Abstract
After epididymal maturation, sperm capacitation, which encompasses a
complex series of molecular events, endows the sperm with the ability to
fertilize an egg. This process can be mimicked in vitro in defined
media, the composition of which is based on the electrolyte concentration of
the oviductal fluid. It is well established that capacitation requires
Na+, 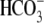 , Ca2+,
and a cholesterol acceptor; however, little is known about the function of
Cl– during this important process. To determine whether
Cl–, in addition to maintaining osmolarity, actively
participates in signaling pathways that regulate capacitation,
Cl– was replaced by either methanesulfonate or gluconate two
nonpermeable anions. The absence of Cl– did not affect sperm
viability, but capacitation-associated processes such as the increase in
tyrosine phosphorylation, the increase in cAMP levels, hyperactivation, the
zona pellucidae-induced acrosome reaction, and most importantly, fertilization
were abolished or significantly reduced. Interestingly, the addition of cyclic
AMP agonists to sperm incubated in Cl–-free medium rescued
the increase in tyrosine phosphorylation and hyperactivation suggesting that
Cl– acts upstream of the cAMP/protein kinase A signaling
pathway. To investigate Cl– transport, sperm incubated in
complete capacitation medium were exposed to a battery of anion transport
inhibitors. Among them, bumetanide and furosemide, two blockers of
Na+/K+/Cl– cotransporters (NKCC),
inhibited all capacitation-associated events, suggesting that these
transporters may mediate Cl– movements in sperm. Consistent
with these results, Western blots using anti-NKCC1 antibodies showed the
presence of this cotransporter in mature sperm.
, Ca2+,
and a cholesterol acceptor; however, little is known about the function of
Cl– during this important process. To determine whether
Cl–, in addition to maintaining osmolarity, actively
participates in signaling pathways that regulate capacitation,
Cl– was replaced by either methanesulfonate or gluconate two
nonpermeable anions. The absence of Cl– did not affect sperm
viability, but capacitation-associated processes such as the increase in
tyrosine phosphorylation, the increase in cAMP levels, hyperactivation, the
zona pellucidae-induced acrosome reaction, and most importantly, fertilization
were abolished or significantly reduced. Interestingly, the addition of cyclic
AMP agonists to sperm incubated in Cl–-free medium rescued
the increase in tyrosine phosphorylation and hyperactivation suggesting that
Cl– acts upstream of the cAMP/protein kinase A signaling
pathway. To investigate Cl– transport, sperm incubated in
complete capacitation medium were exposed to a battery of anion transport
inhibitors. Among them, bumetanide and furosemide, two blockers of
Na+/K+/Cl– cotransporters (NKCC),
inhibited all capacitation-associated events, suggesting that these
transporters may mediate Cl– movements in sperm. Consistent
with these results, Western blots using anti-NKCC1 antibodies showed the
presence of this cotransporter in mature sperm.
Before becoming fertilization-competent, mammalian sperm must undergo a
series of maturational processes in the female reproductive tract
(1). The molecular,
biochemical, and physiological changes that occur in sperm, whereas in the
female tract are collectively referred to as capacitation. These functional
changes associated with capacitation are not one event but are a combination
of sequential and concomitant processes involving modifications at the
molecular level occurring both in the head (i.e. preparation for the
acrosome reaction) and the tail (i.e. motility changes such as
hyperactivation). Molecular events implicated in the initiation of
capacitation can be mimicked in vitro and have been partially
defined. These include removal of cholesterol from the sperm plasma membrane;
modifications in plasma membrane phospholipids; fluxes of
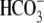 and other intracellular ions;
increased protein tyrosine phosphorylation; and hyperpolarization of the sperm
plasma membrane potential (Em) in mouse and other species
(for review see Ref. 2).
and other intracellular ions;
increased protein tyrosine phosphorylation; and hyperpolarization of the sperm
plasma membrane potential (Em) in mouse and other species
(for review see Ref. 2).
With respect to the changes in the plasma membrane Em
in mouse sperm, it is hypothesized that the capacitation-associated
hyperpolarization results from changes in the activity of ion-selective
channels and transporters. Consistent with this hypothesis, our studies in
sperm from this species have revealed the presence of amiloride-sensitive
epithelial Na+ channels
(ENaCs)2 and the
cystic fibrosis transmembrane regulator (CFTR)
(3,
4). Experiments in those
reports suggest that closing of ENaCs, with the consequent reduction in
Na+ influx, induces the hyperpolarization of the sperm
Em observed during capacitation. Down-regulation of ENaC
activity appears to be a consequence of either the activation of CFTR or the
influx of Cl–. Independent of our work, the presence of CFTR
in sperm was also reported by Xu et al.
(5); this group hypothesized
that in addition to its role as a Cl– transporter, CFTR also
transports 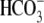 . As CFTR is primarily a
Cl– channel and other anion transporters are likely to be
involved in capacitation (4),
the present work focuses on the role of Cl– on sperm
function.
. As CFTR is primarily a
Cl– channel and other anion transporters are likely to be
involved in capacitation (4),
the present work focuses on the role of Cl– on sperm
function.
Cl– can be transported through various systems across the
plasma membrane including different types of Cl– channels and
a series of specialized carriers. Intracellular Cl– levels
are determined by the relative contributions of all Cl–
transporters present in the plasma membrane of a given cell type.
Cl– channels are a subset of ion channels selective to
Cl– and often permeable to other small monovalent anions.
Four structural Cl– channel families have been identified to
date: 1) Cl–-selective ion channels (ClCs); 2) CFTR channels;
3) the γ-aminobutyric acid- and glycine-gated neurotransmitter
receptors; and 4) Ca2+-activated Cl– channels. In
addition, functional data suggest that other not yet identified
Cl– channels may exist. The Cl– carrier
proteins include: 1) the electroneutral and electrogenic
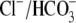 exchanger families (6) and 2)
the electroneutral cation-Cl– cotransporter family. All of
these are secondary active transporters, which means that the translocation of
one ion is coupled to the translocation of another ion in either the opposite
direction (antiporter) or the same direction (cotransporter or symporter). The
flow of the ion translocated up the electrochemical gradient is coupled to the
flow of a second ion down its electrochemical gradient, and thus the energy
required does not come directly from ATP
(7).
exchanger families (6) and 2)
the electroneutral cation-Cl– cotransporter family. All of
these are secondary active transporters, which means that the translocation of
one ion is coupled to the translocation of another ion in either the opposite
direction (antiporter) or the same direction (cotransporter or symporter). The
flow of the ion translocated up the electrochemical gradient is coupled to the
flow of a second ion down its electrochemical gradient, and thus the energy
required does not come directly from ATP
(7).
In the present work we have analyzed whether Cl– ions regulate different events in the capacitation process. The central observations of this work are that: 1) Cl– is necessary for the capacitation-associated increase in cAMP and tyrosine phosphorylation; 2) sperm incubated in a medium without Cl– do not acquire either responsiveness to zona pellucidae (ZP) for the induction of acrosome reaction nor hyperactivated motility; 3) fertilization is inhibited if sperm are incubated under capacitating medium without Cl–; 4) in the absence of Cl–, cAMP-permeable analogs can induce both the increase in tyrosine phosphorylation as well as hyperactivated motility; 5) bumetanide and furosemide, two Na+/K+/Cl– cotransporter (NKCC) inhibitors, block capacitation and the capacitation-associated processes; and 6) at least one member of the NKCC family is present in mature sperm.
EXPERIMENTAL PROCEDURES
Materials—Chemicals were obtained from the following sources. Bovine serum albumin (BSA, fatty acid-free), sodium methanesulfonate, sodium gluconate, potassium gluconate, dibutyryl cyclic AMP, Sp-cAMPS, 8-bromo-cAMP, 3-isobutyl-1-methylxanthine (IBMX), carbonyl cyanide m-chlorophenylhydrazone, valinomycin, chlorotoxin, diphenylamine-2-carboxylate (DPC), 5-[(4-carboxyphenyl)methylene]-2-thioxo-3-[(3-trifluoromethyl)phenyl-4-thiazolidinone (inh-172), bumetanide, furosemide, 9-(hydroxymethyl)anthracene, R(+)-butylindazone, (+)-bicuculline, 5-nitro-2-(3-phenylpropylamino)benzoic acid, indanyloxyacetic acid 94, hydrochlorothiazide, picrotoxin, DIDS, and SITS were purchased from Sigma. The fluorescent dyes 3,3-dipropylthiadicarbocyanine iodide (DiSC3-(5)) and Hoestch 33342 were obtained from Invitrogen. Niflumic acid was from Calbiochem. Cyclic AMP analogs and inhibitors were prepared fresh the day of the experiment in either Milli-Q water or DMSO depending on solubility.
Anti-phosphotyrosine (PY) monoclonal antibody (clone 4G10) was purchased from Upstate Biotechnology (Lake Placid, NY). In addition, for immunodetection of NKCC and β-tubulin, an anti-NKCC1 monoclonal antibody (clone T4) developed by Lytle et al. (8) and an anti-β-tubulin monoclonal antibody (Clone E7) developed by Chu and Klymkowsky (9) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institutes of Health, NICHD, and maintained by The University of Iowa Department of Biological Sciences, Iowa City, IA. For negative control donkey anti-mouse affinity-purified IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Mouse Sperm Preparation—Cauda epididymal mouse sperm were collected from CD1 retired male breeders (Charles River Laboratories, Wilmington, MA) and sacrificed in accordance with the Institutional Animal Care and Use Committee guidelines. Minced cauda epididymis from each animal was placed in 500 μl of a modified Krebs-Ringer medium (Whitten's HEPES-buffered medium) (10). This medium does not support capacitation unless supplemented with 5 mg/ml BSA (fatty acid-free) and 15 mm NaHCO3. After 10 min, the sperm suspension was washed by adding 1 ml of noncapacitating medium and posterior centrifugation at 800 × g for 5 min at room temperature. Sperm were then resuspended to a final concentration of 2 × 107 cells/ml and diluted 10 times in the appropriate medium depending on the experiment performed. In experiments where capacitation was investigated, 5 mg/ml BSA and 15 mm NaHCO3 were added, and sperm were incubated at 37 °C for at least 1 h. To study the role of Cl– in capacitation, NaCl and KCl in the media were replaced either by sodium gluconate and potassium gluconate or by sodium methanesulfonate and KOH. In all cases pH was maintained at 7.2. When different Cl– concentrations were assessed, the total NaCl plus sodium gluconate (or sodium methanesulfonate) was maintained at 100 mm. For the experiments in K+-free media, KCl and KH2PO4 were replaced by NaCl and NaH2PO4, respectively. To test the effect of the different inhibitors in capacitation, they were pre-incubated with sperm for 15 min preceding the beginning of the capacitating incubation. For the in vitro fertilization (IVF) assays, sperm were obtained and incubated for capacitation in Whitten's medium without HEPES containing 22 mm NaHCO3 and 15 mg/ml BSA and equilibrated in a humidified atmosphere of 5% CO2 in air (11).
Sperm Membrane Purification—The preparation of sperm fractions was carried out as described previously (12). Briefly, sperm (20 × 107 cells) were homogenized using 10 strokes with a Teflon Dounce homogenizer in TE buffer (50 mm Tris-HCl, pH 7.5, 1 mm EDTA) supplemented with protease inhibitors (protease inhibitor mixture (Roche Applied Science) as indicated by the manufacturer plus 0.4 mm leupeptin, 0.4 mm aprotinin, 0.1 mm pepstatin, 0.3 m benzamidine, and 0.32 mg/ml calpain I and II inhibitor). After homogenization, the sample was sonicated three times for 15 s on ice at intervals of 1 min. Cell debris was pelleted (1000 × g for 10 min at 4 °C), and the supernatant was centrifuged at 10,000 × g for 10 min at 4 °C. Again, the resultant pellet was saved, and the supernatant then was centrifuged at 100,000 × g for 1 h at 4 °C. The final pellet, which contained the membrane fraction, was resuspended in sample buffer and used for SDS-PAGE and immunoblotting.
SDS-PAGE and Immunoblotting—After incubation under different experimental conditions, sperm were spun down, washed in 1 ml of phosphate-buffered saline (PBS), resuspended in Laemmli sample buffer (13) without β-mercaptoethanol, and boiled for 5 min. After centrifugation, the supernatants were saved, and β-mercaptoethanol was added to a final concentration of 5%. The samples were boiled for 5 min and subjected to SDS-PAGE using 8–10% mini-gels; protein extracts equivalent to 1–2 × 106 sperm were loaded per lane. Each gel contained dual prestained molecular weight standard (Bio-Rad). Electrophoretic transfer of proteins to Immobilon (Bio-Rad) and immunodetection of tyrosine-phosphorylated proteins were carried out using PY monoclonal antibodies as described previously (14). For loading controls, membranes were stripped, and an anti-β-tubulin monoclonal antibody was used at a 5.2 ng/ml. For immunodetection of the NKCC, an anti-NKCC monoclonal antibody was used at a concentration of 2.3 μg/ml. Immunoblots were developed with the appropriate secondary antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories) and ECL chemiluminescence reagents.
Kidneys from CD1 male retired breeders were collected, and proteins were extracted using radioimmune precipitation assay buffer (10 mm Tris, pH 7.2, 150 mm NaCl, 0.1% SDS, 1% Triton X-100, 1% deoxycholate, 5 mm EDTA, and protease and phosphatase inhibitors). Protein concentration was assayed using the BCA kit from Pierce. In each lane 50 μg of total protein was loaded in an SDS-8% polyacrylamide gel. Proteins were transferred onto polyvinylidene difluoride membranes, and Western blotting was performed using anti-NKCC1 antibodies.
Sperm Motility Analysis—Samples of sperm were incubated in Whitten's medium without HEPES at 37 °C and 5% CO2 for 1 h. After incubation, the sperm suspension was loaded on a 20-μm chamber slide (Leja slide, Spectrum Technologies) and placed on a microscope stage heated to 37 °C. Sperm movement was examined using the CEROS computer-assisted semen analysis (CASA) system (Hamilton Throne Research, Beverly, MA). The parameters used were: frames acquired = 30, frame rate = 60 Hz, minimum cell size = 4 pixels, low average path velocity cutoff = 5 mm/s, static head size = 0.2–2.99, static head intensity = 0.26–1.31, and static head elongation = 0–100. Sperm with hyperactivated motility, defined as motility with high amplitude thrashing patterns and short distance of travel, were sorted using the criteria established by Bray et al. (15): 1) curvilinear velocity (VCL, velocity calculated from the sum of track-point to track-point velocity) > 180 μm/s; 2) amplitude of lateral head displacement (ALH, mean width of the sperm head oscillation as the cell swims), > 9.5 μm; 3) linearity (LIN, departure of the cell from a straight line) < 38%.
Acrosome Reaction Assay—Capacitation was measured indirectly by determining the zona pellucidae-induced acrosome reaction based on the premise that only capacitated sperm will undergo exocytosis. Zona pellucidae were prepared from homogenized ovaries of virgin female 22-day-old outbred CD1 mice (Charles River Laboratories) as described (16, 17) and solubilized by the procedures outlined previously (18). The percentage of acrosome reaction was measured using Coomassie Blue G-250 staining as described by Visconti et al. (19). Briefly, following a 45-min incubation at 37 °C under the conditions mentioned for each experiment, 5 zona pellucida equivalents/μl were added. After an additional 30 min of incubation at 37 °C, a fixative solution consisting of 5% final concentration of formaldehyde in PBS was added to each tube. Following fixation, 10-μl aliquots of sperm suspension were spread onto glass slides and air-dried. The slides were then stained with 0.22% Coomassie Blue G-250 in 50% methanol and 10% glacial acetic acid for 3–5 min, gently rinsed with deionized H2O, air-dried, and mounted with 50% (v/v) glycerol in phosphate-buffered saline. To calculate the percentage of acrosome reaction, at least 100 sperm were assayed per experimental condition for the presence or absence of the characteristic dark blue acrosomal crescent. The percentage of acrosome-reacted spermatozoa was calculated for each experimental condition dividing the number of acrosome-reacted spermatozoa by the total number of spermatozoa scored (sum of acrosome-reacted and non-acrosome-reacted) and multiplying this ratio by 100.
Mouse Eggs Collection and IVF Assays—Egg collection was performed as described previously (20). Briefly, metaphase II-arrested eggs were collected from 6–8-week-old superovulated CD1 female mice (Charles River Laboratories) at 13 h after human chorionic gonadotropin (Sigma) injection. Cumulus cells were removed by brief incubation (<5 min) in Whitten's HEPES-buffered medium with 7 mm NaHCO3, 5 mg/ml BSA, and 0.02% type IV-S hyaluronidase (Sigma). After cumulus cell removal, eggs were placed in a drop of Whitten's medium containing 22 mm NaHCO3 and 15 mg/ml BSA and then allowed to recover for 30 min in an incubator with 5% CO2 at 37 °C (11).
Fertilization drops (200 μl each) containing 10–20 eggs were inseminated with sperm (final concentration of 2.5 × 106cells/ml) that had been incubated for 1 h under capacitating conditions. After 4 h of insemination, eggs were washed through three drops of Whitten's medium containing 22 mm NaHCO3 and 15 mg/ml BSA using a thin bore pipette to detach any loosely attached sperm. After another 3 h of incubation, eggs were fixed with 3.7% paraformaldehyde/PBS for 15 min, washed, and stained with Hoestch 33342 (Sigma; 10 μg/ml final concentration) in PBS for 10 min at room temperature. To assess fertilization the three following criteria were considered: 1) the formation of the male and female pronuclei, 2) the emission of the second polar body, and 3) the presence of the sperm tail. The percentage of fertilization was calculated for each fertilization drop by dividing the number of fertilized eggs by the total number of eggs in that drop (sum of fertilized and nonfertilized eggs) and multiplying this ratio by 100.
RNA Isolation and Reverse Transcription-PCR—Total RNA was prepared from isolated mouse pachytene, round and elongated spermatids (60) using TRIzol reagent (Sigma) according to the manufacturer's instructions. cDNA was synthesized from total RNA samples with random hexamer-primed reverse transcription (Superscript II RNase H-reverse transcriptase; Invitrogen). cDNA was then subjected to PCR amplification using Taq DNA polymerase (Invitrogen). The NKCC1 primers were designed using the mouse-reported nucleotide sequence for these genes (mouse NKCC GenBank™ accession number NM_009194.2). Primer sequences for mouse NKCC1 were as follows: forward, 5′-CCT GCT TTA CTT CAT C-3′; reverse, 5′-GTC AAA CCT CCA TCA-3′. The absence of genomic contamination in the RNA samples was confirmed with reverse transcription negative controls (no reverse transcriptase) for each experiment. Amplified products were analyzed by DNA sequencing in order to confirm their identity.
Indirect Immunofluorescence—Sperm obtained by the swim-up method in Whitten's HEPES-buffered medium were washed once, resuspended in PBS at a concentration of 1–2 × 105 sperm/ml, and seeded on 8-well glass slides. After air-drying, sperm were fixed with 3.7% paraformaldehyde in PBS for 15 min at room temperature, washed with PBS (four washes each for 5 min), and permeabilized with 0.5% Triton X-100 for 5 min. Following permeabilization, sperm were treated with 10% BSA in PBS for 1 h at room temperature and then incubated either with the respective primary antibody (1:50–1:250) diluted in PBS containing 1% BSA or with the same concentration of the corresponding affinity-purified IgG. Incubations were then carried out at 4 °C overnight. After incubation, sperm were washed thoroughly with PBS and incubated with the corresponding Alexa 555-conjugated secondary antibody (1:200) diluted in PBS containing 1% BSA for 1 h at room temperature; these solutions also contained Alexa 488-conjugated peanut agglutinin (1:100) for staining acrosomes. Incubation with the secondary antibody was followed by four washes in PBS, mounted using SlowFade Light reagents (Molecular Probes, Eugene, OR), and observation by epifluorescence microscopy using a TE300 Eclipse microscope (magnification ×60) (Nikon). Differential interference contrast images were taken in parallel and served as control for sperm morphology. Negative controls using secondary antibody alone were also used to check for antibody specificity (not shown).
cAMP Measurements—Sperm (5 × 106 sperm/ml) were incubated for 1 h in noncapacitating (without BSA and NaHCO3) or capacitating medium (with BSA and NaHCO3) in the presence or absence of Cl– or bumetanide (1 mm). All treatments were supplemented with IBMX (0.1 mm). Afterward, the sperm were centrifuged for 2 min at 800 × g, the pellet was resuspended in 200 μl of HCl (0.1 mm), vortexed twice for 2 s, and incubated for 20 min at room temperature. Next, sperm were centrifuged at 5000 × g for 5 min, and the supernatant was used to measure cAMP levels by using the BIOMOL format A cyclic AMP “PLUS” EIA kit (BIOMOL International, Plymouth Meeting, PA). A standard curve was run for each assay, and the unknown cAMP concentrations were obtained utilizing a weighted four-parameter logistic curve fitting (as recommended by manufacturer) with the aid of GraphPad software.
Statistical Analysis—The data are expressed as the means ± S.E. The IVF experimental results were normalized to the control values, which were considered as 100%. In order to assume normal distribution, percentages were converted to ratios and all data subjected to the arcsine square root transformation (21). Statistical analysis was performed with the aid of GraphPad software using the parametric t test for simple comparisons and either the Tukey test following one-way analysis of variance or the Bonferroni post-tests after two-way analysis of variance for multiple comparisons.
RESULTS
Cl– Is Necessary for
Capacitation—Cl– is involved in the regulation of
the capacitation-associated hyperpolarization of the sperm
Em (4).
However, it has not been established whether Cl– acts
upstream, downstream, or independently of other signaling pathways involved in
capacitation. To analyze the role of Cl– in capacitation,
media with different Cl– concentrations were prepared by
replacing this anion with gluconate as described under “Experimental
Procedures.” Initially, the capacitation-associated increase in tyrosine
phosphorylation was analyzed by Western blot using anti-PY antibodies. As
shown previously (18), in the
absence of BSA and 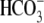 , either with or
without Cl–, there is no increase in tyrosine phosphorylation
(Fig. 1A). When BSA
and
, either with or
without Cl–, there is no increase in tyrosine phosphorylation
(Fig. 1A). When BSA
and 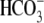 were present, the increase in
tyrosine phosphorylation was observed only in the presence of
Cl– (Fig.
1A). Moreover, the increase in tyrosine phosphorylation
was dependent on the Cl– concentration
(Fig. 1B). Because
hexokinase (116 kDa) is a protein that is constitutively phosphorylated in
tyrosine residues (18), it is
commonly used as a loading control for Western blots with anti-PY antibodies.
To confirm this understanding, as an additional loading control each membrane
was stripped and reproved with an anti-tubulin antibody (described under
“Experimental Procedures”).
were present, the increase in
tyrosine phosphorylation was observed only in the presence of
Cl– (Fig.
1A). Moreover, the increase in tyrosine phosphorylation
was dependent on the Cl– concentration
(Fig. 1B). Because
hexokinase (116 kDa) is a protein that is constitutively phosphorylated in
tyrosine residues (18), it is
commonly used as a loading control for Western blots with anti-PY antibodies.
To confirm this understanding, as an additional loading control each membrane
was stripped and reproved with an anti-tubulin antibody (described under
“Experimental Procedures”).
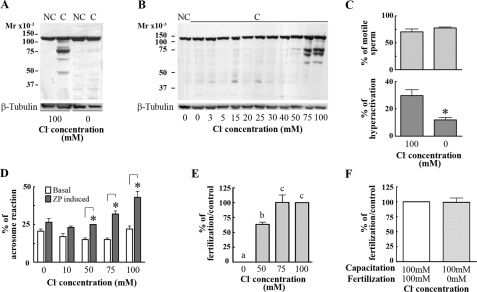
FIGURE 1.
Cl– is necessary for capacitation-associated events and
for fertilization. A, capacitation-associated tyrosine
phosphorylation is inhibited in Cl–-free medium. Mouse sperm
were incubated for 60 min in media that do not support (–BSA,
–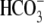 )(NC) or support
capacitation (+BSA,
)(NC) or support
capacitation (+BSA, 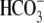 )(C)
in the presence or absence of Cl– (replaced by gluconate).
Subsequently, aliquots from each condition were processed for Western blotting
with anti-PY antibodies (upper panel). The increase in tyrosine
phosphorylated proteins was observed only when sperm were capacitated in the
presence of Cl–. The stripped membranes were reblotted with
an anti-β-tubulin antibody for loading control (lower panel).
B, the increase in tyrosine phosphorylation is dependent on
Cl– concentration. Sperm were incubated in Whitten's media
containing BSA,
)(C)
in the presence or absence of Cl– (replaced by gluconate).
Subsequently, aliquots from each condition were processed for Western blotting
with anti-PY antibodies (upper panel). The increase in tyrosine
phosphorylated proteins was observed only when sperm were capacitated in the
presence of Cl–. The stripped membranes were reblotted with
an anti-β-tubulin antibody for loading control (lower panel).
B, the increase in tyrosine phosphorylation is dependent on
Cl– concentration. Sperm were incubated in Whitten's media
containing BSA, 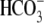 , and different
Cl– concentrations. Sperm proteins were analyzed by Western
blots with anti-PY antibodies (upper panel). After being stripped,
membranes were reblotted with an anti-β-tubulin antibody for loading
control (lower panel). C, the percentage of hyperactivated
sperm is reduced in the absence of Cl–. After capacitation in
the presence or the absence of Cl–, motility parameters were
analyzed by CASA. The percentage of motile cells (upper panel) and
the percentage of hyperactivation (lower panel) were compared between
treatments as explained under “Experimental Procedures.”
D, Cl– is needed for the induction of acrosome
reaction. Sperm were incubated with different Cl–
concentrations in capacitation media, and then solubilized ZP was added and
incubated for induction of acrosome reaction. After fixation and staining with
Coomassie Blue, the status of the acrosome was assessed in at least 100
sperm/treatment. E, IVF is inhibited in the absence of
Cl–. Sperm incubated in capacitating media with different
Cl– concentrations were used in IVF assays. The insemination
drops contained 10–20 eggs and had the same Cl–
concentration as the capacitation media. F, the absence of
Cl– during the co-incubation does not affect the interaction
between gametes. Eggs were co-incubated in the absence of Cl–
with sperm capacitated in control media. The percentage of fertilization in
the control was 75 ± 8. Each experiment was repeated at least three
times. Hyperactivation experiments: *, p < 0.05 versus
control (100 mm Cl–) using two-tailed Student's
t test. Acrosome reaction: *, p < 0.05 comparing basal
with ZP-induced acrosome reaction for each Cl– concentration
using Bonferroni post tests. IVF experiments: Tukey's multiple comparison test
was performed. The means of groups that have different letters differ
significantly (p < 0.05).
, and different
Cl– concentrations. Sperm proteins were analyzed by Western
blots with anti-PY antibodies (upper panel). After being stripped,
membranes were reblotted with an anti-β-tubulin antibody for loading
control (lower panel). C, the percentage of hyperactivated
sperm is reduced in the absence of Cl–. After capacitation in
the presence or the absence of Cl–, motility parameters were
analyzed by CASA. The percentage of motile cells (upper panel) and
the percentage of hyperactivation (lower panel) were compared between
treatments as explained under “Experimental Procedures.”
D, Cl– is needed for the induction of acrosome
reaction. Sperm were incubated with different Cl–
concentrations in capacitation media, and then solubilized ZP was added and
incubated for induction of acrosome reaction. After fixation and staining with
Coomassie Blue, the status of the acrosome was assessed in at least 100
sperm/treatment. E, IVF is inhibited in the absence of
Cl–. Sperm incubated in capacitating media with different
Cl– concentrations were used in IVF assays. The insemination
drops contained 10–20 eggs and had the same Cl–
concentration as the capacitation media. F, the absence of
Cl– during the co-incubation does not affect the interaction
between gametes. Eggs were co-incubated in the absence of Cl–
with sperm capacitated in control media. The percentage of fertilization in
the control was 75 ± 8. Each experiment was repeated at least three
times. Hyperactivation experiments: *, p < 0.05 versus
control (100 mm Cl–) using two-tailed Student's
t test. Acrosome reaction: *, p < 0.05 comparing basal
with ZP-induced acrosome reaction for each Cl– concentration
using Bonferroni post tests. IVF experiments: Tukey's multiple comparison test
was performed. The means of groups that have different letters differ
significantly (p < 0.05).
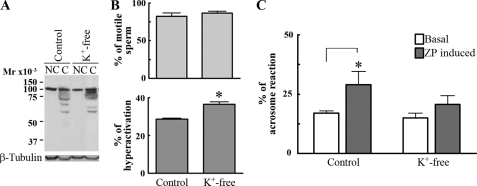
Capacitation is linked to sperm flagellum hyperactivation and to sperm preparation for agonist-induced acrosome reaction. To investigate the effect of Cl– on hyperactivated motility, CASA of sperm incubated in capacitating media with or without Cl– was carried out. Although the percentage of motile sperm did not differ in either medium (with or without Cl–), the percentage of hyperactivated cells was significantly reduced in the absence of Cl– (Fig. 1C). To assure that the absence of Cl– did not affect sperm viability, sperm membrane integrity was tested using propidium iodide, a nonpermeable dye commonly used to assay this parameter. No difference in the percentage of viable sperm was observed between sperm incubated in media with and without Cl– (data not shown).
Second, to test whether the presence of Cl– during capacitation is needed for the acrosome reaction, mouse sperm were incubated in media with or without Cl–, and the ZP-induced acrosome reaction was determined. The ZP-induced acrosome reaction was significantly reduced when Cl– was not present (Fig. 1D). Because Cl– has been reported to be involved in the actual process of acrosome reaction (22–24), to ensure that the observed effect is on capacitation, sperm were incubated in the absence of Cl– for 1 h. At that time the Cl–-free medium was supplemented with 100 mm Cl–, and ZP was added. Under these conditions, the ZP-induced acrosome reaction was still significantly inhibited (data not shown), indicating that the effect observed was related to capacitation.
Finally, only capacitated sperm are able to fertilize eggs in vitro; therefore, the need for Cl– was tested on in vitro fertilization assays. Sperm incubated in capacitating media with different concentrations of Cl– were co-incubated with eggs as described under “Experimental Procedures,” and fertilization was evaluated at the pronuclei stage. In the absence of Cl– the percentage of fertilized eggs was zero (Fig. 1E). To rule out a possible role of Cl– in the direct interaction between gametes, sperm capacitated in media containing Cl– were used to fertilize eggs in a medium without Cl–. Under these conditions the percentage of fertilized eggs in media without Cl– was not significantly different from the control (Fig. 1F). Interestingly, the minimum Cl– concentration needed for the increase in protein tyrosine phosphorylation was between 50 and 75 mm, similar to the Cl– concentration needed for the ZP-induced acrosome reaction and for the IVF assays (Fig. 1B, D, and E). When methanesulfonate was used instead of gluconate to replace Cl– in the incubation media, the same set of capacitation-associated parameters was analyzed, and in all cases similar results were obtained (data not shown).
Previously we showed that 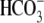 cotransporters are present in sperm
(25). In particular, that
study demonstrated that
cotransporters are present in sperm
(25). In particular, that
study demonstrated that 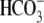 additions
induce membrane potential hyperpolarization in non-capacitated mouse sperm.
Because
additions
induce membrane potential hyperpolarization in non-capacitated mouse sperm.
Because 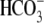 is involved in the
regulation of soluble adenylyl cyclase (sAC), we analyzed whether this initial
is involved in the
regulation of soluble adenylyl cyclase (sAC), we analyzed whether this initial
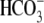 transport was affected in the
absence of Cl–. Similar to findings in control media, the
addition of
transport was affected in the
absence of Cl–. Similar to findings in control media, the
addition of 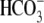 to sperm in the
absence of Cl– elicited a membrane potential
hyperpolarization (data not shown), suggesting that
to sperm in the
absence of Cl– elicited a membrane potential
hyperpolarization (data not shown), suggesting that
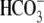 transport through the
transport through the
 cotransporter is independent of the presence of Cl– in the
incubation medium.
cotransporter is independent of the presence of Cl– in the
incubation medium.
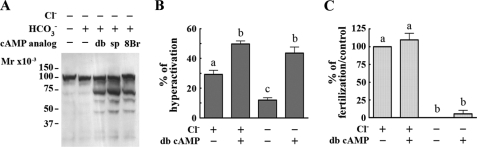
Cyclic AMP Is Downstream of the Effect of Cl– in Capacitation—It has been reported that protein tyrosine phosphorylation in mammalian sperm is downstream of the cAMP pathway (26–29). To assess whether the requirement for Cl– is mediated by cAMP, sperm were incubated in media with or without Cl– and in the presence or absence of cAMP agonists (dibutyryl cAMP, SP-cAMPS, or 8-bromo-cAMP) and the phosphodiesterase inhibitor IBMX as described in the legend for Fig. 2. Under these conditions the three cAMP analogs were able to increase tyrosine phosphorylation in Cl–-free medium (Fig. 2A), suggesting that cAMP is involved in the Cl– requirement for this phosphorylation event.
FIGURE 2.
Cyclic AMP is downstream of the effect of Cl– on capacitation. Sperm were incubated in media without Cl– in the absence or presence of IBMX (0.2 mm) and different cAMP analogs: dibutyryl cyclic AMP (db; 5 mm), sp-cAMPS (sp; 1 mm), or 8-bromo-cAMP (8Br; 0.5 mm). The increase in tyrosine phosphorylation (A) and the percentage of hyperactivation (B) were evaluated. In IVF assays (C), gamete co-incubation was conducted in drops with the same composition as the capacitation media. The percentage of fertilization in the control was 85 ± 6. Each experiment was repeated at least three times. For hyperactivation and IVF experiments, Tukey's multiple comparison tests were performed. The means of groups that have different letters differ significantly (p < 0.01) (B and C).
CASA revealed that in the presence of dibutyryl cAMP and IBMX, the percentage of hyperactivated sperm incubated in Cl– -free medium is similar to the control with Cl– (Fig. 2B), suggesting that the role of Cl– in hyperactivated motility is also mediated by cAMP. In contrast, when IBMX and dibutyryl cAMP were used in IVF experiments, these compounds were not able to increase the percentage of fertilized eggs when the experiment was conducted in the absence of Cl– (Fig. 2C). Because IBMX is dissolved in DMSO, 0.1% DMSO was present under all conditions tested to control for deleterious solvent effect on IVF. The lack of action of cAMP agonists in this case suggests that in addition to its role in the increase in tyrosine phosphorylation and hyperactivation, likely mediated by a cAMP pathway, Cl– might have additional cAMP-independent functions in the capacitation process. Consistent with findings by Branham et al. (30), cAMP-permeable analogs induced the acrosome reaction under all conditions tested, even in the absence of ZP (data not shown). Therefore, no conclusions were obtained in these experiments.
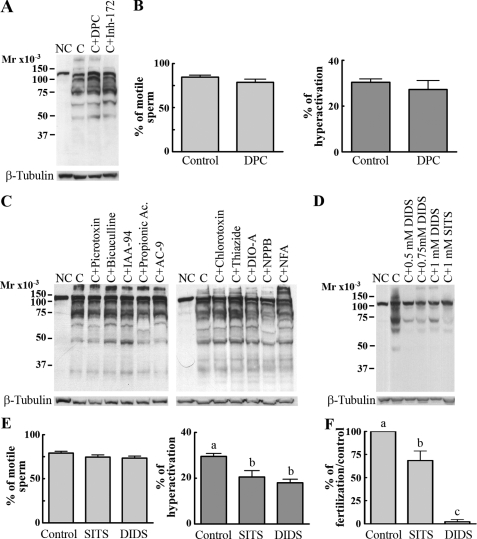
Effect of Cl– Transport Inhibitors on Capacitation-associated Processes—The observation that Cl– was required for the increase in tyrosine phosphorylation, hyperactivation, acrosome reaction, and IVF suggests that Cl– transport in sperm plays an important role in capacitation. Moreover, this hypothesis is consistent with previous results indicating that during capacitation there is an increase in intracellular Cl– concentration (4). Despite these data, how Cl– enters the sperm is not known. To investigate the nature of the transporters mediating Cl– fluxes during capacitation, the increase in tyrosine phosphorylation was used as an end point to test a battery of known inhibitors. Previous observations (4) indicated that the CFTR antagonists DPC and inh-172 are not able to inhibit the increase in tyrosine phosphorylation. Here we show the same lack of effect on capacitation-dependent tyrosine phosphorylation, hyperactivation, and motility even at concentrations higher than the ones effective in CFTR regulation (Fig. 3, A and B, and Table 1). Similarly, bicuculline and picrotoxin, γ-aminobutyric acid-A receptor/Cl– channel antagonists (31); chlorotoxin and niflumic acid, two Ca2+-dependent Cl– channel inhibitors (32, 33); and 5-nitro-2-(3-phenylpropylamino)benzoic acid, 9-(hydroxymethyl)anthracene, indanyloxyacetic acid 94, and propionic acid, known ClC family blockers (34–36), were not able to inhibit the capacitation-associated increase in tyrosine phosphorylation (Fig. 3C and Table 1). Although these results do not rule out the presence of the respective Cl– transporters in sperm, they suggest that these transporters do not play a key role in the events leading to the increase in tyrosine phosphorylation. On the other hand, as expected (25), more general Cl–-transport inhibitors such as stilbene compounds (SITS, DIDS) were able to block the increase in tyrosine phosphorylation as well as significantly reduce sperm hyperactivation and IVF (Fig. 3, D–F, and Table 1). Although these results suggest that anion transport participates in sperm capacitation, it is not possible to conclude which type of transporter is involved because these compounds can inhibit a range of molecules.
FIGURE 3.
Effect of Cl– channel inhibitors in capacitation. A and B, CFTR inhibitors do not inhibit capacitation-associated increase in tyrosine phosphorylation, motility, or hyperactivation. Sperm were incubated in complete capacitating media in the presence of two different CFTR inhibitors: inh-172 (250 μm) or DPC (1 mm), and the increase in tyrosine phosphorylation (A, upper panel) and percentage of motile sperm and hyperactivation (B) were analyzed. C, upper panel, Cl– channel inhibitors with no effect on tyrosine phosphorylation. Sperm were incubated under noncapacitating (NC) or capacitating (C) conditions in the presence of different anionic channel blockers: picrotoxin (1 mm), bicuculline (1 mm), indanyloxyacetic acid 94 (IAA-94; 0.2 mm), propionic acid (1 mm), 9-(hydroxymethyl)anthracene (AC-9; 0.25 mm), chlorotoxin (0.01 mm), thiazide (1 mm), R(+)-butylindazone (DIO-A; 0.2 mm), 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB; 0.2 mm); and niflumic acid (NFA; 0.5 mm). Then, Western blotting was conducted using anti-PY antibodies. D, upper panel, DIDS and SITS block capacitation-associated increase in tyrosine phosphorylation. Sperm were incubated under noncapacitating or capacitating conditions in the presence or absence of DIDS or SITS (disulfonic stilbene derivatives), two broad anionic channel blockers. Next, Western blotting was conducted using anti-PY antibodies. E and F, DIDS and SITS block hyperactivation and IVF. Sperm incubated in capacitating media in the presence or absence of DIDS or SITS (1 mm) were used to assay for hyperactivation (E) and IVF (F). The inhibitors were present also during the gametes co-incubation. The percentage of fertilization in the control was 78 ± 13. All membranes were stripped and reblotted with an anti-β-tubulin antibody for loading control (A, C, and D, lower panels). Each experiment was repeated at least three times. For motility, hyperactivation and IVF experiments, two-tailed Student's t test (B) or Tukey's multiple comparison test (E and F) were performed. The means of groups that have different letters differ significantly (p < 0.01) (E and F).
TABLE 1.
Effect of inhibitors on capacitation associated events and fertilization
The effect of different Cl– channel inhibitors was tested first on capacitation-associated tyrosine phosphorylation. Those inhibitors that affected the increase in phosphorylation were controlled for effects on motility (as a parameter of viability) and further for hyperactivation and fertilization. GABA-A, γ-aminobutyric acid-A; NPPB, 5-nitro-2-(3-phenylpropylamino)benzoic acid; IAA-94, indanyloxyacetic acid 94; AC-9, 9-(hydroxymethyl)anthracene; —, not determined. VRAC, volume-regulated anion channel.
| Inhibitor | Concentration | Effect on tyrosine phosphorylation | Effect on motility | Effect on hyperactivation | Effect on IVF |
|---|---|---|---|---|---|
| DPC (CFTR inhibitor) | 1 mm | None | None | None | None |
| Bumetanide (NKCC inhibitor) | 1-100 μm | None | — | — | — |
| 250 μm—1 mm | Inhibition | None | Inhibition | Inhibition | |
| Furosemide (NKCC inhibitor) | 100-250 μm | None | — | — | — |
| 250 μm—1 mm | Inhibition | None | Inhibition | Inhibition | |
| Thiazide (NCC inhibitor) | 250 μm—1 mm | None | — | — | None |
| R(+)-Butylindazone (KCC inhibitor) | 200 μm | None | — | — | — |
| DIDS (chloride channel blocker) | 500 μm—1 mm | Inhibition | None | Inhibition | Inhibition |
| SITS (chloride channel blocker) | 1 mm | Inhibition | None | Inhibition | Inhibition |
| Chlorotoxin (calcium-activated Cl- channel inhibitor, small conductance Cl- channel inhibitor) | 1 μm—10 μm | None | — | — | — |
| Niflumic acid (calcium-activated Cl-channels inhibitor and VRAC inhibitor) | 250-750 μm | None | — | — | — |
| Picrotoxin (GABA-A receptor/Cl– channel antagonist) | 200 μm—1 mm | None | — | — | — |
| Bicuculline (GABA-A receptor/Cl– channel antagonist) | 10 μm—1 mm | — | — | — | — |
| NPPB (CLC channels inhibitor) | 200 μm | None | None | None | — |
| IAA-94 (CLC channels inhibitor) | 200 μm | None | — | — | — |
| Propionic acid (CLC channels inhibitor) | 1 mm | None | — | — | — |
| AC-9 (CLC channels inhibitor) | 100-250 μm | None | None | None | — |
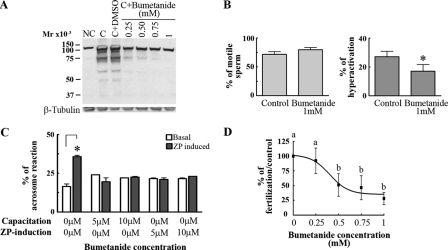
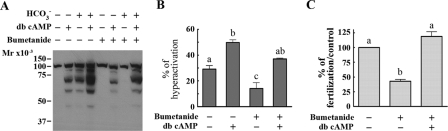
We then tested whether the family of electroneutral Cl– cotransporters participated in the capacitation-associated events. This later transporter family is composed of three subfamilies with a total of seven members (37, 38): one Na+/Cl– cotransporter (NCC), which can be inhibited by diuretics such as hydrochlorothiazide; two NKCCs, inhibited by bumetanide and less potently by furosemide; and four Na+-independent K+/Cl– cotransporters (KCC), which can be specifically blocked by R(+)-butylindazone (39). No effects were observed on tyrosine phosphorylation (Fig. 3C and Table 1) with either hydrochlorothiazide or R(+)-butylindazone. Notably, bumetanide and furosemide had a concentration-dependent inhibitory effect on the increase in protein tyrosine phosphorylation (Fig. 4A; Table 1). In addition, although bumetanide had no effect on the percentage of motile cells (Fig. 4B, left), it significantly reduced the percentage of hyperactivated sperm (Fig. 4B, right) and inhibited ZP-induced acrosome reaction and in vitro fertilization (Fig. 4, C and D).
FIGURE 4.
Effect of bumetanide on capacitation-associated events. A and B, bumetanide, an NKCC inhibitor, blocks the increase in tyrosine phosphorylation and hyperactivation with no effect on the percentage on sperm motility. Sperm were incubated under noncapacitating (NC) or capacitating (C) conditions in the presence of increasing bumetanide concentrations. Subsequently, Western blotting with anti-PY antibodies (A, upper panel) and CASA (B) were carried out. Membranes were stripped and reblotted with an anti-β-tubulin antibody for loading control (A, lower panel). C, bumetanide inhibits the ZP-induced acrosome reaction. Bumetanide was added to sperm during capacitation or at the same time as the ZP. Subsequently, ZP-induced acrosome reaction was evaluated. D, bumetanide inhibits IVF. Eggs were inseminated with sperm capacitated in the presence of different concentrations of bumetanide. The inhibitor was also added to each co-incubation drop at the same concentration as during capacitation. The percentage of fertilization in the control was 80 ± 9. Each experiment was repeated at least three times. For hyperactivation experiments (B), *, p < 0.01 versus control (0 mm bumetanide plus DMSO) using two-tailed Student's t test; for acrosome reaction (C), *, p < 0.05 comparing basal with ZP-induced acrosome reaction for each bumetanide concentration and using Bonferroni post tests; for IVF experiments, Tukey's multiple comparison test was performed. The means of groups that have different letters differ significantly (p < 0.05) (D).
Bumetanide is an NKCC inhibitor; however, its effect on the increase in protein tyrosine phosphorylation, hyperactivation, and IVF was observed at higher concentrations of these compounds than the ones known to inhibit NKCC transporters in other cell types (40). On the other hand, the ZP-induced acrosome reaction was inhibited with lower concentrations saturating at ∼10 μm, a concentration compatible with NKCC inhibition (Fig. 4C). To test whether the effect of low bumetanide concentrations was related to capacitation or directly affecting the ZP-induced acrosome reaction, the inhibitor was either added during capacitation and removed by centrifugation before addition of ZP or added at the same time as the ZP. Under both conditions, the acrosome reaction was inhibited (Fig. 4C). Hence, in addition to the effect of bumetanide and furosemide in the processes that make the sperm able to respond to ZP induction, the actual acrosome reaction process is affected by the inhibitors. Similar results were obtained with furosemide (data not shown).
The role of NKCC in capacitation was further analyzed by eliminating external K+ from capacitation media, a condition that would stop NKCC function. Although the lack of K+ had no effect on the increase in tyrosine phosphorylation (Fig. 5A) and sperm hyperactivation (Fig. 5B), sperm incubated in K+-free medium were unable to acrosome-react in the presence of ZP (Fig. 5C). Consistent with these results, the absence of K+ also inhibited IVF (data not shown). These observations provide additional evidence that NKCC is involved in the regulation of the acrosome reaction. The analysis of Cl– transporter inhibitors in different capacitation-associated parameters is summarized in Table 1. All of the inhibitors were tried at a wide range of concentrations. However, some of them were deleterious to the cells when used at high concentrations, and hence these concentrations were excluded from the table.
FIGURE 5.
Analysis of sperm capacitation in the absence of K+. A and B, the lack of K+ during capacitation does not affect either the increase in tyrosine phosphorylation or sperm hyperactivation. Sperm were incubated under noncapacitating (NC) or capacitating (C) medium with or without K+, and the increase in tyrosine phosphorylation (A, upper panel), the percentage of motility (B, upper panel), and hyperactivation (B, lower panel) were evaluated. Membranes were stripped and reblotted with an anti-β-tubulin antibody for loading control (A, lower panel). C, K+ ions are necessary for the ZP-induced acrosome reaction to occur. Sperm were incubated in control or K+-free capacitating media, and ZP-induced acrosome reaction was assayed. Each experiment was repeated at least three times. For hyperactivation (B), *, p < 0.01 versus control, using two-tailed Student's t test. For acrosome reaction (C), *, p < 0.01 comparing basal with ZP-induced acrosome reaction, using Bonferroni post tests.
As in the case of sperm incubated in Cl–-free medium, the
addition of cAMP agonists to sperm capacitated in the presence of 1
mm bumetanide completely overcame the inhibition of the increase in
protein tyrosine phosphorylation (Fig.
6A), suggesting that the signal transduction machinery is
functional under these conditions. Moreover in the presence of bumetanide,
permeable cAMP analogs increased both the percentage of hyperactivation
(Fig. 6B) and the
percentage of fertilization in IVF assays
(Fig. 6C). This
result, together with the ability of cAMP-permeable analogs to induce
capacitation-associated events in the absence of Cl–,
suggests that Cl– transport is upstream of a cAMP pathway. To
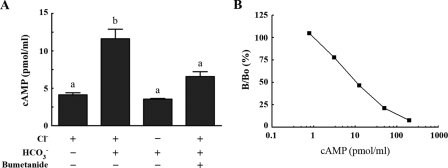
further investigate this hypothesis cAMP accumulation was measured. When
capacitation was carried out in the presence of Cl–, there
was a 2.5-fold increase on the cAMP levels compared with sperm that were kept
under noncapacitating conditions (without BSA and
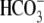 ). On the other hand, when sperm
were incubated in capacitation media either in the absence of
Cl– or in the presence of bumetanide, no significant
increases in the cAMP levels were observed
(Fig. 7A). In all of
these measurements, the cAMP concentration falls into the linear part of the
cAMP standard curve (Fig.
7B).
). On the other hand, when sperm
were incubated in capacitation media either in the absence of
Cl– or in the presence of bumetanide, no significant
increases in the cAMP levels were observed
(Fig. 7A). In all of
these measurements, the cAMP concentration falls into the linear part of the
cAMP standard curve (Fig.
7B).
FIGURE 6.
Cyclic AMP agonists rescue the inhibition of capacitation by bumetanide. Capacitation was carried out in the presence or absence of bumetanide (1 mm), IBMX (0.2 mm), and dibutyryl cyclic AMP (db cAMP; 5mm). Then, protein tyrosine phosphorylation (A), sperm hyperactivation (B), and fertilization (C) were evaluated. The percentage of fertilization in the control was 78 ± 7. Each experiment was repeated at least three times. For hyperactivation and IVF experiments, Tukey's multiple comparison tests were performed. The means of groups that have different letters (a and b) differ significantly (p < 0.01) (B and C).
FIGURE 7.
Capacitation-associated increase in cAMP levels are prevented in the absence of Cl– or in the presence of bumetanide. Capacitation was carried out in the presence or absence of Cl– or bumetanide (1 mm). Then, sperm were centrifuged and resuspended in HCl (0.1 mm) for 20 min. After that, sperm were centrifuged again and the supernatant was used to measure cAMP levels (A). A standard curve was run for each assay. A typical standard curve is depicted (B). Tukey's multiple comparison tests were performed. The means of groups that have different letters (a and b) differ significantly (p < 0.05) (A).
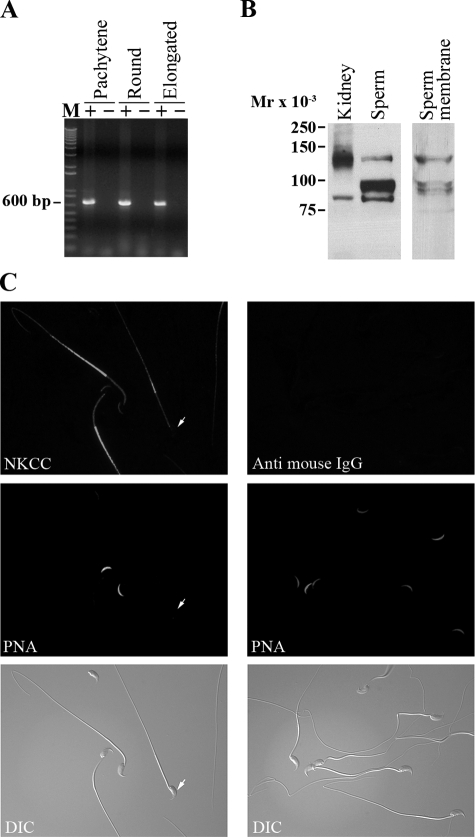
NKCC1 Is Present in Mouse Sperm—Although pharmacological approaches are limited by the specificity of the inhibitors, they restrict the possible transporter candidates. In this case, the inhibition of the ZP-induced acrosome reaction in the absence of K+ and by low concentrations of bumetanide suggests that NKCC transporters might be present in mouse sperm. In particular, NKCC1 is present in pachytene and round spermatids and is involved in spermatogenesis (41) (Fig. 8A). Taking these results into consideration, to analyze the presence of the protein in mature sperm, an anti-NKCC1 monoclonal antibody was used. Western blots indicate the presence of the predicted 145-kDa molecular mass protein (8) in total sperm extracts as well as in the kidney positive control (Fig. 8B). Consistent with its predicted localization, NKCC1 was present in sperm plasma membrane purified fractions. In both kidney and sperm, the anti-NKCC1 antibody recognized additional lower molecular weight proteins. Interestingly, these lower bands are also present in the sperm plasma membrane fraction and could represent degradation products, an alternative form of NKCC1, or additional sperm proteins not related to NKCC. Immunofluorescence experiments using this antibody show staining in different sperm compartments including the principal piece, the mid-piece, and the anterior head (Fig. 8C). Notably, staining in the head was not observed when PNA staining was negative, suggesting that NKCC is present in the plasma membrane overlaying the acrosome.
FIGURE 8.
NKCC is present in mouse sperm. A, NKCC mRNA is present in spermatogenic cells. Reverse transcription-PCR of spermatogenic cells was carried out using oligonucleotides for NKCC1 and cDNA of pachytene, round cells, and elongated spermatids (+); negative controls (–) without reverse transcriptase are also shown for each PCR. B, NKCC1 is present in mature mouse sperm. Total kidney and sperm extracts were analyzed by Western blotting with a monoclonal anti-NKCC1 antibody (left). In addition, sperm membrane purifications were carried out as described under “Experimental Procedures” and SDS extracts from 100,000 × g pellet were analyzed using anti-NKCC antibodies (right). C, NKCC1 localizes to different compartments in sperm including anterior head, mid and principal piece. For immunolocalization assays, mouse sperm were air-dried, fixed, permeabilized, and probed with anti-NKCC1 antibodies. Peanut agglutinin (PNA) was used to follow the acrosomal status. An NKCC1-specific signal in the head is not observed when peanut agglutinin is negative (arrows). Each experiment was repeated at least three times.
DISCUSSION
Mammalian sperm are not able to fertilize eggs immediately after
ejaculation. In 1951, Chang
(42) and Austin
(43) demonstrated
independently that sperm acquire fertilization capacity only after residing in
the female tract for a finite period of time in a process known as sperm
capacitation. Taking these initial investigations into account, sperm
capacitation became defined using fertilization as the end-point. However, a
variety of evidence suggests that the functional changes occurring in sperm
during capacitation are not one event but a combination of sequential and
concomitant processes. Some of them are: 1) sperm hyperactivation, 2)
preparation to undergo an agonist-induced acrosome reaction (e.g.
zona pellucidae or progesterone), and 3) acquisition of the ability to
fertilize the egg. Additionally, at the molecular level, during the last 10
years, a series of studies has established that some signaling pathways are
activated during sperm capacitation. In particular, capacitation has been
associated with a cAMP/PKA-dependent increase in protein tyrosine
phosphorylation (18,
26). This pathway appears to
be downstream of a series of events that occur at the level of the sperm
plasma membrane. Synthesis of cAMP is mediated by an atypical soluble adenylyl
cyclase known as sAC (44,
45). This enzyme was first
reported by Okamura et al.
(46) as a
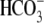 -stimulated cyclase and is
essential for the regulation of multiple aspects of sperm capacitation
including activation of motility
(47), the cAMP/PKA-dependent
increase in protein tyrosine phosphorylation
(18,
26,
48), and hyperactivation
(49,
50). In contrast to all the
evidence indicating a role for
-stimulated cyclase and is
essential for the regulation of multiple aspects of sperm capacitation
including activation of motility
(47), the cAMP/PKA-dependent
increase in protein tyrosine phosphorylation
(18,
26,
48), and hyperactivation
(49,
50). In contrast to all the
evidence indicating a role for 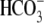 function in the regulation of these signaling pathways, much less is known
about the role of Cl–, the predominant anion in the
capacitation medium.
function in the regulation of these signaling pathways, much less is known
about the role of Cl–, the predominant anion in the
capacitation medium.
Previously, we had shown that Cl– is needed for the membrane potential changes that accompany capacitation (4). We have hypothesized that CFTR mediates the effect of Cl– on the capacitation-associated hyperpolarization downstream of cAMP through PKA activation of this Cl– channel. However, CFTR inhibition does not alter either tyrosine phosphorylation or sperm hyperactivation, two processes that are also downstream of a cAMP pathway. In contrast to these results, sperm incubated in Cl–-free medium did not hyperactivate and did not undergo the capacitation-associated increase in tyrosine phosphorylation. Altogether, these experiments suggest that in addition to CFTR, other Cl– transport systems are present in sperm. To investigate this hypothesis, a battery of Cl– transport inhibitors was tested in capacitation assays. As expected, general Cl– transport blockers such as stilbenes mimicked the absence of Cl–; however, more specific inhibitors were not able to reduce either the increase in tyrosine phosphorylation or hyperactivation. DIDS was significantly more effective than SITS in inhibiting IVF, but it had equal inhibitory power when other sperm parameters such as the increase in tyrosine phosphorylation and hyperactivation were assayed. These compounds are known to react covalently with lysine and cysteine residues (51); however, the ability of DIDS to bind covalently to these residues is much greater than that of SITS (52). In contrast with measurements of tyrosine phosphorylation and hyperactivation, longer incubation times are needed for IVF. Under these conditions covalent binding might be essential for the inhibitory properties of the stilbene compounds.
Bumetanide and furosemide, two NKCC inhibitors, reduced sperm capacitation parameters to levels similar to those observed in the absence of Cl–. However, the concentration necessary for the inhibition of tyrosine phosphorylation and hyperactivation was higher than that reported to be effective in inhibiting NKCC (40, 53, 54). Moreover, even though the presence of Cl– and Na+ (25) is essential for the increase in tyrosine phosphorylation, K+, another ion needed for the function of NKCCs, was not required for these processes. These results suggest that high concentrations of bumetanide might have a target different from NKCC, that is essential for the onset of hyperactivation and the increase in tyrosine phosphorylation. In contrast to these results, the ZP-induced acrosome reaction was dependent on the presence of Cl–, K+ (this study) and Na+ (25) and was inhibited at a much lower concentration of bumetanide and furosemide, suggesting that NKCC might have a role in the capacitation-dependent preparation of sperm for the acrosome reaction or directly in the acrosome reaction process itself. Similarly, DPC and inh-172, two CFTR antagonists used in this work, were not able to inhibit either hyperactivation or the increase in tyrosine phosphorylation, yet they block the ZP-induced acrosome reaction (4). On the other hand, in sAC null mutants, the inhibition of the increase in tyrosine phosphorylation and hyperactivation did not block the ability of the sperm to acrosome react in the presence of ZP (55). Altogether, these experiments suggest that different aspects of capacitation are regulated by different molecular pathways.
The presence of NKCC was studied using Western blot analyses in both total sperm extracts and purified membrane preparations. It is worth noting that in addition to the 145-kDa band that corresponds to the reported NKCC1 (8), a lower molecular mass band was detected in sperm. Interestingly, two NKCC1 transcripts were reported in specific testis cell populations (41, 56). Besides the normal NKCC1 transcript (6.5 kb) present in somatic tissues, an alternate transcript of 4.2 kb was detected in pachytene spermatocytes and round spermatids. Furthermore, null mutants of this protein were shown to have defects in spermatogenesis and are infertile (41). It remains to be investigated whether the shorter transcript codes for the lower molecular weight protein recognized by anti-NKCC1. Alternatively, it is not possible to discard the possibility that the anti-NKCC antibody recognized additional sperm proteins; therefore, the immunofluorescence staining may not correspond completely to NKCC.
As mentioned previously, bumetanide inhibited hyperactivation, the increase in tyrosine phosphorylation, and fertilization at higher concentrations than those needed to block NKCC1 in other systems. It is likely that higher concentrations of this inhibitor target molecule(s) different from NKCC1. Studies in human erythrocytes (39) have shown that most of the classical Cl– transport inhibitors, including furosemide and bumetanide, can inhibit anion exchangers and, to a lesser extent, KCCs when used at high concentrations. Taking into account that thiazide, the specific KCC inhibitor, did not affect tyrosine phosphorylation, it would be more likely that an anion exchanger was affected by bumetanide (and furosemide) when used at high concentration. An alternative hypothesis is that high concentrations of bumetanide are needed to inhibit an additional sperm-specific NKCC and that this protein has less affinity to the inhibitor than the classical NKCC found in somatic tissues. Consistent with this hypothesis, changes in bumetanide binding affinity have been reported in several chimeras of human colonic NKCC1 (57). In addition, although K+ is needed for a functional NKCC in most cases, in a few it has been reported that it can transport only Na+ and Cl– (58). The finding of a lower molecular weight NKCC1 in sperm, as well as the aforementioned smaller NKCC1 transcripts, supports this possibility. Further isolation and identification of the 95-kDa band will be necessary to verify this hypothesis. In contrast to hyperactivation and the increase in tyrosine phosphorylation, the classical NKCC1 appears to have a role in other aspects of capacitation, such as the preparation of the sperm for the ZP-induced acrosome reaction. This conclusion is based on the requirement of external Cl–, K+, and Na+ for capacitation and the exocytotic reaction, as well as on the lower bumetanide concentrations needed to inhibit these processes.
Although it is not yet possible to define the complete set of Cl– transporters regulating Cl– homeostasis in sperm, this study provides strong evidence that this anion is required for capacitation. Moreover, the results presented here indicate that cAMP-permeable analogs are able to overcome the absence of Cl– as well as bumetanide inhibition of capacitation-associated parameters. This result, coupled with the finding that accumulation of cAMP in sperm incubated under capacitating conditions does not occur in the absence of Cl– or in the presence of bumetanide, suggests that the observed increase in Cl– concentration during capacitation (4) is upstream of cAMP signaling pathways. Although less likely, another possibility is that Cl– and cAMP are part of parallel and coincident signaling pathways involved in sperm capacitation and that addition of exogenous permeable cAMP analogs can compensate for the loss of Cl–-mediated signaling.
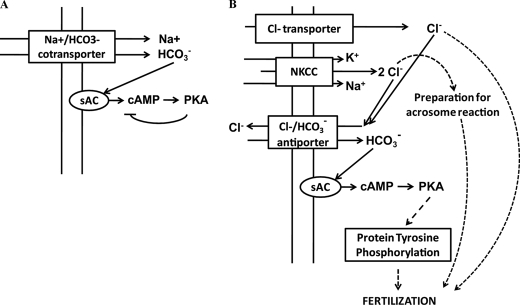
Our working hypothesis (Fig.
9) is that inward translocation of Cl–, to which
NKCC1 is likely to contribute, is coupled to the activity of a
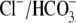 antiporter. Inward fluxes of
antiporter. Inward fluxes of 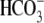 through this exchanger would stimulate sAC and increase the cAMP levels.
Recently, Nolan et al.
(59) generated mice that lack
the sperm-specific PKA catalytic subunit Cα2. These mice are infertile
despite normal mating behavior, and their sperm present defects in motility
and capacitation-associated events such as the increase in tyrosine
phosphorylation. Also, cAMP levels in Cα2 knock-out mice are
up-regulated, suggesting that PKA phosphorylation exerts a negative feedback
on cAMP production. Consistent with these findings, incubation of wild type
sperm with the PKA inhibitor H89 increases the basal levels of cAMP
(59). Because cAMP is needed
for later events associated with capacitation, it is possible to hypothesize
that the negative feedback mechanism on cAMP levels exerted by PKA
phosphorylation is released at some point during the capacitation process.
Because Cl– is required for subsequent cAMP-dependent
processes, we postulate that the release of the negative feedback in cAMP
synthesis is dependent on the activation of other regulatory pathways
downstream of cholesterol release and Cl– transport. Further
investigation should test whether two waves of cAMP synthesis by sAC are
needed in the capacitation process: 1) an acute
Cl–-independent increase in cAMP levels involving sAC
activation by
through this exchanger would stimulate sAC and increase the cAMP levels.
Recently, Nolan et al.
(59) generated mice that lack
the sperm-specific PKA catalytic subunit Cα2. These mice are infertile
despite normal mating behavior, and their sperm present defects in motility
and capacitation-associated events such as the increase in tyrosine
phosphorylation. Also, cAMP levels in Cα2 knock-out mice are
up-regulated, suggesting that PKA phosphorylation exerts a negative feedback
on cAMP production. Consistent with these findings, incubation of wild type
sperm with the PKA inhibitor H89 increases the basal levels of cAMP
(59). Because cAMP is needed
for later events associated with capacitation, it is possible to hypothesize
that the negative feedback mechanism on cAMP levels exerted by PKA
phosphorylation is released at some point during the capacitation process.
Because Cl– is required for subsequent cAMP-dependent
processes, we postulate that the release of the negative feedback in cAMP
synthesis is dependent on the activation of other regulatory pathways
downstream of cholesterol release and Cl– transport. Further
investigation should test whether two waves of cAMP synthesis by sAC are
needed in the capacitation process: 1) an acute
Cl–-independent increase in cAMP levels involving sAC
activation by 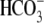 and mediated through
a
and mediated through
a  cotransporter as postulated previously
(25)
(Fig. 9A); and 2) a
sustained increase in cAMP concentration mediated by an inward flux of
Cl– coupled to the activation of a
cotransporter as postulated previously
(25)
(Fig. 9A); and 2) a
sustained increase in cAMP concentration mediated by an inward flux of
Cl– coupled to the activation of a
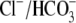 antiporter (Fig.
9B).
antiporter (Fig.
9B).
FIGURE 9.
Model for the involvement of Cl– during
capacitation. A, initial events during capacitation. At first, an
acute Cl–-independent increase in cAMP levels involving sAC
activation by 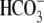 and mediated through
a
and mediated through
a  cotransporter occurs allowing the consequent activation of PKA. Subsequently,
PKA phosphorylation exerts a negative feedback on cAMP levels. B,
later events during sperm capacitation. Because of the need of cAMP for later
events, it is possible to hypothesize that the negative feedback mechanism on
the cAMP levels exerted by PKA phosphorylation is released at some point
during the capacitation process. Our working hypothesis is that inward
translocation of Cl– (through NKCC and other
Cl– transporters) is coupled to the activity of a
cotransporter occurs allowing the consequent activation of PKA. Subsequently,
PKA phosphorylation exerts a negative feedback on cAMP levels. B,
later events during sperm capacitation. Because of the need of cAMP for later
events, it is possible to hypothesize that the negative feedback mechanism on
the cAMP levels exerted by PKA phosphorylation is released at some point
during the capacitation process. Our working hypothesis is that inward
translocation of Cl– (through NKCC and other
Cl– transporters) is coupled to the activity of a
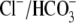 antiporter. Inward fluxes of
antiporter. Inward fluxes of 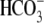 through this exchanger would stimulate sAC and raise the cAMP levels necessary
for the posterior increase in tyrosine phosphorylation. Also, NKCC-dependent
Cl– currents would be necessary to prepare sperm for
ZP-induced acrosome reaction. In addition, Cl–-dependent
unknown mechanisms occurring in sperm are essential for fertilization to
occur.
through this exchanger would stimulate sAC and raise the cAMP levels necessary
for the posterior increase in tyrosine phosphorylation. Also, NKCC-dependent
Cl– currents would be necessary to prepare sperm for
ZP-induced acrosome reaction. In addition, Cl–-dependent
unknown mechanisms occurring in sperm are essential for fertilization to
occur.
Acknowledgments
We thank Matias Okawa for helpful discussion and critical reading of the manuscript and Laura Brassard and Dr. Randolph Lisle for proofreading. We also acknowledge technical assistance from the Animal Care and Sequencing facilities at Instituto de the Biotecnologia-Universidad Nacional Autónoma de México.
This work was supported, in whole or in part, by National Institutes of Health Grants HD38082 and HD44044 (to P. E. V.) from the Eunice Kennedy Shriver NICHD. This work was also supported by Fogarty International Research Collaboration Award Grant RO3 TW006121 (to P. E. V. and A. D.) and by funds from the Consejo Nacional de Ciencia y Tecnología (CONACyT 49113 to A. D.), the Dirección General de Asuntos del Personal Académico-Universidad Nacional Autónoma de México (DGAPA IN225406 to A. D. and IN227806-3 to C. T.), and the Wellcome Trust. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ENaC, epithelial Na+ channel; NKCC, Na+/K+/Cl– cotransporter; CFTR, cystic fibrosis transmembrane regulator; ZP, zona pellucida; Sp-cAMPS, Sp-diastereomer of adenosine 3′,5′-cyclic monophosphothiorate; IBMX, 3-isobutyl-1-methylxanthine; DPC, diphenylamine-2-carboxylate; inh-172, 5-[(4-carboxyphenyl)methylene]-2-thioxo-3-[(3-trifluoromethyl)phenyl-4-thiazolidinone; DIDS, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid; SITS, disodium 4-acetamido-4′-isothiocyanato-stilben-2,2′-disulfonate; DMSO, dimethyl sulfoxide; BSA, bovine serum albumin; PBS, phosphate-buffered saline; IVF, in vitro fertilization; PY, phosphotyrosine; CASA, computer-assisted semen analysis; ClC, Cl–-selective ion channel; KCC, K+/Cl– cotransporter; sAC, soluble adenylyl cyclase; PKA, protein kinase A.
References
- 1.Yanagimachi, R. (1994) in The Physiology of Reproduction (Knobil, E., and Neill, J. D., eds) pp. 189–317, Raven Press, Ltd., New York
- 2.Visconti, P. E., Westbrook, V. A., Chertihin, O., Demarco, I., Sleight, S., and Diekman, A. B. (2002) J. Reprod. Immunol. 53 133–150 [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Gonzalez, E. O., Sosnik, J., Edwards, J., Acevedo, J. J., Mendoza-Lujambio, I., Lopez-Gonzalez, I., Demarco, I., Wertheimer, E., Darszon, A., and Visconti, P. E. (2006) J. Biol. Chem. 281 5623–5633 [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Gonzalez, E. O., Trevino, C. L., Castellano, L. E., de la Vega-Beltran, J. L., Ocampo, A. Y., Wertheimer, E., Visconti, P. E., and Darszon, A. (2007) J. Biol. Chem. 282 24397–24406 [DOI] [PubMed] [Google Scholar]
- 5.Xu, W. M., Shi, Q. X., Chen, W. Y., Zhou, C. X., Ni, Y., Rowlands, D. K., Yi Liu, G., Zhu, H., Ma, Z. G., Wang, X. F., Chen, Z. H., Zhou, S. C., Dong, H. S., Zhang, X. H., Chung, Y. W., Yuan, Y. Y., Yang, W. X., and Chan, H. C. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 9816–9821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steward, M. C., Ishiguro, H., and Case, R. M. (2005) Annu Rev. Physiol. 67 377–409 [DOI] [PubMed] [Google Scholar]
- 7.Akabas, M. H. (2001) in Encyclopedia of Life Sciences, pp. 1–7 Macmillan Reference Ltd., London
- 8.Lytle, C., Xu, J. C., Biemesderfer, D., and Forbush, B., III (1995) Am. J. Physiol. 269 C1496–C1505 [DOI] [PubMed] [Google Scholar]
- 9.Chu, D. T., and Klymkowsky, M. W. (1989) Dev. Biol. 136 104–117 [DOI] [PubMed] [Google Scholar]
- 10.Moore, G. D., Ayabe, T., Visconti, P. E., Schultz, R. M., and Kopf, G. S. (1994) Development (Camb.) 120 3313–3323 [DOI] [PubMed] [Google Scholar]
- 11.Tutuncu, L., Stein, P., Ord, T. S., Jorgez, C. J., and Williams, C. J. (2004) Dev. Biol. 270 246–260 [DOI] [PubMed] [Google Scholar]
- 12.Visconti, P. E., Olds-Clarke, P., Moss, S. B., Kalab, P., Travis, A. J., de las Heras, M., and Kopf, G. S. (1996) Mol. Reprod. Dev. 43 82–93 [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. (1970) Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- 14.Kalab, P., Visconti, P., Leclerc, P., and Kopf, G. S. (1994) J. Biol. Chem. 269 3810–3817 [PubMed] [Google Scholar]
- 15.Bray, C., Son, J. H., Kumar, P., and Meizel, S. (2005) Biol. Reprod. 73 807–814 [DOI] [PubMed] [Google Scholar]
- 16.Ward, C. R., Storey, B. T., and Kopf, G. S. (1992) J. Biol. Chem. 267 14061–14067 [PubMed] [Google Scholar]
- 17.López-González, I., De La Vega-Beltran, J. L., Santi, C. M., Florman, H. M., Felix, R., and Darszon, A. (2001) Dev. Biol. 236 210–219 [DOI] [PubMed] [Google Scholar]
- 18.Visconti, P. E., Bailey, J. L., Moore, G. D., Pan, D., Olds-Clarke, P., and Kopf, G. S. (1995) Development (Camb.) 121 1129–1137 [DOI] [PubMed] [Google Scholar]
- 19.Visconti, P. E., Galantino-Homer, H., Ning, X., Moore, G. D., Valenzuela, J. P., Jorgez, C. J., Alvarez, J. G., and Kopf, G. S. (1999) J. Biol. Chem. 274 3235–3242 [DOI] [PubMed] [Google Scholar]
- 20.McAvey, B. A., Wortzman, G. B., Williams, C. J., and Evans, J. P. (2002) Biol. Reprod. 67 1342–1352 [DOI] [PubMed] [Google Scholar]
- 21.Sokal, R. R., and Rolf, J. F. (1995) Biometry: The Principles and Practice of Statistics in Biological Research, 3rd Ed., W. H. Freeman, New York
- 22.Yoshimatsu, N., and Yanagimachi, R. (1988) Dev. Growth Differ. 30 651–659 [DOI] [PubMed] [Google Scholar]
- 23.Melendrez, C. S., and Meizel, S. (1995) Biol. Reprod. 53 676–683 [DOI] [PubMed] [Google Scholar]
- 24.Meizel, S. (2004) Biol. Rev. Camb. Philos. Soc. 79 713–732 [DOI] [PubMed] [Google Scholar]
- 25.Demarco, I. A., Espinosa, F., Edwards, J., Sosnik, J., De La Vega-Beltran, J. L., Hockensmith, J. W., Kopf, G. S., Darszon, A., and Visconti, P. E. (2003) J. Biol. Chem. 278 7001–7009 [DOI] [PubMed] [Google Scholar]
- 26.Visconti, P. E., Moore, G. D., Bailey, J. L., Leclerc, P., Connors, S. A., Pan, D., Olds-Clarke, P., and Kopf, G. S. (1995) Development (Camb.) 121 1139–1150 [DOI] [PubMed] [Google Scholar]
- 27.Leclerc, P., de Lamirande, E., and Gagnon, C. (1998) J. Androl. 19 434–443 [PubMed] [Google Scholar]
- 28.Galantino-Homer, H. L., Visconti, P. E., and Kopf, G. S. (1997) Biol. Reprod. 56 707–719 [DOI] [PubMed] [Google Scholar]
- 29.Kalab, P., Peknicova, J., Geussova, G., and Moos, J. (1998) Mol. Reprod. Dev. 51 304–314 [DOI] [PubMed] [Google Scholar]
- 30.Branham, M. T., Mayorga, L. S., and Tomes, C. N. (2006) J. Biol. Chem. 281 8656–8666 [DOI] [PubMed] [Google Scholar]
- 31.Shi, Q. X., and Roldan, E. R. (1995) Biol. Reprod. 52 373–381 [DOI] [PubMed] [Google Scholar]
- 32.Mouhat, S., Jouirou, B., Mosbah, A., De Waard, M., and Sabatier, J. M. (2004) Biochem. J. 378 717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White, M. M., and Aylwin, M. (1990) Mol. Pharmacol. 37 720–724 [PubMed] [Google Scholar]
- 34.d'Anglemont de Tassigny, A., Souktani, R., Ghaleh, B., Henry, P., and Berdeaux, A. (2003) Fundam. Clin. Pharmacol. 17 539–553 [DOI] [PubMed] [Google Scholar]
- 35.Estevez, R., Schroeder, B. C., Accardi, A., Jentsch, T. J., and Pusch, M. (2003) Neuron 38 47–59 [DOI] [PubMed] [Google Scholar]
- 36.Weber-Schurholz, S., Wischmeyer, E., Laurien, M., Jockusch, H., Schurholz, T., Landry, D. W., and al-Awqati, Q. (1993) J. Biol. Chem. 268 547–551 [PubMed] [Google Scholar]
- 37.Simard, C. F., Daigle, N. D., Bergeron, M. J., Brunet, G. M., Caron, L., Noel, M., Montminy, V., and Isenring, P. (2004) J. Biol. Chem. 279 48449–48456 [DOI] [PubMed] [Google Scholar]
- 38.Haas, M., and Forbush, B., III (2000) Annu. Rev. Physiol. 62 515–534 [DOI] [PubMed] [Google Scholar]
- 39.Culliford, S., Ellory, C., Lang, H. J., Englert, H., Staines, H., and Wilkins, R. (2003) Cell Physiol. Biochem. 13 181–188 [DOI] [PubMed] [Google Scholar]
- 40.Russell, J. M. (2000) Physiol. Rev. 80 211–276 [DOI] [PubMed] [Google Scholar]
- 41.Pace, A. J., Lee, E., Athirakul, K., Coffman, T. M., O'Brien, D. A., and Koller, B. H. (2000) J. Clin. Investig. 105 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang, M. C. (1951) Nature 168 697–698 [DOI] [PubMed] [Google Scholar]
- 43.Austin, C. R. (1951) Aust. J. Sci. Res. (B) 4 581–596 [DOI] [PubMed] [Google Scholar]
- 44.Buck, J., Sinclair, M. L., Schapal, L., Cann, M. J., and Levin, L. R. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wuttke, M. S., Buck, J., and Levin, L. R. (2001) J. Pancreas 2 Suppl. 4, 154–158 [PubMed] [Google Scholar]
- 46.Okamura, N., Tajima, Y., Soejima, A., Masuda, H., and Sugita, Y. (1985) J. Biol. Chem. 260 9699–9705 [PubMed] [Google Scholar]
- 47.Carlson, A. E., Hille, B., and Babcock, D. F. (2007) Dev. Biol. 312 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visconti, P. E., Johnson, L. R., Oyaski, M., Fornes, M., Moss, S. B., Gerton, G. L., and Kopf, G. S. (1997) Dev. Biol. 192 351–363 [DOI] [PubMed] [Google Scholar]
- 49.Neill, J. M., and Olds-Clarke, P. (1987) Gamete Res. 18 121–140 [DOI] [PubMed] [Google Scholar]
- 50.Boatman, D. E., and Robbins, R. S. (1991) Biol. Reprod. 44 806–813 [DOI] [PubMed] [Google Scholar]
- 51.Gatto, C., Lutsenko, S., and Kaplan, J. H. (1997) Arch. Biochem. Biophys. 340 90–100 [DOI] [PubMed] [Google Scholar]
- 52.Proks, P., Jones, P., and Ashcroft, F. M. (2001) Br. J. Pharmacol. 132 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garg, P., Martin, C. F., Elms, S. C., Gordon, F. J., Wall, S. M., Garland, C. J., Sutliff, R. L., and O'Neill, W. C. (2007) Am. J. Physiol. 292 H2100–H2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasannejad, H., Takeda, M., Taki, K., Shin, H. J., Babu, E., Jutabha, P., Khamdang, S., Aleboyeh, M., Onozato, M. L., Tojo, A., Enomoto, A., Anzai, N., Narikawa, S., Huang, X. L., Niwa, T., and Endou, H. (2004) J. Pharmacol. Exp. Ther. 308 1021–1029 [DOI] [PubMed] [Google Scholar]
- 55.Hess, K. C., Jones, B. H., Marquez, B., Chen, Y., Ord, T. S., Kamenetsky, M., Miyamoto, C., Zippin, J. H., Kopf, G. S., Suarez, S. S., Levin, L. R., Williams, C. J., Buck, J., and Moss, S. B. (2005) Dev. Cell 9 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delpire, E., Rauchman, M. I., Beier, D. R., Hebert, S. C., and Gullans, S. R. (1994) J. Biol. Chem. 269 25677–25683 [PubMed] [Google Scholar]
- 57.Isenring, P., and Forbush, B. (2001) Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 130 487–497 [DOI] [PubMed] [Google Scholar]
- 58.Ianowski, J. P., Christensen, R. J., and O'Donnell, M. J. (2004) J. Exp. Biol. 207 3707–3716 [DOI] [PubMed] [Google Scholar]
- 59.Nolan, M. A., Babcock, D. F., Wennemuth, G., Brown, W., Burton, K. A., and McKnight, G. S. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 13483–13488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellve, A. R. (1993) Methods Enzymol. 225 84–113 [DOI] [PubMed] [Google Scholar]