Abstract
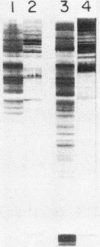
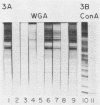
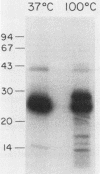
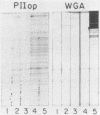
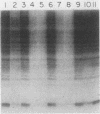
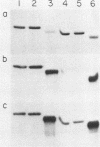
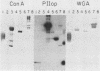
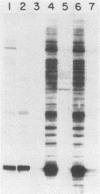
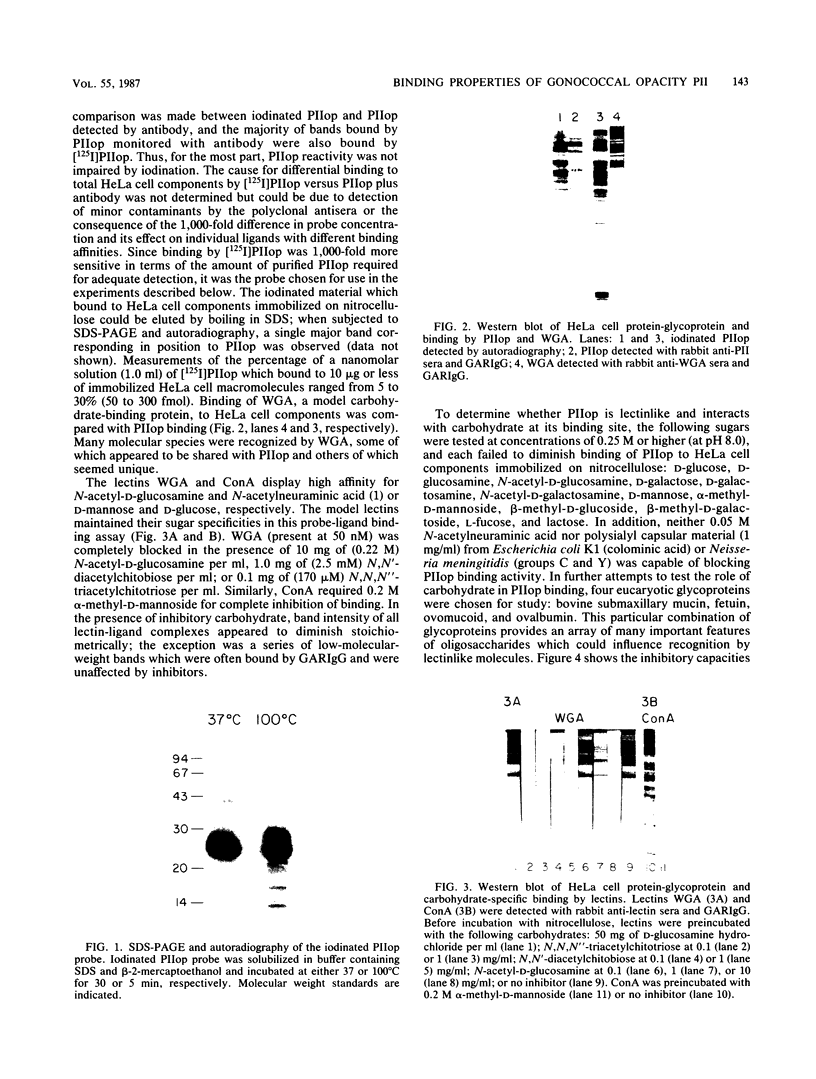
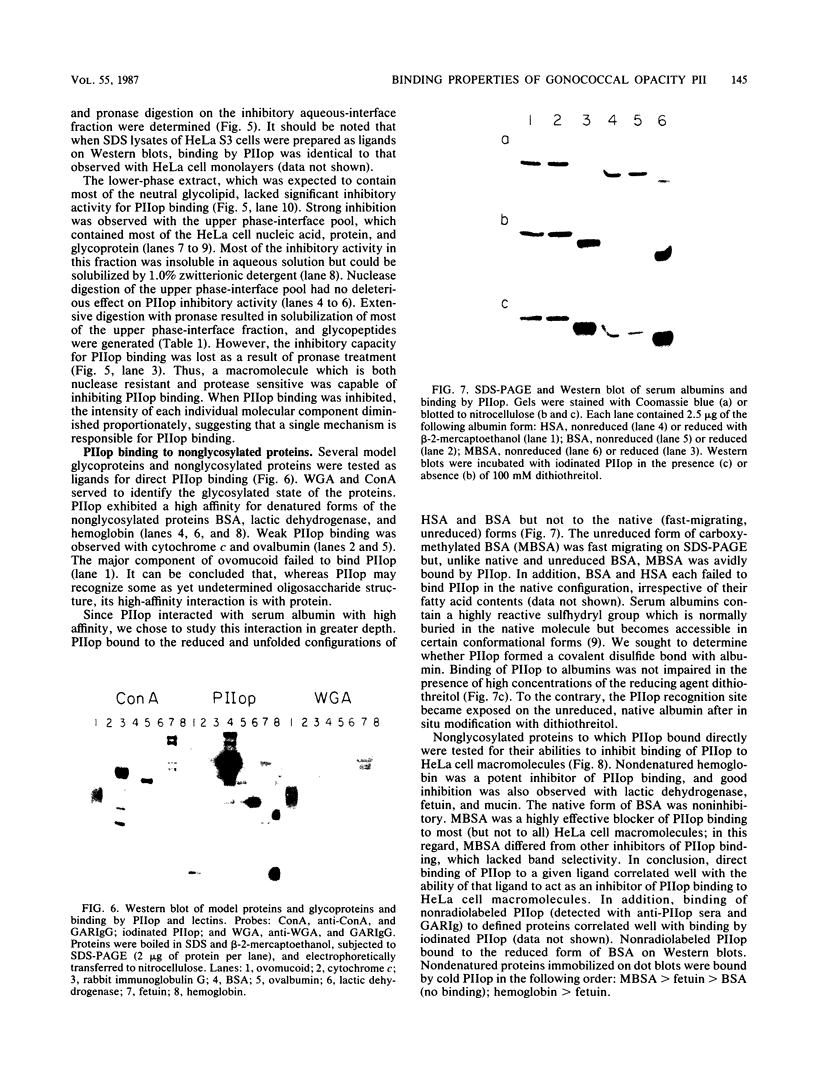
Binding of an opacity-associated protein II (PIIop) from Neisseria gonorrhoeae to eucaryotic macromolecules was studied. HeLa cell extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose, and purified PIIop bound to approximately 50 distinct molecular species. The binding of PIIop to HeLa cell components was stable in high salt and nonionic detergent and was not inhibited by a variety of monosaccharides and polyionic substances. PIIop binding behavior was compared with that of two model carbohydrate-binding proteins, wheat germ agglutinin (WGA) and concanavalin A (ConA). Model glycoproteins (ovomucoid, fetuin, mucin, ovalbumin) inhibited binding by PIIop, WGA, and ConA to various degrees. HeLa cell glycopeptides, generated by pronase digestion of chloroform-methanol-extracted cells, were tested for their ability to inhibit binding by PIIop to Western blots of HeLa cell macromolecules. HeLa cell extracts inhibited PIIop binding before pronase treatment, but inhibitory activity was lost as a result of pronase digestion. Direct binding to defined glycosylated and nonglycosylated proteins revealed that ConA and WGA bound only glycoproteins, whereas PIIop bound to proteins lacking carbohydrate as well. PIIop binding to human and bovine serum albumins was of high affinity and required partial unfolding of albumin; native albumin was not bound by PIIop; however, both the denatured, reduced form of albumin and the compact, nonreduced form of carboxymethylated albumin were bound strongly by PIIop. Albumin-PIIop interaction did not involve covalent bond formation through sulfhydryl groups. The predominant binding interactions of PIIop found in this study were with protein rather than carbohydrate, and the chemical nature of the interactions is more complex than involvement of purely ionic or hydrophobic forces.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C., Swanson J. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1981 Jul;33(1):212–222. doi: 10.1128/iai.33.1.212-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Finne J., Krusius T. Preparation and fractionation of glycopeptides. Methods Enzymol. 1982;83:269–277. doi: 10.1016/0076-6879(82)83020-6. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Magnusson K. E., Davies J., Grundström T., Kihlström E., Normark S. Surface charge and hydrophobicity of Salmonella, E. coli, Gonococci in relation to their tendency to associate with animal cells. Scand J Infect Dis Suppl. 1980;Suppl 24:135–140. [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Lee N., Bowden C. Stimulation of human leukocytes by protein II+ gonococci is mediated by lectin-like gonococcal components. Infect Immun. 1985 Oct;50(1):116–122. doi: 10.1128/iai.50.1.116-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Swanson J. 125I-labeled peptide mapping of some heat-modifiable proteins of the gonococcal outer membrane. Infect Immun. 1980 Apr;28(1):54–64. doi: 10.1128/iai.28.1.54-64.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Barrera O. Immunological characteristics of gonococcal outer membrane protein II assessed by immunoprecipitation, immunoblotting, and coagglutination. J Exp Med. 1983 May 1;157(5):1405–1420. doi: 10.1084/jem.157.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Ceccarini C., Atkinson P. H. Labeling complex carbohydrates of animal cells with monosaccharides. Methods Enzymol. 1978;50:175–204. doi: 10.1016/0076-6879(78)50019-0. [DOI] [PubMed] [Google Scholar]