expressing CRFR1 (Fig. 1B). The cross-linked receptor has an apparent molecular mass above the highest standard used in this gel (Fig. 1B); this was previously characterized to be ∼80 kDa (27). Co-incubation of the membranes with 100 nm of unlabeled YQL-SVG completely inhibited the binding 125I-YQB-SVG and the intensity of the cross-linked band (Fig. 1B).
FIGURE 1.
Properties of the Bpa-substituted sauvagine, [Tyr0, Gln1, Bpa17]SVG (YQB-SVG). A, binding and signaling properties. Intact LLCPK-1 cells expressing mCRFR1 were incubated with 125I-YQ-SVG (∼100,000 cpm) in the presence of increasing concentrations of YQB-SVG or YQ-SVG for 2 h at 18 C; the cells were rinsed (three times) with binding buffer; the cells were lysed with 1 m NaOH (0.25 ml × 3), and the amount of bound radioligand was determined by counting in a γ-counter. The data (B/B0%) are calculated from the means ± S.D. of triplicates. Stimulation of cAMP accumulation by YQB-SVG and YQ-SVG was performed in LLCPK-1 cells expressing CRFR1. The cells were challenged with increasing concentration of the agonist for 20 min in presence of 2 mm 3-isobutyl-1-methylxanthene, supernatant was removed, and intracellular cAMP was extracted by lysing the cells with 100 mm HCl and measured by specific radioimmunoassay. The data are the means ± S.D. of triplicates. B, photoaffinity cross-linking of 125I-YQ-SVG to LLCPK-1 cells stably expressing mCRFR1. The cells were incubated with 125I-YQB-SVG (200,000 cpm/well) in presence of increasing concentrations of YQB-SVG for 2 h at 18 C; the cells were then rinsed with binding buffer and exposed to UV light. The receptor-ligand complexes were extracted with SDS sample buffer and loaded on 5–20% SDS-PAGE, followed by autoradiography.
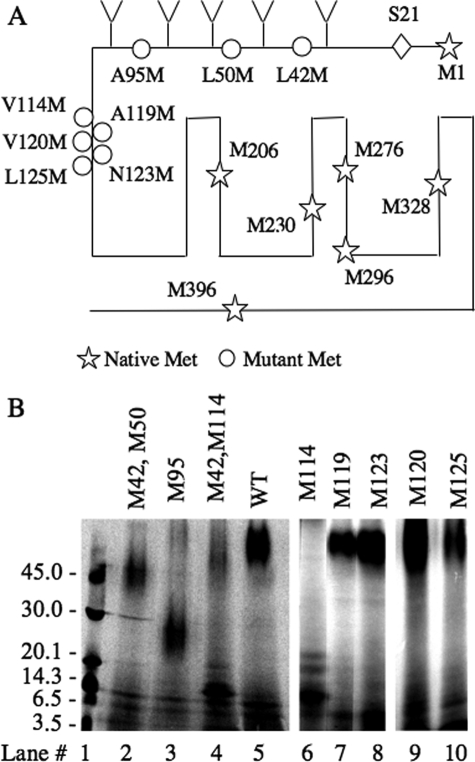
To identify the site of molecular cross-linking between the ligand and the receptor, we introduced Met substitution at different locations on the backbone of c-myc-tagged mCRFR1 and used CNBr cleavage to characterize the cross-linked receptor fragment. The hydrophobic residues were systematically replaced at desired locations with Met. Each receptor mutant was expressed in COS-7 cells and analyzed for expression level, apparent ligand binding Kd, and stimulation of cAMP accumulation; only functional receptor mutants were used for mapping of the cross-linking site (Table 1). The different receptor mutants, which were functional in binding and signaling assays, were then photo-cross-linked to 125I-YQB-SVG; the receptor-ligand complexes were cleaved by CNBr, and the receptor fragments were analyzed by SDS-PAGE followed by autoradiography.
TABLE 1.
Expression, ligand binding and cAMP stimulation in the wild type and Met mutants of mCRFR1 expressed in COS-7 cells The cells were transiently transfected with a plasmid construct encoding the wild type or the Met-substituted mCRFR1 mutants using the DEAE-dextran transfection method. Three days after transfection, cell surface expression of the receptor mutants was assessed using the specific monoclonal antibody directed against the c-myc epitope in the extracellular domain of the backbone of CRFR1 followed by 125I-labeled second antibody. The ligand binding properties, apparent Kd, and number of binding sites were derived from Scatchard analysis on radio-receptor assays performed on cell membranes prepared from COS-7 cells expressing the different receptor mutants using 125I-YQL-SVG as described under “Experimental Procedures.” Stimulation of cAMP accumulation by YQL-SVG was performed in 24-well plates incubated with increasing concentrations of agonists (0, 0.01, 0.1, 1, 10, and 100 nm) in serum-free culture medium containing 0.1% bovine serum albumin and 2 mm 3-isobutyl-1-methylxanthene at 37 °C for 20 min; intracellular cAMP was extracted with 0.5 m HCl and assayed for cAMP with a specific radioimmunoassay. All of the assays were performed in triplicate, and the means are shown in the table. Expression data were normalized to their respective values in the wild type receptor. Apparent binding Kd and ligand concentrations that caused half-maximal cAMP stimulation (EC50) are reported in nm. Maximal cAMP stimulation was normalized to the maximal stimulation caused by 1 μm forskolin (Fsk) on mock transfected cells. The data are means of triplicates.
| CRFR1 construct | Expression | Kd | EC50 | cAMP |
|---|---|---|---|---|
| % of wild type | nm | nm | % of Fsk | |
| Wild type | 100.3 | 3.4 | 1.5 | 112.0 |
| Met42/50 | 165.3 | 1.1 | 4.0 | 119.1 |
| Met95 | 126.8 | 1.5 | 1.3 | 125.4 |
| Met114 | 100.8 | 1.0 | 14.0 | 136.3 |
| Met115 | 87.5 | 1.0 | 4.0 | 65.7 |
| Met117 | 115.8 | 4.4 | 6.8 | 135.5 |
| Met119 | 142.5 | 2.5 | 1.5 | 147.3 |
| Met120 | 101.8 | 5.7 | 2.3 | 99.1 |
| Met123 | 108.0 | 1.3 | 2.0 | 68.2 |
| Met125 | 173.2 | 1.1 | 0.2 | 86.3 |
| Met42/114 | 147.9 | 1.9 | 4.0 | 141.7 |
| Met114/117 | 127.7 | 7.5 | 1.1 | 74.1 |
| Met114/120 | 116.2 | 9.3 | 2.0 | 93.8 |
| Met116/117 | 114.4 | 7.2 | 1.0 | 125.8 |
The 125I-YQB-SVG-cross-linked CNBr-cleaved CRFR1 fragment migrates as a broad band on Tricine SDS-PAGE with an apparent molecular mass of >60 Kd (Fig. 2B, lane 5); this band corresponds to a glycosylated receptor fragment extending from residue 24 of the mature CRFR1 protein (40, 41) to the first naturally occurring Met residue (Met206) within the TM3 of CRFR1 (Fig. 2A and Table 2). The predicted MM of this fragment is ∼70 kDa (Table 2), which is equal to the MM of its backbone peptide (∼20 kDa), the MM of the cross-linked ligand (4.7 kDa), and the MM of five polysaccharide chains attached at Asn38, Asn45, Asn78, Asn90, and Asn98 (Fig. 2A); each contributes to the apparent MM by ∼10 kDa (17). Introduction of Met residues at positions 42/50 or 95 produced smaller CNBr-cleaved labeled fragments with apparent MM of ∼48 and ∼28 kDa, respectively (Fig. 2B, lanes 2 and 3). CNBr cleavage of 125I-YQB-SVG-cross-linked M42/M50 receptor mutant is predicted to generate an 125I-YQB-SVG-cross-linked fragment with an apparent MM of 18 kDa plus the MM of three polysaccharide chains attached at Asn78, Asn90, and Asn98 (Fig. 2A and Table 2) with a predicted MM of ∼48 kDa, which is similar to that seen in lane 2 of Fig. 2B. CNBr cleavage of 125I-YQB-SVG-cross-linked M95 mutant is predicted to have a backbone peptide with an apparent MM of 18 kDa and one polysaccharide chain at Asn90 (Fig. 2A and Table 2); this results in a cross-linked fragment of a predicted size of ∼28 kDa, as seen in lane 3 of Fig. 2B.
FIGURE 2.
Mapping of the cross-linked sites using CNBr cleavage of CRFR1 bearing Met substitution. A, the locations of native methionine residues in the CRFR1 backbone are depicted with stars, and the sites of the methionine substitutions are illustrated with circles; Ser24 is predicted to be the first residue of the mature protein (40, 41). B, CRFR1 or CRFR1 with methionine residues substitutions are expressed in COS-7 cells. Binding and cross-linking of 125I-YQB-SVG to the different CRFR1 mutants, expressed in COS-7 cells, was performed to as in Fig. 1B. The cell lysates were then cleaved with CNBr. The cleaved products were lyophilized, resuspended in sample buffer, and analyzed on Tricine-PAGE followed by autoradiography. Lane 1 shows the elution profile of the molecular mass markers. WT, wild type.
TABLE 2.
Predicted molecular masses of the CNBr-cleaved products Shown are the predicted molecular weights of the CNBr-cleaved products of the different CRFR1 mutants used in Fig 2, taking into account the following assumptions: 1) all potential glycosylation (Chinese hamster ovary) sites (Asn38, Asn45, Asn78, Asn90, and Asn98) are glycosylated, and each Chinese hamster ovary group adds ∼10 kDa to the molecular mass of the cleaved fragment; as we previously reported (17); 2) CNBr cleaves the peptidic bonds that are C-terminal to the Met residue-releasing fragment that ends with a homoserine lactone (64); 3) the CRFR1 signal peptide is cleaved resulting in a mature receptor starting at position 24 (40, 41); and 4) the molecular mass of the cross-linked ligand (4.7 kDa).
| Construct | Residue positions | Glycosylation (Chinese hamster ovary) sites included in the cleaved fragment | Molecular mass of CNBr-cleaved, fragments + YQL-SVG | Molecular mass of CNBr-cleaved, Fragments + YQL-SVG + Chinese hamster ovary groups |
|---|---|---|---|---|
| kDa | kDa | |||
| Wild type | 24–206 | Asn38, Asn45, Asn78, Asn90, and Asn98 | ∼25 | ∼70 |
| Met42/50 | 51–206 | Asn78, Asn90, and Asn98 | ∼23 | ∼48 |
| Met95 | 96–206 | Asn98 | ∼18 | ∼28 |
| Met42/114 | 115–206 | 0 | ∼16 | ∼16 |
| Met114 | 115–206 | 0 | ∼16 | ∼16 |
| Met115 | 116–206 | 0 | ∼16 | ∼16 |
| Met117, Met119, Met120, Met123, and Met125 | 24–117 | Asn38, Asn45, Asn78, Asn90, and Asn98 | ∼16 | ∼66 |
Introduction of Met residue at position 114 produced an 125I-YQB-SVG-cross-linked CNBr-cleaved fragment with an apparent molecular mass of ∼16 kDa, plus two minor inconsistent bands with apparent molecular masses of 19 and 21 kDa (Fig. 2B, lanes 4 and 6). The sharp appearance of the ∼16-kDa band suggests that it is not glycosylated, as predicted from its backbone peptide sequence (Table 2 and Fig. 2A). The less abundant and inconsistent 19- and 21-kDa bands represent incomplete CNBr cleavage at other Met residues within the receptor backbone. The pattern of the CNBr-cleaved fragments caused by the introduction of Met residues at positions 50, 95, and 114 (Fig. 2, lanes 2, 3, and 4 or 6) indicate that 125I-YQB-SVG is cross-linked to a receptor fragment flanked by the naturally occurring Met at position 206 and the introduced mutation at position 114.
To further map the cross-linking site, Met residues were introduced at positions 119, 120, 123, and 125 in the CRFR1 backbone. Cross-linking of 125I-YQB-SVG followed by CNBr cleavage resulted in labeled bands with apparent MMs of >60 kDa; this indicates that the cross-linked receptor fragments are glycosylated (Fig. 2B, lanes 7–10). These data indicate that the cross-linking site in these mutants is N-terminal to position 125, 123, 120, or 119 (Fig. 2A and Table 2).
To summarize, Met substitution at position 114 resulted in a small fragment, which indicates that the cross-linking site is C-terminal to position 114. In contrast, Met substitution at position 119 resulted in a large glycosylated fragment, which indicates that the cross-linking site is N-terminal to residue 119. Taken together, the data from Met substitution at positions 114 and 119 suggest that 125I-YQB-SVG cross-links to sequences flanked by positions 119 and 114, i.e., position 118, 117, 116, or 115.
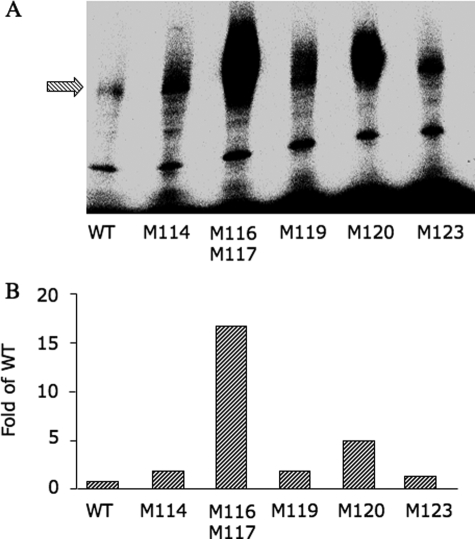
It has been shown that UV cross-linking of Bpa-substituted ligands favors Met residues existing in a close proximity to the cross-linking site (42). Therefore we compared the cross-linking efficiencies of different CRFR1 mutants bearing Met substitutions in this region (Fig. 3). Clearly, the double mutant Met116/Met117 shows the highest cross-linking efficiency compared with the other mutants; this suggests that one (or both) of these residues is (are) involved in the cross-linking reaction.
FIGURE 3.
Cross-linking efficiency of CRFR1 mutants. The different mutants were expressed in COS-7 cells; binding and cross-linking to 125I-YQB-SVG was performed as in Fig. 1B. Similar amounts of bound receptor-ligand complex (1200 cpm) were analyzed on SDS-PAGE followed by autoradiography using phosphorimaging (A). In B, cross-linking efficiency was calculated as the ratios of the receptor band of the different receptor mutants divided by the receptor band in the wild type. WT, wild type.
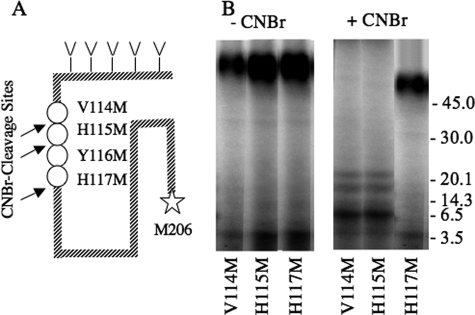
To further define the residues of the receptor and of the ligand that are involved in the cross-linking reaction, we introduced a single Met substitution at position 115, 116, or 117; however, only CRFR1 mutants bearing Met at positions 115 and 117 were functional (Table 1). The CRFR1 mutants bearing Met at positions 114, 115, and 117 were cross-linked to 125I-YQB-SVG, and their CNBr-cleaved products were analyzed on Tricine SDS-PAGE and autoradiography (Fig. 4B). CNBr cleavage of the Met117 cross-linked CRFR1 mutants resulted in a large fuzzy band that was ∼66 kDa in size (Fig. 4B), which is ∼50 kDa higher than the predicted size of the backbone peptide. The SDS-PAGE characteristic of the band and its apparent MM indicate that the fragment is glycosylated (Table 2); these data indicate that 125I-YQB-SVG cross-links to position 117 or to a residue that is N-terminal to position 117. In contrast, the CNBr cleavage products of the CRFR1 mutant bearing Met at position 114 or 115 show a small nonglycosylated band that was ∼16 kDa in size (Fig. 4B); this indicates that cross-linking site is C-terminal to position 115. Taken together, the data suggest that the cross-linking site occurs at position 116 and/or 117.
FIGURE 4.
Characterization of cross-linked CNBr-cleaved fragments of V114M, H115M and H117M CRFR1 mutants. A, scheme showing the location of the three CRFR1 methionine (M) mutations, the endogenous methionine at position 206, and the potential cross-linked site. B, the different CRFR1 mutants, expressed in COS-7 cells were cross-linked to 125I-YQB-SVG as in Fig. 1B and then analyzed on Tricine-PAGE before (-CNBr) and after (+CNBr) CNBr cleavage. The cleaved products were lyophilized, resuspended in sample buffer, and analyzed on Tricine-PAGE followed by autoradiography. The positions of the molecular mass markers are shown on the right side of the gel. The Y116M receptor mutant was not functional, and cross-linking could not be performed.
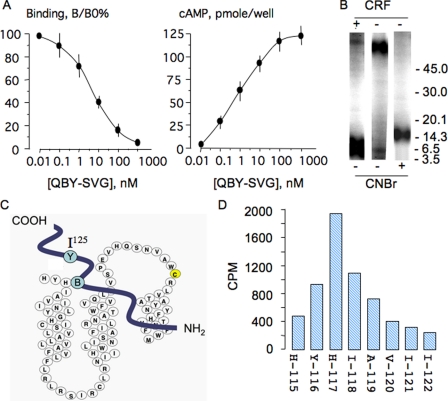
To identify the cross-linked residue we developed a method for radiosequencing of the CNBr-cleaved CRFR1 bearing Met substitution at position 114 photo-cross-linked to 125I-[Gln1, Bpa17, Tyr23]SVG (125I-QBY-SVG). CNBr cleavage of the Met114 mutant produces an 125I-labeled receptor fragment that starts at position 115; this places the predicted cross-linked residues (Tyr116 or His117) within two or three cycles of the Edman degradation sequencing reaction. We first characterized the binding and signaling property of QBY-SVG in LLCPK-1 cells stably expressing mCRFR1. The apparent binding Kd and the EC50 for stimulation of cAMP accumulation were 8 and 1 nm, respectively (Fig. 5A). Excess CRF (1 μm) completely blocked cross-linking of 125I-QBY-SVG to CRFR1 (Fig. 5B). CNBr cleavage of a photo-cross-linked CRFR1 mutant bearing a Met substitution at position 114 resulted in a single fragment of ∼16 kDa, which corresponds to the predicted MMs of the backbone fragment (∼11 kDa) and the ligand (∼4.7 kDa) (Table 2 and Fig. 5B). Because the predicted cross-linking site is located at position 116 or 117, N-terminal sequencing of the 125I-QBY-SVG cross-linked and CNBr-cleaved ∼16-kDa fragment is predicted to release the radioactivity after few cycles of sequencing (Fig. 5C). As predicted, most of the radioactivity was released at the third sequencing cycle with His117 (Fig. 5D). Taken together, the data indicate that residue 17 from the ligand cross-links to residue 117 of the receptor.
FIGURE 5.
A, competition binding with [Gln1, Bpa17, Tyr23]SVG (QBY-SVG) against 125I-QBY-SVG and stimulation of cAMP accumulation in LLCPK-1 cells stably expressing CRFR1; the experimental conditions are similar to those in Fig. 1A. The data are the means ± S.D. of triplicates. B, photoaffinity cross-linking of 125I-QBY-SVG to COS-7 cells expressing the Met114 CRFR1 mutant in presence and absence of excess ovine CRF (1 μm). CNBr cleavage of the photo-cross-linked materials were resolved on Tricine-PAGE. C, scheme showing the relative position of Tyr23 and Bpa17 in the QBY-SVG analog and the cross-linked site His117 in CRFR1. D, radiosequencing of cross-linked receptor-ligand fragment purified from the 16-kDa band depicted in B and sequenced using Edman degradation; at each cycle the radioactivity released was quantified in a γ-counter for 5 min; ∼60% of radioactivity submitted for sequencing was recovered in the fractions.
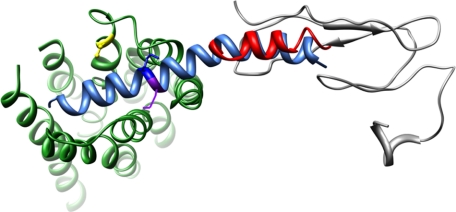
The model of the sauvagine-CRFR1 complex based on the structural data available for UCN1, the N terminus of the CRFR2 receptor, and multiple cross-linking points of similar hormone systems (i.e. PTH, secretin) is illustrated in Fig. 6. The placement of the N terminus of the receptor relative to the TM bundle is not well defined, because 19 amino acids are missing between the C-terminal end of the NMR-based structure and the beginning of TM1 (this is denoted as a dotted blue line in Fig. 6). Based on the observation that this region adopts two α-helices in the related PTH receptor (as determined by NMR (43), we have placed these two end points projecting toward each other (the distance between them is determined by the ligand). The other undefined regions of the model are the structural preferences of the extracellular loops. Concerning EC2, Cys258, directly in the middle of EC2, is involved in a disulfide bond with Cys188 on the extracellular end of TM3. This forces EC2 to fold over the core region of the TM bundle (approximated in Fig. 6 by a dotted orange line), and in close proximity to Lys16 of sauvagine consistent with our previous photoaffinity labeling results (27). The generation of this model results in Met17 of sauvagine projecting toward the proximal N terminus of CRFR1 and the extracellular end of TM1 in full accord with the photoaffinity labeling results presented here.
FIGURE 6.
Molecular model of sauvagine binding to CRF1R. The structure of sauvagine, as determined by homology with human urocortin-1, is shown in aqua, with Lys16 and Met17 denoted in dark blue and purple, respectively. The orientation of sauvagine to CRFR1 was guided by the NMR structure of the N terminus of CRFR2 (gray) bound to the C-terminal portion of astressin (red). The 19 residues missing between the CRF2R N terminus as determined by NMR and the beginning of TM1 is denoted by a dashed blue line. Likewise, the EC2 consisting of KLYYDNEKCWFGKRPGVYT is denoted as an orange dashed line, with Cys258 forming a disulfide bond with Cys188 at the extracellular end of TM3 (shown in yellow).
DISCUSSION
Extensive efforts have been directed into understanding the nature of hormone-receptor interaction within group B of the G protein receptor family. After the molecular cloning of the CRFR1 and CRFR2, it was possible to study the molecular interaction between ligands and receptors using receptor mutagenesis and mammalian and bacterial expression systems to study binding properties of the mutant receptors. Thus it was recognized that the N-terminal extracellular domains of CRFR1 and CRFR2 contain most of the binding determinant for binding of CRF, UCN1, and SVG (16, 30, 31, 33, 41, 44–48). Comparison of kinetics of binding and activation of wild type CRFR1 or of CRFR1 extracellular domain fused to the transmembrane domain of the activin II receptor suggested a two-binding domain model with the receptor N-domain binding CRF with moderate to high microaffinity, substantially increasing the local concentration of agonist and so allowing weak agonist-J-domain interaction, which is then allosterically enhanced by receptor-G protein interaction (45, 49). The NMR structure of the CRFR2 extracellular domain is also consistent with the above model that the extracellular domain captures the C-terminal segment of the ligand, whose N terminus then penetrates into the transmembrane region of the receptor to initiate signaling (28, 50). Cross-linking with Bpa-substituted UCN1 analogs showed that a Bpa group located at positions 0 or 12 cross-links to the second extracellular receptor loop, whereas Bpa at positions 17 or 22 cross-links to the first extracellular receptor loop (26); these data suggest that the N terminus of UCN1 binds closer to the CRFR1 extracellular surface. It is also possible that UCN1 binding to CRFR1 expressed in mammalian cells as shown by UCN1 cross-linking (26) is different from the binding of the free N-terminal extracellular domain of CRFR1, which is shown to be both necessary and sufficient for binding N-terminally truncated CRF, sauvagine, and UCN1 based analogs (15, 28, 30, 31, 40, 41, 45, 48, 50).
We have previously shown, using the bifunctional chemical cross-linker disuccinimidyl suberate, that Lys16 in sauvagine cross-links to Lys257 within the second extracellular loop of CRF1R (27), and we now show that Bpa-substituted position 17 in sauvagine cross-links to His117 in the TM1 of CRFR1. These data suggest that the molecular distance between Lys257 in the second extracellular loop and His117 in TM1 is within the span of Bpa at position 16 and Lys17 of the sauvagine analogs, plus the molecular distance of the disuccinimidyl suberate chain and one peptide bond, and that residues 16 and 17 of sauvagine are oriented in such a way that positions residue 16 closer to the second extracellular loop and residue 17 closer to TM1. These data force a model for SVG-CRFR1 interactions whereby the N terminus of the ligand fits between TM1 and TM7, allowing the mid-region of the ligand to be in a close contact to TM1 and second extracellular loop (Fig. 6). This model is consistent with the NMR structure of the CRFR2 N-terminal extracellular domain (28), with the functional data showing full binding capacity of the N-terminal extracellular domain (15, 28, 30, 31, 40, 41, 45, 48, 50), and with the cross-linking data of Bpa-substituted UCN1 at positions 17 and 22, which showed cross-linking to the first extracellular loop (26). However, the model does not fit the cross-linking data of Bpa-substituted at position 0 and 12, which showed cross-linking to the second extracellular loop (26). Alternatively, the fact that position 16 of sauvagine (27) and positions 0 and 12 of UCN1 (26) cross-link to the second extracellular loop may indicate that CRFR1 adopts distinct binding conformations with different agonists, which may have different signaling potentials. In this regard, it has been shown that only UCN1 (not CRF) stimulates MAPK activity in hCRF1-α expressed in HEK293 cells, although both ligands bind with a high affinity to the CRFR1-α (19).
Recently, the extracellular domain of the PTH1R, fused to the bacterial mannose binding protein and bound to PTH(15–34), was crystallized (51). The crystal structure showed an interesting pattern with the PTH1R extracellular domain engulfing PTH(15–34) as a “hot dog in a bun” (51). The crystal structure of the PTH1R extracellular domain-PTH(15–34) complexes provides direct evidence for the model that the C-terminal domain of the ligand binds to the N terminus of the receptor, thus positioning the N terminus of the ligand at a favorable distance to allosterically activate the J-domain of the receptor (52). Therefore, it is interesting to compare the data from photoaffinity cross-linking of PTH1R (22–25, 53–60) with those of CRFR1. Similar to our finding that residues 16 and 17 of sauvagine cross-link to the J-domain of CRFR1, residues within the C terminus of PTH(1–34), such as 19 and 27, are reported to cross-link to the second transmembrane domain and within the first extracellular loop, respectively; both are within the J-domain of PTH1R (58).
The photoaffinity results presented here and by us previously (27) clearly define the topological orientation of the ligand with respect to entry into the TM bundle between TM1 and TM2, the midsection of the ligand (residues 16 and 17) interacting with both the extracellular end of TM1 and EC2. A similar topological orientation has been generated for the PTH receptor system and is consistent with the cross-linking data for secretin (61). The interaction between residue Lys16 of sauvagine and Lys257 of CRFR1 is easily accounted for by the folding of the EC2 loop over the core of the TM bundle. Indeed, in the x-ray structure of rhodopsin, EC2 adopts a well ordered structure, forming a number of interactions with the retinal (62); similar interactions have been reported for the β-adrenergic receptor (63).
As illustrated in Fig. 6, the cross-linking results provide important insight into the orientation of the N terminus relative to the helical bundle. Using the superposition of the ligands (sauvagine and astressin in the CRFR2-astressin complex) and the necessary connection of the N terminus to TM1 limits the topological arrangements possible. The assumption of the proximal N terminus adopting two α-helices, as observed for PTH (43), places further restraints on the relative arrangement of these components.
In conclusion, our data are consistent with the proposed model for ligand-receptor interaction whereby the N terminus of the ligand binds to the CRFR1 J-domain and the C terminus of the ligand binds to the N terminus extracellular domain of CRFR1. Our data, however, provide useful constraints for ligand-receptor interaction, indicating that the mid-region of the ligand (residues 16 and 17) is in close proximity to the second extracellular loop and top of TM1.
This work was supported, in whole or in part, by National Institutes of Health Grants 7RO1 DK63211 (to A. B. A.-S.) and GM54082 (to D. F. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CRF, corticotropin-releasing factor; SVG, sauvagine; UCN, urocortin; CRFR1, CRF receptor type 1; Bpa, p-benzoylphenylalanine; MAPK, mitogen-activated protein kinase; J-domain, juxtamembrane domain; TM, transmembrane-spanning domain; HPLC, high pressure liquid chromatography; m, murine; PBS, phosphate-buffered saline; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine; PTH, parathyroid hormone; MM, molecular mass(es).
References
- 1.Vale, W., Spiess, J., Rivier, C., and Rivier, J. (1981) Science 213 1394-1397 [DOI] [PubMed] [Google Scholar]
- 2.Chang, C. P., Pearse, R. V., II, O'Connell, S., and Rosenfeld, M. G. (1993) Neuron 11 1187-1195 [DOI] [PubMed] [Google Scholar]
- 3.Chen, R., Lewis, K. A., Perrin, M. H., and Vale, W. W. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 8967-8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishimoto, T., Pearse, R. V., II, Lin, C. R., and Rosenfeld, M. G. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 1108-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovenberg, T. W., Liaw, C. W., Grigoriadis, D. E., Clevenger, W., Chalmers, D. T., De Souza, E. B., and Oltersdorf, T. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 836-840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin, M., Donaldson, C., Chen, R., Blount, A., Berggren, T., Bilezikjian, L., Sawchenko, P., and Vale, W. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 2969-2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenzel, P., Kesterson, R., Yeung, W., Cone, R., Rittenberg, M., and Senzel-Poore, M. (1995) Mol. Endocrinol. 9 637-645 [DOI] [PubMed] [Google Scholar]
- 8.Arai, M., Assil, I. Q., and Abou-Samra, A. B. (2001) Endocrinology 142 446-454 [DOI] [PubMed] [Google Scholar]
- 9.Montecucchi, P. C., Anastasi, A., deCastiglione, R., and Espamer, V. (1980) Int. J. Peptide Protein Res. 16 191-199 [PubMed] [Google Scholar]
- 10.Lederis, K., Letter, A., McMaster, D., and Moore, G. (1982) Science 218 162-164 [DOI] [PubMed] [Google Scholar]
- 11.Vaughan, J., Donaldson, C., Bittencourt, J., Perrin, M. H., Lewis, K., Sutton, S., Chan, R., Turnbull, A. V., Lovejoy, D., Rivier, C., Sawchenko, P. E., and Vale, W. W. (1995) Nature 378 287-292 [DOI] [PubMed] [Google Scholar]
- 12.Reyes, T. M., Lewis, K., Perrin, M. H., Kunitake, K. S., Vaughan, J., Arias, C. A., Hogenesch, J. B., Gulyas, J., Rivier, J., Vale, W. W., and Sawchenko, P. E. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 2843-2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis, K., Li, C., Perrin, M. H., Blount, A., Kunitake, K., Donaldson, C., Vaughan, J., Reyes, T. M., Gulyas, J., Fischer, W., Bilezikjian, L., Rivier, J., Sawchenko, P. E., and Vale, W. W. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 7570-7575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu, S. Y., and Hsueh, A. J. (2001) Nat. Med. 7 605-611 [DOI] [PubMed] [Google Scholar]
- 15.Perrin, M. H., Grace, C. R., Digruccio, M. R., Fischer, W. H., Maji, S. K., Cantle, J. P., Smith, S., Manning, G., Vale, W. W., and Riek, R. (2007) J. Biol. Chem. 282 37529-37536 [DOI] [PubMed] [Google Scholar]
- 16.Qi, L. J., Leung, A. T., Xiong, Y., Marx, K. A., and Abou-Samra, A. B. (1997) Biochemistry 36 12442-12448 [DOI] [PubMed] [Google Scholar]
- 17.Assil, I. Q., and Abou-Samra, A. B. (2001) Am. J. Physiol. 281 E1015-E1021 [DOI] [PubMed] [Google Scholar]
- 18.Xiong, Y., Xie, L. Y., and Abou-Samra, A. B. (1995) Endocrinology 136 1828-1834 [DOI] [PubMed] [Google Scholar]
- 19.Grammatopoulos, D. K., Randeva, H. S., Levine, M. A., Katsanou, E. S., and Hillhouse, E. W. (2000) Mol. Endocrinol. 14 2076-2091 [DOI] [PubMed] [Google Scholar]
- 20.Markovic, D., Punn, A., Lehnert, H., and Grammatopoulos, D. K. (2008) Mol. Endocrinol. 22 689-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams, A. E., Bisello, A., Chorev, M., Rosenblatt, M., and Suva, L. J. (1998) Mol. Endocrinol. 12 1673-1683 [DOI] [PubMed] [Google Scholar]
- 22.Bisello, A., Adams, A. E., Mierke, D. F., Pellegrini, M., Rosenblatt, M., Suva, L. J., and Chorev, M. (1998) J. Biol. Chem. 273 22498-22505 [DOI] [PubMed] [Google Scholar]
- 23.Behar, V., Bisello, A., Rosenblatt, M., and Chorev, M. (1999) Endocrinology 140 4251-4261 [DOI] [PubMed] [Google Scholar]
- 24.Greenberg, Z., Bisello, A., Mierke, D. F., Rosenblatt, M., and Chorev, M. (2000) Biochemistry 39 8142-8152 [DOI] [PubMed] [Google Scholar]
- 25.Gensure, R. C., Shimizu, N., Tsang, J., and Gardella, T. J. (2003) Mol. Endocrinol. 17 2647-2658 [DOI] [PubMed] [Google Scholar]
- 26.Kraetke, O., Holeran, B., Berger, H., Escher, E., Bienert, M., and Beyermann, M. (2005) Biochemistry 44 15569-15577 [DOI] [PubMed] [Google Scholar]
- 27.Assil-Kishawi, I., and Abou-Samra, A. B. (2002) J. Biol. Chem. 277 32558-32561 [DOI] [PubMed] [Google Scholar]
- 28.Grace, C. R., Perrin, M. H., DiGruccio, M. R., Miller, C. L., Rivier, J. E., Vale, W. W., and Riek, R. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 12836-12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoare, S. R., Sullivan, S. K., Pahuja, A., Ling, N., Crowe, P. D., and Grigoriadis, D. E. (2003) Peptides 24 1881-1897 [DOI] [PubMed] [Google Scholar]
- 30.Klose, J., Fechner, K., Beyermann, M., Krause, E., Wendt, N., Bienert, M., Rudolph, R., and Rothemund, S. (2005) Biochemistry 44 1614-1623 [DOI] [PubMed] [Google Scholar]
- 31.Perrin, M. H., DiGruccio, M. R., Koerber, S. C., Rivier, J. E., Kunitake, K. S., Bain, D. L., Fischer, W. H., and Vale, W. W. (2003) J. Biol. Chem. 278 15595-15600 [DOI] [PubMed] [Google Scholar]
- 32.Assil, I. Q., Shomali, M. E., and Abou-Samra, A. B. (2001) Peptides 22 1055-1061 [DOI] [PubMed] [Google Scholar]
- 33.Assil, I. Q., Qi, L. J., Arai, M., Shomali, M., and Abou-Samra, A. B. (2001) Biochemistry 40 1187-1195 [DOI] [PubMed] [Google Scholar]
- 34.Abou-Samra, A. B., Harwood, J. P., Manganiello, V. C., Catt, K. J., and Aguilera, G. (1987) J. Biol. Chem. 262 1129-1136 [PubMed] [Google Scholar]
- 35.Tawfeek, H. A., Qian, F., and Abou-Samra, A. B. (2002) Mol. Endocrinol. 16 1-13 [DOI] [PubMed] [Google Scholar]
- 36.Grace, C. R., Perrin, M. H., Cantle, J. P., Vale, W. W., Rivier, J. E., and Riek, R. (2007) J. Am. Chem. Soc. 129 16102-16114 [DOI] [PubMed] [Google Scholar]
- 37.Grace, C. R., Perrin, M. H., Gulyas, J., Digruccio, M. R., Cantle, J. P., Rivier, J. E., Vale, W. W., and Riek, R. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 4858-4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wittelsberger, A., Thomas, B. E., Mierke, D. F., and Rosenblatt, M. (2006) FEBS Lett. 580 1872-1876 [DOI] [PubMed] [Google Scholar]
- 39.Dong, M., Lam, P. C., Pinon, D. I., Sexton, P. M., Abagyan, R., and Miller, L. J. (2008) Mol. Pharmacol. 74 413-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann, B. A., Sydow, S., Jahn, O., van Werven, L., Liepold, T., Eckart, K., and Spiess, J. (2001) Protein Sci. 10 2050-2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrin, M. H., Fischer, W. H., Kunitake, K. S., Craig, A. G., Koerber, S. C., Cervini, L. A., Rivier, J. E., Groppe, J. C., Greenwald, J., Moller Nielsen, S., and Vale, W. W. (2001) J. Biol. Chem. 276 31528-31534 [DOI] [PubMed] [Google Scholar]
- 42.O'Neil, K. T., Erickson-Viitanen, S., and DeGrado, W. F. (1989) J. Biol. Chem. 264 14571-14578 [PubMed] [Google Scholar]
- 43.Pellegrini, M., Bisello, A., Rosenblatt, M., Chorev, M., and Mierke, D. F. (1998) Biochemistry 37 12737-12743 [DOI] [PubMed] [Google Scholar]
- 44.Hoare, S. R., Sullivan, S. K., Fan, J., Khongsaly, K., and Grigoriadis, D. E. (2005) Peptides 26 457-470 [DOI] [PubMed] [Google Scholar]
- 45.Hoare, S. R., Sullivan, S. K., Schwarz, D. A., Ling, N., Vale, W. W., Crowe, P. D., and Grigoriadis, D. E. (2004) Biochemistry 43 3996-4011 [DOI] [PubMed] [Google Scholar]
- 46.Klose, J., Wendt, N., Kubald, S., Krause, E., Fechner, K., Beyermann, M., Bienert, M., Rudolph, R., and Rothemund, S. (2004) Protein Sci. 13 2470-2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liaw, C. W., Grigoriadis, D. E., Lovenberg, T. W., De Souza, E. B., and Maki, R. A. (1997) Mol. Endocrinol. 11 980-985 [DOI] [PubMed] [Google Scholar]
- 48.Perrin, M. H., Sutton, S., Bain, D. L., Berggren, W. T., and Vale, W. W. (1998) Endocrinology 139 566-570 [DOI] [PubMed] [Google Scholar]
- 49.Hoare, S. R., Fleck, B. A., Gross, R. S., Crowe, P. D., Williams, J. P., and Grigoriadis, D. E. (2008) Mol. Pharmacol. 73 1371-1380 [DOI] [PubMed] [Google Scholar]
- 50.Perrin, M. H., Grace, C. R., Riek, R., and Vale, W. W. (2006) Ann. N. Y. Acad. Sci. 1070 105-119 [DOI] [PubMed] [Google Scholar]
- 51.Pioszak, A. A., and Xu, H. E. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 5034-5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Potts, J. T., and Gardella, T. J. (2007) Ann. N. Y. Acad. Sci. 1117 196-208 [DOI] [PubMed] [Google Scholar]
- 53.Barbier, J. R., Gardella, T. J., Dean, T., MacLean, S., Potetinova, Z., Whitfield, J. F., and Willick, G. E. (2005) J. Biol. Chem. 280 23771-23777 [DOI] [PubMed] [Google Scholar]
- 54.Behar, V., Bisello, A., Bitan, G., Rosenblatt, M., and Chorev, M. (2000) J. Biol. Chem. 275 9-17 [DOI] [PubMed] [Google Scholar]
- 55.Dean, T., Khatri, A., Potetinova, Z., Willick, G. E., and Gardella, T. J. (2006) J. Biol. Chem. 281 32485-32495 [DOI] [PubMed] [Google Scholar]
- 56.Dean, T., Linglart, A., Mahon, M. J., Bastepe, M., Juppner, H., Potts, J. T., Jr., and Gardella, T. J. (2006) Mol. Endocrinol. 20 931-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gensure, R. C., Gardella, T. J., and Juppner, H. (2001) J. Biol. Chem. 276 28650-28658 [DOI] [PubMed] [Google Scholar]
- 58.Gensure, R. C., Gardella, T. J., and Juppner, H. (2005) Biochem. Biophys. Res. Commun. 328 666-678 [DOI] [PubMed] [Google Scholar]
- 59.Pham, V. I., and Sexton, P. M. (2004) J. Pept. Sci. 10 179-203 [DOI] [PubMed] [Google Scholar]
- 60.Shimizu, N., Dean, T., Tsang, J. C., Khatri, A., Potts, J. T., Jr., and Gardella, T. J. (2005) J. Biol. Chem. 280 1797-1807 [DOI] [PubMed] [Google Scholar]
- 61.Dong, M., Pinon, D. I., Cox, R. F., and Miller, L. J. (2004) J. Biol. Chem. 279 1167-1175 [DOI] [PubMed] [Google Scholar]
- 62.Palczewski, K., Kumasaka, T., Hori, T., Behnke, C. A., Motoshima, H., Fox, B. A., Le Trong, I., Teller, D. C., Okada, T., Stenkamp, R. E., Yamamoto, M., and Miyano, M. (2000) Science 289 739-745 [DOI] [PubMed] [Google Scholar]
- 63.Warne, T., Serrano-Vega, M. J., Baker, J. G., Moukhametzianov, R., Edwards, P. C., Henderson, R., Leslie, A. G., Tate, C. G., and Schertler, G. F. (2008) Nature 454 486-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inglis, A. S., and Edman, P. (1970) Anal. Biochem. 37 73-80 [DOI] [PubMed] [Google Scholar]