Abstract
The ectodomain shedding of syndecan-1, a major cell surface heparan sulfate proteoglycan, modulates molecular and cellular processes central to the pathogenesis of inflammatory diseases. Syndecan-1 shedding is a highly regulated process in which outside-in signaling accelerates the proteolytic cleavage of syndecan-1 ectodomains at the cell surface. Several extracellular agonists that induce syndecan-1 shedding and metalloproteinases that cleave syndecan-1 ectodomains have been identified, but the intracellular mechanisms that regulate syndecan-1 shedding are largely unknown. Here we examined the role of the syndecan-1 cytoplasmic domain in the regulation of agonist-induced syndecan-1 shedding. Our results showed that the syndecan-1 cytoplasmic domain is essential because mutation of invariant cytoplasmic Tyr residues abrogates ectodomain shedding, but not because it is Tyr phosphorylated upon shedding stimulation. Instead, our data showed that the syndecan-1 cytoplasmic domain binds to Rab5, a small GTPase that regulates intracellular trafficking and signaling events, and this interaction controls the onset of syndecan-1 shedding. Syndecan-1 cytoplasmic domain bound specifically to Rab5 and preferentially to inactive GDP-Rab5 over active GTP-Rab5, and shedding stimulation induced the dissociation of Rab5 from the syndecan-1 cytoplasmic domain. Moreover, the expression of dominant-negative Rab5, unable to exchange GDP for GTP, interfered with the agonist-induced dissociation of Rab5 from the syndecan-1 cytoplasmic domain and significantly inhibited syndecan-1 shedding induced by several distinct agonists. Based on these data, we propose that Rab5 is a critical regulator of syndecan-1 shedding that serves as an on-off molecular switch through its alternation between the GDP-bound and GTP-bound forms.
Syndecans comprise a major family of cell surface heparan sulfate proteoglycans (1–3). There are four syndecans in mammals and all adherent cells express at least one syndecan on their cell surface. Syndecans have been shown or proposed to bind to and regulate various bioactive molecules, such as growth factors, cytokines, chemokines, enzymes, and cell adhesion molecules, in a heparan sulfate (HS)2-dependent manner (1, 4, 5). Although all syndecans contain the ligand-binding HS chains, they show distinct temporal and spatial expression patterns and, thus, are likely to function specifically in vivo (1, 6, 7). For instance, in adult tissues, syndecan-1 is predominantly expressed by epithelial and plasma cells, and to a lesser degree by other cell types (e.g. endothelial cells, fibroblasts). The overall structural design of syndecans is similar: starting at the NH2 terminus, the three major domains are the extracellular ectodomain where HS chains attach distally to the plasma membrane, followed by the highly conserved transmembrane and short cytoplasmic domains.
Syndecans function as a coreceptor on the cell surface and also as a soluble heparan sulfate proteoglycan in the extracellular environment because its ectodomain, replete with all its HS chains, can be shed by metalloproteinases (1, 2). Current evidence suggests that the ectodomain shedding of syndecan-1 is an innate host response to tissue injury and inflammation. Syndecan-1 shedding is stimulated in vitro by several inflammatory factors and in vivo under certain pathological conditions. Agonists of syndecan-1 shedding include epidermal growth factor family growth factors, chemokines, stress-related agonists, heparanase, and bacterial virulence factors (8–17). In humans, elevated levels of syndecan-1 ectodomains are found in skin wound fluids, serum of patients with acute graft-versus-host disease, and in plasma of myeloma patients, among other fluids from injured or inflamed tissues (18–22). In mouse models of inflammatory diseases, elevated levels of syndecan-1 ectodomains are found in lung and skin homogenates of mice infected with Pseudomonas aeruginosa (23, 24), in incisional skin wound fluid (20, 25), and in bronchoalveolar lavage fluids of mice challenged with bleomycin (26) or allergens (27).
Results from animal studies suggest that syndecan-1 shedding modulates the extent and outcome of inflammatory processes. For example, in the mouse model of acute P. aeruginosa pneumonia, syndecan-1 shedding enhanced by LasA, a virulence factor for P. aeruginosa lung infection, promotes bacterial colonization and infectious pneumonia by dysregulating host defense mechanisms in an HS-dependent manner (11, 24). In contrast, in animal models of non-infectious tissue injury and inflammation, syndecan-1 shedding apparently protects the host from inflammatory tissue damage by regulating inflammatory factors and assuring the correct functioning of inflammation. In the mouse model of allergic lung inflammation, syndecan-1 ectodomain attenuates inflammation by inhibiting CC chemokine (CCL7, 11, 17)-induced homing of Th2 cells to the lung, a central process in the development of allergic diseases (27), whereas syndecan-1 shedding coordinates the generation of a KC (CXCL1) chemokine gradient that guides the transepithelial migration of neutrophils into the airspace in bleomycin-induced acute lung injury (26). These data highlight the diverse and critical functions of syndecan-1 shedding in modulating inflammatory disorders in vivo.
Despite its importance in modulating a variety of key inflammatory processes, the underlying mechanisms of how syndecan-1 shedding is regulated are incompletely understood. Much is known about the identity of extracellular agonists of syndecan-1 shedding, and several syndecan-1 sheddases, such as matrix metalloproteinase-7 (MMP-7, matrilysin) (26, 28), MMP-9 (gelatinase B) (15), and MMP-14 (MT1-MMP) (29) have been identified, but there is a gap in our understanding of intracellular mechanisms that connect the activities of the syndecan-1 shedding agonists to the metalloproteinase-mediated cleavage of syndecan-1 ectodomains at the cell surface. Chemical inhibitor studies have implicated ERK (extracellular signal-regulated kinase) and JNK (c-Jun NH2-terminal kinase) MAP kinases (9) and protein-tyrosine kinases (PTKs) in the regulation of syndecan-1 shedding (9–11, 16), but precisely how these signaling factors regulate syndecan-1 shedding is not known.
The cytoplasmic domain of syndecans is highly conserved and it has been shown to interact with several signaling and scaffolding proteins, including c-Src (30), cortactin (30), protein kinase A (31), protein kinase Cα (32), syntenin (33), and CASK/LIN2A (34, 35). The syndecan cytoplasmic domain is composed of two invariant regions (C1 and C2), separated by a central variable region (V) that is distinct for each family member (1). Furthermore, the syndecan cytoplasmic domain contains several signaling motifs, including one invariant Ser, four invariant Tyr, and a Glu-Phe-Tyr-Ala PDZ binding domain at the COOH terminus. Collectively, these observations suggest that the cytoplasmic domain plays a vital role in the regulation of syndecan functions in vivo, including syndecan-1 shedding.
In this study, we sought to define the regulatory role of syndecan-1 cytoplasmic domain in agonist-induced syndecan-1 ectodomain shedding. Our results indicate that the cytoplasmic domain is essential, but not because it is Tyr phosphorylated upon shedding stimulation. Instead, our results show that the syndecan-1 cytoplasmic domain interacts specifically with the small GTPase Rab5, and the oscillation between the GDP- and GTP-bound forms of Rab5 serves as an intracellular on-off molecular switch of syndecan-1 shedding.
EXPERIMENTAL PROCEDURES
Materials—Phorbol 12-myristate 13-acetate (PMA) and C2 ceramide were obtained from Calbiochem (La Jolla, CA). Protein G-agarose beads, enhanced chemiluminescence (ECL) reagents, and Ultralink affinity chromatography resins were purchased from Pierce Chemical. Immobilon Ny+ (cationic nylon) and Immobilon P (polyvinylidene difluoride) membranes were from Millipore (Bedford, MA), and nitrocellulose membrane was from Schleicher & Schuell (Keene, NH). Culture medium, fetal calf serum, and other tissue culture supplements were obtained from Mediatech (Herndon, VA). Reduced glutathione, thrombin cleavage kit, bovine serum albumin (BSA), TPCK-treated trypsin, and soybean trypsin inhibitor were from Sigma. Glutathione-Sepharose 4B and pGEX4T-1 were obtained from GE Healthcare. Purified Staphylococcus aureus β toxin was from Toxin Technology (Sarasota, FL). ViraPower Adenovirus Expression System, AccuPrime Pfx DNA polymerase, pENTER/SD/D-TOPO, pAd/CMV/V5-DEST, LR clonase, and Lipofectamine 2000 were from Invitrogen. PacI was from New England BioLabs (Ipswich, MA). All other materials and reagents were obtained from either Fisher (Pittsburgh, PA) or VWR (Arlington Heights, IL).
Cells and Immunochemicals—Normal murine mammary gland (NMuMG) epithelial, human lung adenocarcinoma (A549), and human epidermoid carcinoma (A431) cells were from our collection and cultured as described previously (11). NMuMG cells were used between passages 18 and 23 for all assays. 293A cells were purchased from Invitrogen. Rat monoclonal anti-mouse syndecan-1 ectodomain antibody (clone 281-2) and mouse monoclonal anti-Rab5 (clone 15), anti-Rab8 (clone 4), anti-β1 integrin (clone 18), anti-β-catenin (clone 14), anti-phosphoinositide-3 kinase (PI3K) (clone 4), anti-early endosome antigen 1 (clone 14), and anti-Stat3 (clone 84) antibodies were from BD Biosciences. Rabbit monoclonal anti-Src antibody (clone 36D10) and rabbit polyclonal anti-Rab5, anti-Akt, and anti-p44/42 MAP kinase antibodies were from Cell Signaling (Danvers, MA). Mouse monoclonal anti-fibroblast growth factor receptor-1 antibody (VBS1) was from Millipore and the mouse monoclonal anti-human syndecan-1 ectodomain antibody (B-B4) was from Serotec (Raleigh, NC). The anti-phosphotyrosine antibodies PY20 and Tyr(P)-100 were from BD Biosciences and Cell Signaling, respectively. Rat monoclonal anti-mouse syndecan-4 ectodomain antibody (Ky8.2) was purified from the conditioned medium of hybridoma cultures by protein G affinity chromatography (11). Affinity purified rabbit polyclonal anti-mouse syndecan-1 cytoplasmic domain antibody directed against the COOH-terminal 16 amino acids was prepared as described previously (31). Horseradish peroxidase-conjugated donkey anti-rat IgG, goat anti-rabbit IgG, and goat anti-mouse IgG secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Rabbit anti-GFP and Alexa 594-conjugated goat anti-rat IgG antibodies were from Invitrogen.
Generation of Recombinant Syndecan-1 Cytoplasmic Domain and Recombinant Human Rab5—PCR-amplified cDNA encoding the entire cytoplasmic domain (Tyr277–Ala311) of mouse syndecan-1 and whole CDS encoding human Rab5a were subcloned in-frame into the GST fusion protein expression vector pGEX 4T-1. The GST-syndecan-1 cytoplasmic domain and GST-Rab5 fusion proteins were expressed and purified according to the manufacturer's instructions. Briefly, Escherichia coli BL21 was transformed with the recombinant constructs, grown to logarithmic growth phase, and induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 4 h at 37 °C. Cells were lysed by sonication in phosphate-buffered saline with 0.5% (v/v) Triton X-100. Cell lysates were cleared by centrifugation and fractionated by glutathione-Sepharose 4B chromatography, and bound proteins were eluted with 15 mm reduced glutathione. In some experiments, the GST moiety was removed from the recombinant proteins by incubating overnight at 4 °C with 50 μl of thrombin-agarose slurry in cleavage buffer (50 mm Tris, pH 8, 10 mm CaCl2) and removing the cleaved GST by glutathione-Sepharose 4B chromatography. To generate recombinant FLAG-tagged syndecan-1 cytoplasmic domain, the entire mouse syndecan-1 cytoplasmic domain was amplified by PCR using a 5′ primer containing the sequence encoding FLAG tag (DYKDDDDK). The resulting PCR fragment was inserted in-frame into the BamHI site of pET11a. FLAG-tagged syndecan-1 cytoplasmic domain was expressed as described above for GST-tagged syndecan-1 cytoplasmic domain and purified by anti-FLAG M2 affinity chromatography using 100 μg/ml FLAG peptide to elute the bound recombinant protein.
Adenoviral Expression of Wild Type and Dominant-negative GFP-Rab5—Adenoviruses harboring GFP-wild type (pAd WT Rab5) or S34N dominant-negative human Rab5 (pAd DN Rab5) were generated using the ViraPower Adenovirus Expression System according to the manufacturer's instructions. Briefly, full-length cDNAs of GFP-WT Rab5 and GFP-DN Rab5 were amplified by PCR with Accuprime using pEGFP human Rab5a (36) and pEGFP S34N human Rab5a (36) as templates, respectively. Amplified cDNA fragments were subcloned into the pENTER/SD/D-TOPO vector using the pENTER Directional TOPO Cloning kit. After verifying the sequence, the inserts were transferred into the pAd/CMV/V5-DEST vector by the Gateway system using LR clonase II. To obtain virus particles, plasmids were linearized by PacI digestion and transfected into 293A cells with Lipofectamine 2000. When most cells were detached (∼7–10 days), cells were harvested by gentle pipetting in culture medium, lysed by three cycles of freeze/thawing, and centrifuged to collect the supernatant containing crude viral lysates. To amplify virus titers, 100 μl of crude viral lysates were added to fresh 293A cells, and cultured for several days until all cells were detached. Virus-enriched supernatants were collected and viral titers were determined by the plaque-forming assay with 293A cells.
Co-sedimentation Assay—Recombinant syndecan-1 cytoplasmic domain free of GST (Sdc1-CPD) was coupled to Ultralink affinity resin according to the manufacturer's instructions. NMuMG total cell lysate was prepared by sonicating cells in lysis buffer A (50 mm HEPES, pH 7.4, 80 mm KCl, 4 mm MgCl2, 2 mm EGTA, 1 mm dithiothreitol, 0.5% Triton X-100) followed by centrifugation at 14,000 × g for 30 min. The cleared total cell lysate (5 mg) was incubated overnight with 10 μg of Sdc1-CPD coupled to Ultralink in the presence of 1 mg of recombinant GST-tagged syndecan-1 cytoplasmic domain or 1 mg of BSA at 4 °C and washed three times with lysis buffer A. Bound proteins were eluted by adding SDS sample buffer and analyzed by SDS-PAGE and immunoblotting.
In some experiments, GST-tagged human Rab5 coupled to Ultralink, preloaded with GTPγS or GDP, was used to precipitate syndecan-1 cytoplasmic domain from mildly trypsinized NMuMG total cell lysates. NMuMG cells were treated with 10 μg/ml TPCK-treated trypsin and sonicated in lysis buffer A to obtain cell lysates enriched in syndecan-1 cytoplasmic domain. GST-tagged human Rab5 was coupled to Ultralink and incubated in buffer (20 mm HEPES, pH 7.5, 100 mm NaCl, 1 mm dithiothreitol, 10 mm EDTA, 5 mm MgCl2) containing 1 mm GTPγS or 5 mm GDP at room temperature for 1 h under rotation. The beads were washed and incubated with stabilization buffer (20 mm HEPES, pH 7.5, 100 mm NaCl, 1 mm dithiothreitol, 2 mm MgCl2) containing 100 μm GTPγS or GDP for 10 min at room temperature. NMuMG cell lysate (1 mg) was incubated with either GTPγS or GDP preloaded Rab5-Ultralink beads for 4 h at 4 °C. Beads were washed and bound proteins were eluted with SDS sample buffer.
Co-immunoprecipitation—NMuMG cells treated with or without shedding agonists were lysed in lysis buffer B (50 mm HEPES, pH 7.4, 80 mm KCl, 4 mm MgCl2, 2 mm EGTA, 0.5% Triton X-100). The lysate was centrifuged at 14,000 × g for 30 min and the supernatant was incubated overnight with 3 μg of 281-2 anti-syndecan-1 or Ky8.2 anti-syndecan-4 antibody at 4 °C with gentle agitation. Protein G-agarose beads (20 μl) were added and incubated for 2 h at 4 °C. In some experiments, immunoprecipitation was performed with 281-2 antibodies directly coupled to Ultralink beads. Beads were pelleted by centrifugation, washed three times with lysis buffer B, and resuspended in non-reducing SDS-PAGE sample buffer. Immunoprecipitation was also performed in the presence of excess recombinant syndecan-1 cytoplasmic domain devoid of GST (150 μg/ml). Samples were resolved by SDS-PAGE, Western blotted onto nitrocellulose, and probed with rabbit anti-Rab5 or rabbit anti-GFP antibodies. For Western immunoblotting of syndecan-1, samples were resuspended in reducing SDS-PAGE sample buffer, resolved by SDS-PAGE, transferred to Immobilon Ny+ membranes, and developed with the 281-2 antibody.
Direct Binding Assay—Direct binding between syndecan-1 cytoplasmic domain and Rab5 was measured by incubating purified FLAG-tagged syndecan-1 cytoplasmic domain (100 ng/ml) with GDP or GTPγS pre-loaded Rab5 coupled to Ultralink in 20 mm HEPES, pH 7.5, 100 mm NaCl, 1 mm dithiothreitol, 2 mm MgCl2, and 1 mg/ml BSA for 4 h at 4 °C. Beads were washed and bound proteins were eluted with SDS sample buffer and analyzed by SDS-PAGE and immunoblotting.
Measurement of Cell Surface and Shed Syndecan-1—Syndecan-1 shedding was assessed as described previously (10) with slight modifications. Briefly, confluent cultures of NMuMG, A431, or A549 epithelial cells were transduced with adenovirus harboring LacZ, GFP-WT human Rab5, or GFP-DN human Rab5 at a multiplicity of infection of 10 for NMuMG and A431 cells and 2 for A549 cells. At day 3, cells were incubated with various test samples for the indicated times at 37 °C. To quantify shedding, the conditioned medium was collected, spun down to remove cells, and acidified by addition of NaOAc (pH 4.5), NaCl, and Tween 20 to a final concentration of 50 mm, 150 mm, and 0.1% (v/v), respectively. Various volumes of the acidified samples were dot blotted onto Immobilon Ny+ and developed with specific antibodies and ECL.
Quantification of cell surface syndecan-1 was performed by mild trypsinization and measurement of the released syndecan-1 ectodomains by dot immunoblotting as described previously (31). Briefly, confluent NMuMG cells transduced with or without adenovirus harboring LacZ, WT Rab5, or DN Rab5 in 96-well plates were washed once with ice-cold Tris-buffered saline containing 0.5 mm EDTA and incubated for 10 min at 4 °C with 10 μg/ml TPCK-treated trypsin in Tris-buffered saline with 0.5 mm EDTA. Trypsin was inactivated by the addition of an equal volume of 100 μg/ml soybean trypsin inhibitor. Trypsinates were spun down to remove detached cells, acidified, and dot immunoblotted with the 281-2 antibody.
GST Pull-down—1 mg of NMuMG total cell lysate in lysis buffer A was incubated with 10 μg of GST-tagged recombinant syndecan-1 cytoplasmic domain in the absence or presence of 0.1 mm GTPγS or 1 mm GDP at 4 °C for 6 h, followed by incubation with 10 μl of glutathione-Sepharose 4B beads for 2 h at 4 °C. Beads were washed 3 times with lysis buffer A and bound proteins were eluted by adding SDS sample buffer.
Immunostaining—Confluent NMuMG cells transduced with or without adenovirus harboring LacZ, WT Rab5, or DN Rab5 in 8-well chamber slides were fixed with 4% paraformaldehyde in PEM buffer (80 mm PIPES, pH 6.8, 5 mm EGTA, 2 mm MgCl2) for 30 min at room temperature. Cells were permeabilized with 0.5% Triton X-100 in PEM buffer for 30 min. Slides were blocked with 1% BSA and incubated with 281-2 antibody (5 mg/ml) for 4 h at room temperature and Alexa 594 goat anti-rat IgG antibody for 1 h at room temperature. Images were obtained by CCD camera-equipped fluorescence microscopy.
RESULTS
The Syndecan-1 Cytoplasmic Domain Is Essential in Agonist-induced Syndecan-1 Shedding—Most agonists of syndecan-1 shedding induce shedding in a PTK-dependent manner, but how PTKs modulate syndecan-1 shedding is not understood. The cytoplasmic domain of syndecan-1 contains four invariant Tyr residues, and several studies have suggested that Tyr phosphorylation of the syndecan-1 cytoplasmic domain positively regulates syndecan-1 shedding (37, 38). To test the regulatory role of Tyr residues in agonist-induced syndecan-1 ectodomain shedding, we generated adenovirus vectors harboring full-length WT mouse syndecan-1 or mutant mouse syndecan-1 with all four Tyr residues in the cytoplasmic domain substituted with Phe, and expressed them in human A431 cells. Mouse syndecan-1 is expressed on the cell surface and shed in a regulated manner in human cells and vice versa, and the 281-2 anti-mouse syndecan-1 ectodomain antibody does not cross-react with human syndecan-1 (9, 31).
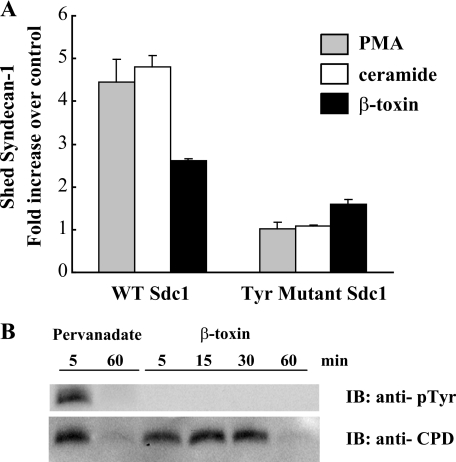
A431 cells expressing similar levels of WT or mutant syndecan-1 were stimulated with PMA, ceramide, or S. aureus β-toxin, and shedding was quantified by measuring the concentration of syndecan-1 ectodomains in the conditioned medium. PMA, ceramide, and β-toxin increased the shedding of WT syndecan-1 by ∼4-, 5-, and 3-fold, respectively (Fig. 1A). In contrast, shedding of mutant syndecan-1 harboring a Phe substitution in all of its four invariant Tyr residues was markedly reduced in response to the three shedding agonists (Fig. 1A). These data suggest that Tyr residues in the cytoplasmic domain are essential in agonist-induced syndecan-1 shedding.
FIGURE 1.
The syndecan-1 cytoplasmic domain is essential in agonist-induced syndecan-1 shedding. A, confluent human A431 cells in 96-well plates were transduced with adenovirus harboring wild type mouse syndecan-1 (WT Sdc1) or mutant mouse syndecan-1 with all four of the invariant Tyr residues in the cytoplasmic domain substituted with Phe (Tyr mutant Sdc1). Cells were stimulated with fresh culture medium (control), PMA (1 μm), ceramide (100 μm), or S. aureus β-toxin (5 μg/ml) for 2 h at 37 °C. Syndecan-1 shedding was assessed by quantifying the concentration of mouse syndecan-1 ectodomains in the conditioned medium by dot immunoblotting. Results are shown as mean -fold increase over control ± S.E. of triplicate measurements. B, confluent NMuMG cells were stimulated with pervanadate (0.1 mm) or β-toxin (10 μg/ml) for the indicated times. Total cell lysates were immunoprecipitated with anti-syndecan-1 cytoplasmic domain antibodies (anti-CPD) and protein A-agarose, and immunoblotted (IB) with anti-phosphotyrosine (anti-pTyr) or anti-CPD antibodies.
However, we unexpectedly found that the syndecan-1 cytoplasmic domain is not Tyr phosphorylated upon stimulation with shedding agonists (Fig. 1B). We examined if stimulation of NMuMG, A549, and A431 cells with β-toxin will result in Tyr phosphorylation of the syndecan-1 cytoplasmic domain by immunoblotting total cell lysates or immunoprecipitates from anti-syndecan-1 cytoplasmic domain or 281-2 anti-syndecan-1 ectodomain antibody immunoprecipitation with two different anti-phosphotyrosine antibodies (PY20 and Tyr(P)-100). We found that the syndecan-1 cytoplasmic domain is rapidly Tyr phosphorylated upon pervanadate treatment, but not with β-toxin between 0 and 60 min post-treatment (Fig. 1B). The syndecan-1 cytoplasmic domain was also not Tyr phosphorylated upon stimulation with PMA between 0 and 120 min post-treatment (not shown). These data are similar to the shedding of Alzheimer amyloid precursor protein and L1 adhesion molecule where their ectodomain shedding is inhibited by PTK inhibitors, but their cytoplasmic domains are not Tyr phosphorylated upon shedding stimulation (39, 40). Collectively, these findings indicate that the syndecan-1 cytoplasmic domain is critical for agonist-induced shedding, but for reasons other than phosphorylation of its Tyr residues.
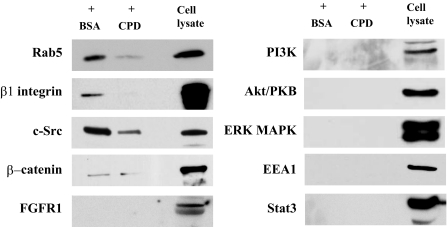
Syndecan-1 Cytoplasmic Domain Binds Specifically to Rab5—Based on the above data, we examined if the cytoplasmic domain is important because it binds to factors that are critical in the regulation of syndecan-1 shedding. To address this, total cell lysates from NMuMG cells were incubated with the recombinant syndecan-1 cytoplasmic domain coupled to Ultralink resin in the presence of excess BSA or recombinant syndecan-1 cytoplasmic domain peptides, and bound proteins were immunoblotted for various cell surface and intracellular signaling molecules. We found that the syndecan-1 cytoplasmic domain associates strongly with Rab5, β1 integrin, and c-Src, and weakly with β-catenin, but not with FGFR1, PI3K, Akt/PKB, p42/44 MAP kinase, early endosome antigen 1, and Stat3 (Fig. 2). More importantly, the association of the syndecan-1 cytoplasmic domain with Rab5, β1 integrin, c-Src, and β-catenin was determined to be specific because co-immunoprecipitation of these molecules was significantly inhibited in the presence of excess cytoplasmic domain peptides, but not in the presence of excess BSA (Fig. 2). These data suggest that Rab5, β1 integrin, c-Src, and β-catenin are potential regulators of syndecan-1 shedding.
FIGURE 2.
Specific association of the syndecan-1 cytoplasmic domain with cell surface and intracellular molecules. NMuMG total cell lysate was incubated overnight at 4 °C with syndecan-1 cytoplasmic domain peptide coupled to Ultralink resins in the presence of either 1 mg/ml BSA (+BSA) or recombinant syndecan-1 cytoplasmic domain peptide (+CPD). Bound proteins were eluted with SDS sample buffer, resolved by SDS-PAGE, and Western immunoblotted for the indicated molecules using specific antibodies. Untreated total cell lysate served as positive controls for the antibodies used.
Rab5 Specifically Associates with the Syndecan-1 Cytoplasmic Domain under Biological Conditions—Although our results showed that the syndecan-1 cytoplasmic domain associates specifically with Rab5, β1 integrin, c-Src, and β-catenin, it is not known if the cytoplasmic domain interacts directly with these molecules. Src has been shown to bind to the C1 region in the cytoplasmic domain of syndecan-3 (30) and this region is identical among the four mammalian syndecans, suggesting that Src binds to the C1 region in the syndecan-1 cytoplasmic domain. Syntenin, an intracellular scaffolding protein with two PDZ domains, associates with both syndecan-1 and β-catenin (41), implying that β-catenin binds to the syndecan-1 cytoplasmic domain using syntenin as a bridge. Binding of β1 integrin to syndecan-1 has not been reported, but several studies have shown that syndecan-1 and β1 integrin coordinately modulate several cellular activities, such as epithelial cell morphology and adhesion (42–44). Because the interaction with Rab5 was wholly unexpected, we decided to initially examine the role of the Rab5-syndecan-1 cytoplasmic domain interaction in regulating syndecan-1 shedding.
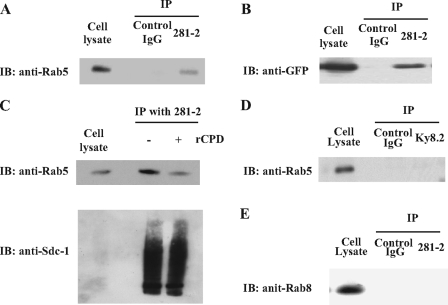
Rab5 is one of the Rab small GTPases that regulates a variety of intracellular trafficking and signaling events, such as receptor endocytosis (45, 46), membrane traffic into and between early endosomes (47–49), and vesicle transport along microtubules (48). Rab5 achieves these functions through alternating between inactive guanosine 5′-diphosphate (GDP)-bound and activated guanosine 5′-triphosphate (GTP)-bound forms. To confirm that Rab5 indeed associates with the syndecan-1 cytoplasmic domain under biological conditions, we assessed for binding using several co-immunoprecipitation approaches. We first immunoprecipitated syndecan-1 from NMuMG total cell lysates with the 281-2 antibody and found that Rab5 co-immunoprecipitates with syndecan-1 (Fig. 3A). We then ectopically expressed GFP-WT human Rab5 in NMuMG cells, immunoprecipitated syndecan-1 from cell lysates with 281-2 antibodies, and found that WT human Rab5 co-immunoprecipitates with mouse syndecan-1 by anti-GFP immunoblotting (Fig. 3B). We also found that co-immunoprecipitation of endogenous Rab5 from NMuMG cell lysates by 281-2 antibodies is blocked in the presence of excess syndecan-1 cytoplasmic domain peptide (Fig. 3C), indicating that the Rab5-syndecan-1 cytoplasmic domain interaction is specific. The specificity of the interaction was further demonstrated when we found by co-immunoprecipitation that Rab5 does not bind to syndecan-4 (Fig. 3D) and Rab8 does not bind to syndecan-1 (Fig. 3E).
FIGURE 3.
Rab5 interacts specifically with the syndecan-1 cytoplasmic domain under biological conditions. Binding of Rab5 to the syndecan-1 cytoplasmic domain was tested under biological conditions using several immunoprecipitation (IP) approaches. A, NMuMG total cell lysate was immunoprecipitated (IPed) with 281-2 anti-syndecan-1 antibodies or control rat IgG, and immunoblotted (IB) for Rab5 with mouse anti-Rab5 antibodies. B, NMuMG cells were transduced with adenovirus harboring GFP-human Rab5 and total cell lysate was immunoprecipitated with 281-2 antibodies and immunoblotted with rabbit anti-GFP antibodies. C, NMuMG total cell lysate was immunoprecipitated with 281-2 antibodies in the absence or presence of 0.1 mg/ml recombinant syndecan-1 cytoplasmic domain peptide (rCPD), and immunoblotted with anti-Rab5 antibodies. The lower panel shows that similar amounts of syndecan-1 were immunoprecipitated from samples incubated with or without excess cytoplasmic domain peptide. D, NMuMG total cell lysate was immunoprecipitated with Ky8.2 anti-syndecan-4 ectodomain antibodies or control rat IgG and immunoblotted with anti-Rab5 antibodies. E, NMuMG total cell lysate was immunoprecipitated with 281-2 antibodies or control rat IgG and immunoblotted with anti-Rab8 antibodies.
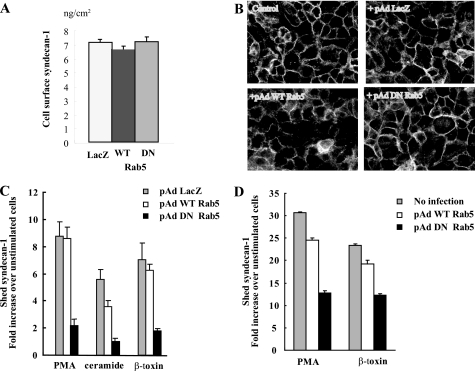
Inhibition of Rab5 GDP-GTP Exchange Inhibits Agonist-induced Syndecan-1 Shedding—We next examined the functional significance of Rab5 in syndecan-1 shedding by testing the effects of expressing a dominant-negative (DN) construct of Rab5 on agonist-induced shedding. NMuMG cells were transduced with adenovirus harboring GFP-LacZ, GFP-WT Rab5, or GFP-DN Rab5 (S34N, GDP-locked) (36, 49). At a multiplicity of infection of 10, the efficiency of transduction for all three constructs was ∼90% by immunoblotting and GFP fluorescence (not shown). Furthermore, adenoviral transduction of LacZ, WT Rab5, or DN Rab5 apparently did not affect the cell surface expression of syndecan-1, as determined by dot immunoblotting of mild trypsin digests (Fig. 4A) and immunostaining of transduced NMuMG cells (Fig. 4B). These results indicate that Rab5 bound to the syndecan-1 cytoplasmic domain does not induce endocytosis of cell surface syndecan-1. However, expression of DN Rab5, but not LacZ or WT Rab5, significantly reduced syndecan-1 shedding induced by PMA, ceramide, or β-toxin by more than 4-fold (Fig. 4C). Expression of DN Rab5 had a similar inhibitory effect on syndecan-1 shedding induced by PMA or β-toxin in A549 cells (Fig. 4D). These data indicate that the activation of Rab5 from GDP-Rab5 to GTP-Rab5 is essential in inducing syndecan-1 shedding.
FIGURE 4.
Dominant-negative Rab5 inhibits agonist-induced syndecan-1 shedding. NMuMG cells were transduced with or without adenovirus harboring LacZ, GFP-WT Rab5, or GFP-DN Rab5 and cell surface syndecan-1 expression was assessed by: A, mild trypsin digestion and dot immunoblotting of trypsinates (mean ± S.E., n = 4) and B, immunostaining with 281-2 anti-syndecan-1 antibodies and Alexa 594 donkey anti-rat IgG antibodies. C, NMuMG cells were infected with the indicated adenovirus vectors and stimulated with or without PMA (1 μm), ceramide (0.1 mm), or S. aureus β-toxin (5 μg/ml) for 4 h at 37 °C and levels of syndecan-1 ectodomains in the conditioned medium were quantified by dot immunoblotting (mean ± S.E., n = 4). D, A549 cells were transduced with the indicated adenovirus vectors and stimulated with PMA or S. aureus β-toxin. The concentration of syndecan-1 ectodomains in the conditioned medium was measured by dot immunoblotting using the B-B4 anti-human syndecan-1 ectodomain antibody (mean ± S.E., n = 4).
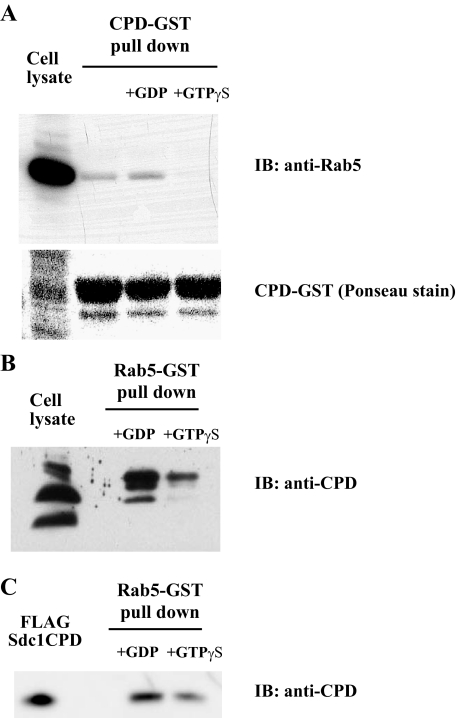
Syndecan-1 Cytoplasmic Domain Interacts Directly and Preferentially with GDP-Rab5 over GTP-Rab5—Rab5 can function as a molecular switch depending on the form of the bound guanosine nucleotide (50). The inhibitory effects of DN Rab5 on agonist-induced syndecan-1 shedding, absence of syndecan-1 internalization upon overexpression of WT Rab5, and the absence of syndecan-1 cytoplasmic domain binding to early endosome antigen 1 (marker of early endosomes), suggested that the syndecan-1 cytoplasmic domain interacts with GDP-Rab5, the inactive membrane form of Rab5. To test this, we examined the binding of cell lysates enriched in either GDP-Rab5 or GTP-Rab5 to the syndecan-1 cytoplasmic domain. NMuMG cell lysates pre-loaded with either 1 mm GDP or 0.1 mm GTPγS (a non-hydrolyzable analog of GTP) were incubated with GST-syndecan-1 cytoplasmic domain, precipitated with glutathione-Sepharose beads, and the precipitated material was immunoblotted for Rab5. Rab5 co-precipitated with the GST-syndecan-1 cytoplasmic domain from GDP preloaded, but not GTPγS pre-loaded, NMuMG cell lysates (Fig. 5A). Rab5 also co-precipitated with the GST-syndecan-1 cytoplasmic domain from untreated NMuMG cell lysates (Fig. 5A), consistent with the fact that the majority of Rab5 is in the GDP-bound form in unstimulated cells (51).
FIGURE 5.
Syndecan-1 cytoplasmic domain binds directly and preferentially to GDP-bound Rab5 over GTP-bound Rab5. A, NMuMG total cell lysate was pre-loaded with either 1 mm GDP or 0.1 mm GTPγS, incubated with GST-tagged syndecan-1 cytoplasmic domain, and subjected to GST pull-down. Pulled down samples were resolved by SDS-PAGE and immunoblotted (IB) with anti-Rab5 (upper panel) or stained with Ponceau S (lower panel). B, NMuMG cells were subjected to mild trypsin digestion and total cell lysates were incubated with Rab5-Ultralink beads preloaded with either GDP or GTPγS. Precipitated samples were resolved by SDS-PAGE and immunoblotted with affinity-purified anti-syndecan-1 cytoplasmic domain antibodies (anti-CPD). C, purified FLAG-tagged syndecan-1 cytoplasmic domain (FLAG-Sdc1CPD) was incubated with Rab5-Ultralink beads pre-loaded with GDP or GTPγS, and bound samples were analyzed by SDS-PAGE and immunoblotting.
We also performed the reverse experiment where GST-Rab5 coupled to Ultralink beads were pre-loaded with GDP or GTPγS, incubated with cell lysates prepared from mild trypsin-treated NMuMG cells, and bound proteins were immunoblotted for syndecan-1 cytoplasmic domain. Mild trypsin treatment removes the syndecan-1 ectodomain and enriches cell lysates for the cell-associated syndecan-1 fragment containing the transmembrane and cytoplasmic domains. GDP pre-loaded GST-Rab5 pulled down substantially more syndecan-1 cytoplasmic domain fragments compared with GTPγS pre-loaded GST-Rab5 (Fig. 5B). Furthermore, we also found that the purified FLAG-tagged syndecan-1 cytoplasmic domain binds more avidly to Rab5-Ultralink beads pre-loaded with GDP over those that were pre-loaded with GTPγS (Fig. 5C). These data demonstrate that the syndecan-1 cytoplasmic domain binds directly and preferentially to GDP-Rab5 over GTP-Rab5. Moreover, these results provide additional evidence that the on-off molecular switch of Rab5 is vital in regulating syndecan-1 shedding.
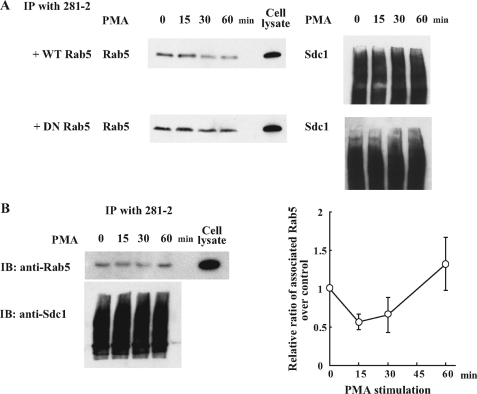
Rab5 Dissociates from Syndecan-1 Cytoplasmic Domain upon Shedding Stimulation—The observed preferential binding of GDP-Rab5 to the syndecan-1 cytoplasmic domain and inhibition of agonist-induced syndecan-1 shedding by expression of DN Rab5 suggested that syndecan-1 shedding is tightly controlled by the exchange of GDP to GTP on Rab5 and the subsequent dissociation of GTP-Rab5 from the syndecan-1 cytoplasmic domain. To test this, we first examined the effects of expressing DN-Rab5 on the dissociation of endogenous Rab5 upon shedding stimulation. Expression of DN Rab5 sequestrates endogenous Rab5 guanosine nucleotide exchange factors and inhibits the conversion of endogenous GDP-Rab5 to GTP-Rab5. NMuMG cells were transduced with adenovirus harboring GFP-WT Rab5 or GFP-DN Rab5 and stimulated with PMA. At various times post-PMA, cell lysates were immunoprecipitated with 281-2 antibodies and immunoblotted for endogenous Rab5. A noticeable proportion of endogenous Rab5 started to dissociate from the syndecan-1 cytoplasmic domain as early as 15 min post-PMA, and ∼80% were dissociated at 30 min post-PMA in cells expressing GFP-WT Rab5 (Fig. 6A). In contrast, expression of GFP-DN Rab5 inhibited the PMA-induced dissociation of endogenous Rab5 from the syndecan-1 cytoplasmic domain (Fig. 6A). Similar amounts of syndecan-1 were immunoprecipitated from cell lysates of cells transduced with WT Rab5 and DN Rab5 (Fig. 6A).
FIGURE 6.
Stimulation of syndecan-1 shedding induces the dissociation of Rab5 from syndecan-1 cytoplasmic domain. A, NMuMG cells transduced with adenovirus harboring either GFP-WT Rab5 or GFP-DN Rab5 were stimulated with PMA (1 μm) for the indicated time periods. Total cell lysate was immunoprecipitated (IP) with 281-2 antibody coupled to Ultralink resins and immunoblotted (IB) with anti-Rab5 or 281-2 antibodies. The band shown is endogenous Rab5 (∼25 kDa), which is readily distinguished from transduced GFP-Rab5 (∼55 kDa). The smear shown is immunoprecipitated syndecan-1. B, NMuMG cells were stimulated with PMA and at the indicated times post-PMA, total cell lysates were prepared, immunoprecipitated with 281-2 antibody-Ultralink beads, and immunoblotted with anti-Rab5 (upper panel) or anti-syndecan-1 antibodies (lower panel with typical proteoglycan smear). The dissociation of Rab5 from the syndecan-1 cytoplasmic domain was quantified by densitometry of the Rab5 band. Data shown are mean ± S.E. from three separate experiments.
We next examined if shedding activation induces the dissociation of endogenous Rab5 from the syndecan-1 cytoplasmic domain in the absence of transduced Rab5. At various times post-PMA, NMuMG cell lysates were immunoprecipitated for syndecan-1 and immunoblotted for Rab5. Approximately 50% of Rab5 were dissociated from the syndecan-1 cytoplasmic domain at 15 and 30 min post-PMA stimulation (Fig. 6B). By 60 min post-PMA, the majority of Rab5 was again bound to the syndecan-1 cytoplasmic domain. This kinetics of rapid dissociation and re-association of endogenous Rab5 are consistent with the kinetics of PMA-stimulated syndecan-1 shedding, where maximal shedding is observed by 30 min post-stimulation (9). Altogether, these data indicate that the oscillation between the GDP- and GTP-bound forms of Rab5 controls its interaction with the syndecan-1 cytoplasmic domain, which in turn serves as an on-off molecular switch of syndecan-1 ectodomain shedding at the cell surface.
DISCUSSION
Accumulating evidence suggests that regulated syndecan-1 shedding is one of the key mechanisms that modulates inflammatory responses. Syndecan-1 shedding is induced by several inflammatory mediators, which in turn stimulates the metalloproteinase-mediated cleavage event at the cell surface, but little is known about how shedding is regulated inside the cell. PTKs are thought to play a pivotal role in the proximal stages of the shedding mechanism because the majority of syndecan-1 shedding agonists are inhibited by PTK antagonists (9–11). In this study, we initially tested the hypothesis that PTKs are essential because they potentiate syndecan-1 shedding at the cell surface by phosphorylating Tyr residues in the syndecan-1 cytoplasmic domain. Consistent with this mechanism, our results showed that mutant syndecan-1 harboring a Tyr to Phe substitution in its cytoplasmic domain is not shed upon stimulation with syndecan-1 shedding agonists.
However, we unexpectedly found that the syndecan-1 cytoplasmic domain is not Tyr phosphorylated upon shedding stimulation, indicating that the cytoplasmic domain is important, but for reasons other than phosphorylation of its Tyr residues. Based on these data, we postulated that the syndecan-1 cytoplasmic domain is important because it interacts with critical regulators of shedding, and found that several cell surface and intracellular proteins bind to syndecan-1 cytoplasmic domain. Among these, we focused on Rab5 and found that: (i) Rab5 binds specifically to the syndecan-1 cytoplasmic domain under biological conditions; (ii) expression of DN Rab5 inhibits agonist-induced syndecan-1 shedding; (iii) the syndecan-1 cytoplasmic domain binds directly and preferentially to the inactive GDP-bound form over the active GTP-bound form of Rab5; and (iv) shedding stimulation induces the concomitant dissociation of endogenous Rab5 from the syndecan-1 cytoplasmic domain, which is inhibited by the expression of DN Rab5. These data indicate that Rab5 is a pivotal regulator of syndecan-1 shedding that serves as an on-off switch of shedding through its alternation between GDP- and GTP-bound conformations.
Our data suggested that Rab5 regulates syndecan-1 shedding by serving as a molecular switch that associates with the syndecan-1 cytoplasmic domain as a GDP-bound form and dissociates from the cytoplasmic domain when activated into the GTP-bound form. A corollary to this mechanism is that shedding stimulation facilitates the exchange of GDP for GTP on Rab5, but how seemingly unrelated agonists of syndecan-1 shedding activate Rab5 is not understood. Consistent with our data showing that PMA induces the dissociation of Rab5 from the syndecan-1 cytoplasmic domain, PMA has been shown to increase the proportion of membrane-associated Rab5 and concomitantly decrease the proportion of cytosolic Rab5 (52), indicating that PMA activates Rab5. However, to our knowledge, other inducers of syndecan-1 shedding (e.g. ceramide, stress-related agonists, and bacterial toxins) have not yet been reported to facilitate the switch from GDP-Rab5 to GTP-Rab5. The exchange of GDP for GTP in Rab5 is catalyzed by Rab5 guanosine nucleotide exchange factors that include Rabex-5, ALS2/Alsin, Rin1, Rin2, and GAPex-5. Thus, it is likely that syndecan-1 shedding agonists work through one or several of these Rab5 guanosine nucleotide exchange factors to activate Rab5, but this remains to be determined.
Our results also suggested that the dissociation of Rab5 from the syndecan-1 cytoplasmic domain triggers syndecan-1 ectodomain shedding at the cell surface. This mechanism is similar to the regulated shedding of L-selectin and angiotensin converting enzyme where calmodulin bound to the cytoplasmic tail of substrates inhibits shedding, and the dissociation of calmodulin induced by calmodulin kinase enhances shedding (53, 54). Alternatively, the dissociation of Rab5 may uncover a cryptic docking site and allow association of an intracellular factor essential for shedding induction, such as in PMA-induced shedding of L-selectin where PMA stimulation induces the binding of moesin to the cytoplasmic domain of L-selectin, which in turn enhances L-selectin shedding at the cell surface (55).
However, in light of the fact that the primary function of Rab5 is to modulate intracellular trafficking events, such as receptor endocytosis, Rab5 may trigger syndecan-1 shedding by inducing the internalization of a cell surface receptor closely associated with cell surface syndecan-1. Syndecan-1 shedding is regulated post-translationally and, more importantly, the cleavage site in syndecan-1 is specified by the distance from the plasma membrane, and not by a specific sequence (1, 9). Furthermore, several MMPs possess the capacity to shed syndecan-1 ectodomains (15, 26, 28, 29). These observations suggest that a cell surface protein closely associated with syndecan-1 protects the cleavage site from MMP sheddases. In this regard, it is interesting to note that the α subunit of β1 integrin binds preferentially to the GTP-bound form of Rab5 and Rab21 over their GDP-bound counterparts, and Rab5 and Rab21 facilitate the internalization of β1 integrin (46). Moreover, our studies identified β1 integrin as one of the proteins that associates specifically with the syndecan-1 cytoplasmic domain. Based on these observations, it is plausible that upon shedding stimulation, activated GTP-Rab5 dissociates from the syndecan-1 cytoplasmic domain, associates with β1 integrin, and stimulates the internalization of β1 integrin and dissociation of β1 integrin from syndecan-1, exposing the cleavage site in the ectodomain to MMP syndecan-1 sheddases.
In summary, our current study provides a new understanding of how syndecan-1 shedding is regulated by the interaction between the syndecan-1 cytoplasmic domain and Rab5. Our data indicate that the oscillation between GDP- and GTP-bound conformations allows Rab5 to associate and dissociate from the syndecan-1 cytoplasmic domain, which in turn serves as an on-off switch for syndecan-1 shedding. Furthermore, because overexpression of either WT or DN Rab5 did not alter the expression pattern of syndecan-1, our results also suggest that the syndecan-1 cytoplasmic domain serves as a binding platform for Rab5 that facilitates the targeting of Rab5 to the plasma membrane. Additional studies are needed to precisely define how the dissociation of Rab5 leads to enhanced syndecan-1 shedding at the cell surface, and to determine whether syndecan-1 regulates the localization and biological activities of Rab5.
Acknowledgments
We thank Atsuko Hayashida for technical assistance and Dr. Paul Kincade (Oklahoma Medical Research Foundation, Oklahoma City, OK) for providing the Ky8.2 rat anti-mouse syndecan-4 hybridoma.
This work was supported, in whole or in part, by National Institutes of Health Grants HL69050 and HL81474. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HS, heparan sulfate; BSA, bovine serum albumin; DN, dominant-negative; MMP, matrix metalloproteinase; NMuMG, normal murine mammary gland; PDZ, post-synaptic density protein (PSD95), Drosophila disc large tumor suppressor, and Zonula occludens-1 protein; PMA, phorbol 12-myristate 13-acetate; PTK, protein-tyrosine kinase; WT, wild type; MAP, mitogen-activated protein kinase; TPCK, l-1-tosylamido-2-phenylethyl chloromethyl ketone; GFP, green fluorescent protein; GST, glutathione S-transferase; GTPγS, guanosine 5′-3-O-(thio)triphosphate; PIPES, 1,4-piperazinediethanesulfonic acid.
References
- 1.Bernfield, M., Götte, M., Park, P. W., Reizes, O., Fitzgerald, M. L., Lincecum, J., and Zako, M. (1999) Annu. Rev. Biochem. 68 729–777 [DOI] [PubMed] [Google Scholar]
- 2.Park, P. W., Reizes, O., and Bernfield, M. (2000) J. Biol. Chem. 275 29923–29926 [DOI] [PubMed] [Google Scholar]
- 3.Carey, D. J. (1997) Biochem. J. 327 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fears, C. Y., and Woods, A. (2006) Matrix Biol. 25 443–456 [DOI] [PubMed] [Google Scholar]
- 5.Gotte, M. (2003) FASEB J. 17 575–591 [DOI] [PubMed] [Google Scholar]
- 6.Bernfield, M., Kokenyesi, R., Kato, M., Hinkes, M. T., Spring, J., Gallo, R. L., and Lose, E. J. (1992) Annu. Rev. Cell Biol. 8 365–393 [DOI] [PubMed] [Google Scholar]
- 7.Kim, C. W., Goldberger, O. A., Gallo, R. L., and Bernfield, M. (1994) Mol. Biol. Cell 5 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramanian, S. V., Fitzgerald, M. L., and Bernfield, M. (1997) J. Biol. Chem. 272 14713–14720 [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald, M. L., Wang, Z., Park, P. W., Murphy, G., and Bernfield, M. (2000) J. Cell Biol. 148 811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park, P. W., Foster, T. J., Nishi, E., Duncan, S. J., Klagsbrun, M., and Chen, Y. (2004) J. Biol. Chem. 279 251–258 [DOI] [PubMed] [Google Scholar]
- 11.Park, P. W., Pier, G. B., Preston, M. J., Goldberger, O., Fitzgerald, M. L., and Bernfield, M. (2000) J. Biol. Chem. 275 3057–3064 [DOI] [PubMed] [Google Scholar]
- 12.Chen, Y., Hayashida, A., Bennett, A. E., Hollingshead, S. K., and Park, P. W. (2007) J. Biol. Chem. 282 159–167 [DOI] [PubMed] [Google Scholar]
- 13.Andrian, E., Grenier, D., and Rouabhia, M. (2005) J. Cell. Physiol. 204 178–183 [DOI] [PubMed] [Google Scholar]
- 14.Charnaux, N., Brule, S., Chaigneau, T., Saffar, L., Sutton, A., Hamon, M., Prost, C., Lievre, N., Vita, C., and Gattegno, L. (2005) Glycobiology 15 119–130 [DOI] [PubMed] [Google Scholar]
- 15.Brule, S., Charnaux, N., Sutton, A., Ledoux, D., Chaigneau, T., Saffar, L., and Gattegno, L. (2006) Glycobiology 16 488–501 [DOI] [PubMed] [Google Scholar]
- 16.Chung, M. C., Popova, T. G., Millis, B. A., Mukherjee, D. V., Zhou, W., Liotta, L. A., Petricoin, E. F., Chandhoke, V., Bailey, C., and Popov, S. G. (2006) J. Biol. Chem. 281 31408–31418 [DOI] [PubMed] [Google Scholar]
- 17.Yang, Y., Macleod, V., Miao, H. Q., Theus, A., Zhan, F., Shaughnessy, J. D., Jr., Sawyer, J., Li, J. P., Zcharia, E., Vlodavsky, I., and Sanderson, R. D. (2007) J. Biol. Chem. 282 13326–13333 [DOI] [PubMed] [Google Scholar]
- 18.Joensuu, H., Anttonen, A., Eriksson, M., Makitaro, R., Alfthan, H., Kinnula, V., and Leppa, S. (2002) Cancer Res. 62 5210–5217 [PubMed] [Google Scholar]
- 19.Kainulainen, V., Wang, H., Schick, C., and Bernfield, M. (1998) J. Biol. Chem. 273 11563–11569 [DOI] [PubMed] [Google Scholar]
- 20.Kato, M., Wang, H., Kainulainen, V., Fitzgerald, M. L., Ledbetter, S., Ornitz, D. M., and Bernfield, M. (1998) Nat. Med. 4 691–697 [DOI] [PubMed] [Google Scholar]
- 21.Seidel, C., Ringdén, O., and Remberger, M. (2003) Transplantation 76 423–426 [DOI] [PubMed] [Google Scholar]
- 22.Yang, Y., Yaccoby, S., Liu, W., Langford, J. K., Pumphrey, C. Y., Theus, A., Epstein, J., and Sanderson, R. D. (2002) Blood 100 610–617 [DOI] [PubMed] [Google Scholar]
- 23.Haynes, A., 3rd, Ruda, F., Oliver, J., Hamood, A. N., Griswold, J. A., Park, P. W., and Rumbaugh, K. P. (2005) Infect. Immun. 73 7914–7921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, P. W., Pier, G. B., Hinkes, M. T., and Bernfield, M. (2001) Nature 411 98–102 [DOI] [PubMed] [Google Scholar]
- 25.Kainulainen, V., Nelimarkka, L., Jarvelainen, H., Laato, M., Jalkanen, M., and Elenius, K. (1996) J. Biol. Chem. 271 18759–18766 [DOI] [PubMed] [Google Scholar]
- 26.Li, Q., Park, P. W., Wilson, C. L., and Parks, W. C. (2002) Cell 111 635–646 [DOI] [PubMed] [Google Scholar]
- 27.Xu, J., Park, P. W., Kheradmand, F., and Corry, D. B. (2005) J. Immunol. 174 5758–5765 [DOI] [PubMed] [Google Scholar]
- 28.Ding, K., Lopez-Burks, M., Sanchez-Duran, J. A., Korc, M., and Lander, A. D. (2005) J. Cell Biol. 171 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo, K., Takino, T., Miyamori, H., Kinsen, H., Yoshizaki, T., Furukawa, M., and Sato, H. (2003) J. Biol. Chem. 278 40764–40770 [DOI] [PubMed] [Google Scholar]
- 30.Kinnunen, T., Kaksonen, M., Saarinen, J., Kalkkinen, N., Peng, H. B., and Rauvala, H. (1998) J. Biol. Chem. 273 10702–10708 [DOI] [PubMed] [Google Scholar]
- 31.Hayashida, K., Johnston, D. R., Goldberger, O., and Park, P. W. (2006) J. Biol. Chem. 281 24365–24374 [DOI] [PubMed] [Google Scholar]
- 32.Oh, E. S., Woods, A., and Couchman, J. R. (1997) J. Biol. Chem. 272 8133–8136 [DOI] [PubMed] [Google Scholar]
- 33.Grootjans, J. J., Zimmermann, P., Reekmans, G., Smets, A., Degeest, G., Durr, J., and David, G. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 13683–13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen, A. R., Woods, D. F., Marfatia, S. M., Walther, Z., Chishti, A. H., and Anderson, J. M. (1998) J. Cell Biol. 142 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsueh, Y. P., Yang, F. C., Kharazia, V., Naisbitt, S., Cohen, A. R., Weinberg, R. J., and Sheng, M. (1998) J. Cell Biol. 142 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, G., and Stahl, P. D. (1993) J. Biol. Chem. 268 24475–24480 [PubMed] [Google Scholar]
- 37.Ott, V. L., and Rapraeger, A. C. (1998) J. Biol. Chem. 273 35291–35298 [DOI] [PubMed] [Google Scholar]
- 38.Reiland, J., Ott, V. L., Lebakken, C. S., Yeaman, C., McCarthy, J., and Rapraeger, A. C. (1996) Biochem. J. 319 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heiz, M., Grunberg, J., Schubiger, P. A., and Novak-Hofer, I. (2004) J. Biol. Chem. 279 31149–31156 [DOI] [PubMed] [Google Scholar]
- 40.Slack, B. E., Breu, J., Petryniak, M. A., Srivastava, K., and Wurtman, R. J. (1995) J. Biol. Chem. 270 8337–8344 [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann, P., Tomatis, D., Rosas, M., Grootjans, J., Leenaerts, I., Degeest, G., Reekmans, G., Coomans, C., and David, G. (2001) Mol. Biol. Cell 12 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hozumi, K., Suzuki, N., Nielsen, P. K., Nomizu, M., and Yamada, Y. (2006) J. Biol. Chem. 281 32929–32940 [DOI] [PubMed] [Google Scholar]
- 43.Iba, K., Albrechtsen, R., Gilpin, B., Frohlich, C., Loechel, F., Zolkiewska, A., Ishiguro, K., Kojima, T., Liu, W., Langford, J. K., Sanderson, R. D., Brakebusch, C., Fassler, R., and Wewer, U. M. (2000) J. Cell Biol. 149 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato, M., Saunders, S., Nguyen, H., and Bernfield, M. (1995) Mol. Biol. Cell 6 559–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbieri, M. A., Roberts, R. L., Gumusboga, A., Highfield, H., Alvarez-Dominguez, C., Wells, A., and Stahl, P. D. (2000) J. Cell Biol. 151 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pellinen, T., Arjonen, A., Vuoriluoto, K., Kallio, K., Fransen, J. A., and Ivaska, J. (2006) J. Cell Biol. 173 767–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorvel, J. P., Chavrier, P., Zerial, M., and Gruenberg, J. (1991) Cell 64 915–925 [DOI] [PubMed] [Google Scholar]
- 48.Zerial, M., and McBride, H. (2001) Nat. Rev. Mol. Cell. Biol. 2 107–117 [DOI] [PubMed] [Google Scholar]
- 49.Stenmark, H., Parton, R. G., Steele-Mortimer, O., Lutcke, A., Gruenberg, J., and Zerial, M. (1994) EMBO J. 13 1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Somsel Rodman, J., and Wandinger-Ness, A. (2000) J. Cell Sci. 113 183–192 [DOI] [PubMed] [Google Scholar]
- 51.Takai, Y., Sasaki, T., and Matozaki, T. (2001) Physiol. Rev. 81 153–208 [DOI] [PubMed] [Google Scholar]
- 52.Vita, F., Soranzo, M. R., Borelli, V., Bertoncin, P., and Zabucchi, G. (1996) Exp. Cell Res. 227 367–373 [DOI] [PubMed] [Google Scholar]
- 53.Kahn, J., Walcheck, B., Migaki, G. I., Jutila, M. A., and Kishimoto, T. K. (1998) Cell 92 809–818 [DOI] [PubMed] [Google Scholar]
- 54.Matala, E., Alexander, S. R., Kishimoto, T. K., and Walcheck, B. (2001) J. Immunol. 167 1617–1623 [DOI] [PubMed] [Google Scholar]
- 55.Ivetic, A., Deka, J., Ridley, A., and Ager, A. (2002) J. Biol. Chem. 277 2321–2329 [DOI] [PubMed] [Google Scholar]