SYNOPSIS
Objective
Pyrethrin and pyrethroid insecticides are commonly applied in homes and businesses and on some agricultural crops. This research used a two-state regional approach to analyze reports of acute pesticide poisonings due to pyrethrin and pyrethroid insecticides.
Methods
The Washington State Department of Health and the Oregon Public Health Division collected pesticide poisoning surveillance data from 2001 through 2005. Cases were included if they involved exposure to at least one pyrethrin or pyrethroid insecticide. Descriptive statistics were calculated; differences between categories were assessed using Chi-square analysis.
Results
A total of 407 cases fit our definition. Overall, the rate of poisoning in Oregon was significantly higher than in Washington (incidence rate ratio 1.70, 95% confidence interval 1.40, 2.07), and rates for both states generally increased during the time period. For both states, most exposures resulted in low severity illnesses (92%), and most were classified as possible cases (73%). Only about one-fourth of cases were related to a person's work. The most common category of clinical signs and symptoms of illness was respiratory (52% of cases), followed by neurological (40% of cases). Exposure route was predominantly inhalation; there was no association between route and case severity. There was a significant association between illness severity and losing time from work or regular activities (p<0.0001).
Conclusions
Although the majority of pyrethrin and pyrethroid poisoning cases were low in severity, adverse reactions have occurred, as transpired in Oregon in 2005. Regional analysis has the potential to improve the surveillance system and provide unique opportunities for targeting preventive interventions.
Pesticides have become a part of the modern living environment and are commonly applied both by commercial applicators and consumers. In the United States in 2001, 11% of the estimated 888 million pounds of active pesticide ingredient were used in the home and garden market sector. An estimated 74% of all U.S. households applied some type of pesticide to their home in 2000, with approximately 56% applying an insecticide.1
The types of pesticides used in the U.S. have evolved over time. Use of organophosphate insecticides has transitioned to use of less hazardous chemicals, as well as integrated pest management approaches, during the last two decades.2 Pyrethrins and their synthetic derivatives, pyrethroids, have become the predominant insecticide class for public health (mosquito) and residential uses due to low environmental persistence and slow resistance development in pests.3,4 Although a discussion of the chemistry of these compounds is beyond the scope of this article, it is important to recognize the differences between them.
Pyrethrins refer to extracts of the chrysanthemum flower (Tenacetum cinerariifolium) that have insecticidal properties. Because these chemicals degrade rapidly, longer-lasting synthetic versions (pyrethroids) were created. Type I pyrethroids have a cyclopropane carboxylic acid structure; type II pyrethroids have an alpha-cyano group attached to the benzylic carbon, which enhances the insecticidal properties.2,5 Type II pyrethroids are more toxic to mammals, and most clinical reports of human poisoning have been due to these substances.4
The mechanism of action for both compounds is the delay of closure of voltage-sensitive sodium channels. Insects, with smaller body sizes and increased sodium channel sensitivity, are highly susceptible to these substances and experience massive nervous system overstimulation. Mammals, which have poor dermal absorption of pyrethroids, larger body sizes, higher body temperatures, and rapid conversion of these substances to nontoxic metabolites, are less susceptible to acute toxic effects.5,6 Signs and symptoms attributable to pyrethrins and pyrethroids have been documented elsewhere in the literature.4,5,7,8 Briefly, they include paresthesias (burning, tingling, itchiness, or numbness, especially on the face), contact dermatitis, anorexia, fatigue, dizziness, muscular fasciculations (involuntary contraction), salivation, irritation of the upper and lower airways, allergic reactions, asthma, and, at higher doses, coma, seizures, and pulmonary edema.4,6,9 Symptoms reported by flight attendants after pyrethroid spraying on airplanes included confusion, weakness, and heart palpitations.6
With pyrethrins and pyrethroids being increasingly encountered in the environment, case-based surveillance is an important public health tool to monitor trends in adverse effects associated with exposure. Data from surveillance systems are used by regulatory agencies to identify hazardous products and improve their safety. Data are also used locally to identify high-risk populations and target prevention and education.
This analysis used pesticide illness surveillance data from the states of Oregon and Washington from 2001 through 2005 to describe the scope and nature of acute illnesses associated with currently used products. This article also serves to alert health-care providers, commercial pesticide applicators, and regulatory agencies to the potential for serious reactions. Existing literature using state-based surveillance systems often focused on specific industries or occupations.6,10,11 Including nonoccupational acute pesticide-poisoning cases in this study provided us the opportunity to examine the burden of exposure to all residents.
METHODS
Data were collected from two similar pesticide illness surveillance systems operated by the Washington Department of Health (WDOH) and the Oregon Public Health Division (OPHD) from 2001 through 2005. The WDOH and OPHD pesticide surveillance systems are similar in that both (1) are mature systems that have operated for more than 15 years, (2) collect reports through mandatory physician reporting laws, (3) use National Institute for Occupational Safety and Health (NIOSH) standard variables for pesticide illness surveillance, (4) have similar climates and pest pressure, (5) receive electronic reporting from poison centers and individual referrals from other state and local agencies, and (6) accept self-reports of pesticide-related illness. The systems differ somewhat in that DOH identifies more cases from industrial insurance claims (workers' compensation) and captures more occupational incidents. In contrast, OPHD receives the bulk of its cases (80%) from the state Poison Control Center.
When a report is received at either program, a staff member investigates to ascertain the nature of the exposure, the pesticides involved, and the medical outcome. Investigations usually include an interview with the symptomatic person and a review of his or her medical records. Information collected on each person's case includes demographics, date of exposure, route of exposure, location of exposure, type of activity or work being done at the time of exposure, medical signs and symptoms, and pesticide product(s) and/or active ingredient(s) involved.
Illness severity was assigned to all cases for both states using standardized criteria.12 Severity was based on signs (objective findings reported by a health-care provider) and symptoms (subjective findings reported by the exposed person), medical care sought and/or received, and time lost from work or normal activities. Four categories of illness severity are defined as follows: (1) Low severity illness or injury usually results in three or fewer missed days, and consists of health effects that likely do not require treatment. (2) Moderate severity illness or injury consists of health effects that are not life-threatening, but that result in some time lost from work, usually less than five days. (3) High severity illness or injury consists of life-threatening health effects that usually require hospitalization, and time lost from work or normal activities tends to be much higher (more than five days). (4) Death is the fourth severity category, assigned to fatalities due to exposure to one or more pesticides.
Cases for both states were classified using a standardized case definition (developed under the NIOSH Sentinel Event Notification System for Occupational Risk Pesticides Program).10,11,13 Cases were classified into the following categories: definite, probable, possible, suspicious, unlikely, insufficient information, exposed but asymptomatic, unrelated, or unknown. Classification was conducted by each state's surveillance expert(s) and was dependent upon (1) strength of evidence for pesticide exposure, (2) whether health effects were reported as signs by a health-care provider or symptoms by the exposed person, and (3) consistent evidence of a causal relationship between the pesticide and the health effects based on known toxicological evidence. Cases classified as definite, probable, or possible were ones in which the affected individuals had health effects that were consistent with the current toxicological information on the pesticide.
This analysis was restricted to 2001 (when Washington began using the standard case definition) through 2005 cases of acute illness or injury determined to be definitely, probably, or possibly related to pesticide exposure. Cases were included if they involved exposure to at least one pyrethrin or pyrethroid insecticide, regardless of the other chemicals involved in the incident. The analysis included occupational and nonoccupational exposures.
Oregon and Washington use different platforms for data collection, requiring data manipulation prior to analysis. All analyses were performed with SAS® version 9.1.14 Frequency tables were produced, and differences between categories were assessed with Chi-square analysis (or Fisher's exact test when cell sizes were small). Significance level was defined as p≤0.05. Incidence rates by year were calculated; the numerator was the number of pesticide-related illnesses for the category of interest, and the denominator was calculated from state population estimates from 2001 through 2005 U.S. Census data. Incidence rate ratios (IRRs) and 95% confidence intervals (CIs) were used to determine the risk of pesticide poisoning between the two states.
RESULTS
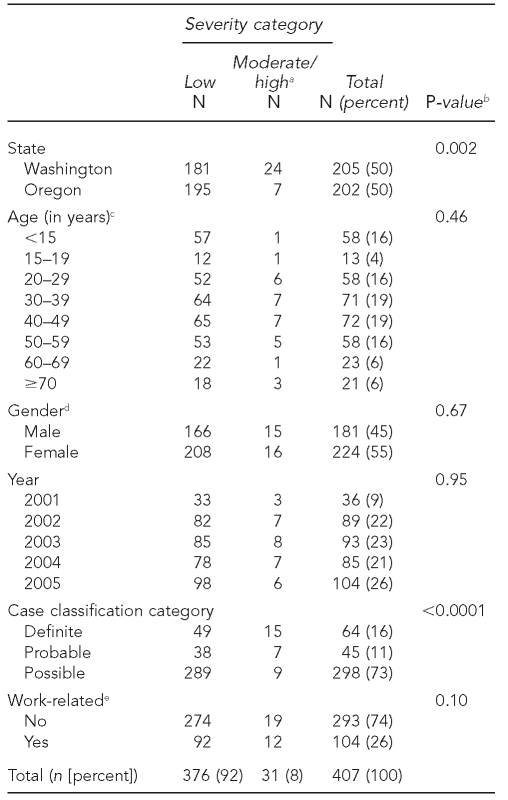
For the five-year time period of 2001 through 2005, a total of 407 cases fit our case definition (205 from Washington and 202 from Oregon). Cases peaked seasonally, with the largest number of cases occurring in the summer months. The demographics of people meeting the case definitions by illness severity are presented in Table 1. Cases were categorized as follows: definite, 64 (16%); probable, 45 (11%); and possible, 298 (73%). Approximately one-quarter of total cases were work-related. Females accounted for a slightly higher proportion of case subjects (55%) than did males (Table 1); however, the IRRs for males vs. females for all age categories were not significantly different (data not shown). The median age of people meeting the case definition was 37.6 years (range of 7 months to 99 years of age); the median age by illness severity was 36.6 years of age for people in the low severity category and 41.8 years of age for people in the higher severity categories (including moderate and high severity as well as death) (data not shown). The highest proportion of people meeting the case definition were in the age ranges of 40 to 49 years (n=72, 19%) and 30 to 39 years (n=71, 19%).
Table 1.
Demographic data by severity category for 407 cases of acute pyrethrin or pyrethroid pesticide poisoning, Oregon and Washington, 2001–2005
aIncludes one fatality
bP-value from Chi-square analysis; p≤0.05 considered statistically significant
cA total of 33 cases with unknown age were excluded.
dTwo cases with unknown gender were excluded.
eTen cases with unknown or not applicable work status were excluded.
Illness severity is presented in Table 1. Most cases were low severity (92%). One death occurred; this case is included in the moderate/high category (8%). Severity (low vs. moderate/high) did not differ by age group, gender, year of event, or work-related status. Severity did differ by state, with Washington more likely than Oregon to have moderate or high severity cases (p=0.002). Case classification differed by illness severity, with a much larger proportion of definite cases having moderate or high illness severity (p<0.0001).
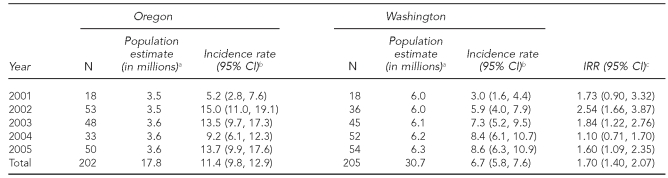
Overall, the incidence rate of acute pyrethrin or pyrethroid poisoning was significantly higher in Oregon compared with Washington (IRR=1.70, 95% CI 1.40, 2.07) (Table 2). Both states had significant increases in poisoning rates from 2001 to 2005 (Mantel-Haenszel Chi-square, p=0.049 for Oregon and p<0.001 for Washington).
Table 2.
Number of pyrethrin/pyrethroid poisoning cases among Oregon and Washington State residents, incidence rates, and incidence rate ratios, by year, 2001–2005 (n=407)
aState population estimates from U.S. Census Bureau
bIncidence rate per million residents
cCompares rate of pyrethrin/pyrethroid poisoning cases among Oregon residents for a given year to Washington residents
CI = confidence interval
IRR = incidence rate ratio
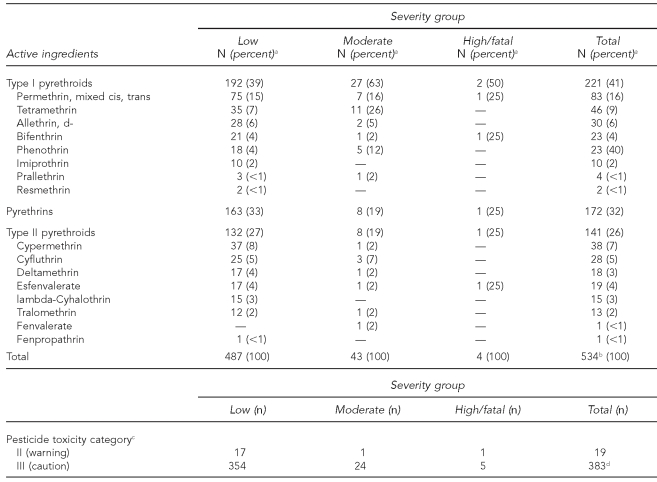
For active ingredients, type I pyrethroids were the most commonly reported for all severity groups (n=221, 41%) (Table 3). The most commonly reported type I pyrethroid was permethrin (n=83, 16%), followed by tetramethrin (n=46, 9%). Pyrethrins were the second most common type of active ingredient for all severity groups (n=172, 32%). Type II pyrethroids were the least common type of active ingredient reported for all severity groups (n=141, 26%). The most commonly reported type II pyrethroid was cypermethrin (n=38, 7%), followed by cyfluthrin (n=28, 5%). When case severity was classified into two groups (low vs. higher) and compared with the active ingredient category, we found that individuals whose cases had moderate, high, or fatal outcomes were more likely to be exposed to type 1 pyrethroids compared with those whose cases were classified as low severity (p=0.0117). Table 3 also shows that in the majority of cases, individuals were exposed to low toxicity products, based on U.S. Environmental Protection Agency (EPA) toxicity ratings.
Table 3.
Frequency of active ingredients and pesticide toxicity category for 407 cases of pyrethrin or pyrethroid pesticide exposure by case severity grouping, Oregon and Washington, 2001–2005
aIndicates column percentage
bThere could be multiple active ingredients per case.
cBased on U.S. Environmental Protection Agency categorization
dThere could be more than one product per case, no products per case (if only active ingredients were reported), or products besides pyrethrins or pyrethroids per case if more than one product was used.
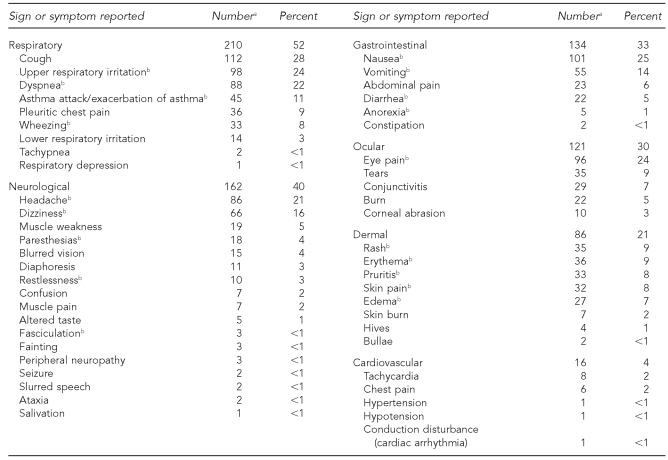
Table 4 lists clinical signs and symptoms reported by people with cases of acute pesticide poisoning from pyrethrins or pyrethroids. The most common category of signs or symptoms reported was respiratory (n=210, 52%), followed by neurological (n=162, 40%), and gastrointestinal (n=134, 33%). The most common individual signs or symptoms are shown following each major division in Table 4.
Table 4.
Clinical signs and symptoms reported by Oregon and Washington State residents exposed to pyrethrin or pyrethroid pesticides, 2001–2005 (n=407)
aThe sums of specific clinical signs/symptoms do not total the number reported for the system because more than one symptom might have been reported in some cases.
bSign or symptom related to pyrethrin or pyrethroid exposure in the published literature: Sutton PM, Vergara X, Beckman J, Nicas M, Das R. Pesticide illness among flight attendants due to aircraft disinsection. Am J Ind Med 2007;50:345-56. Calvert GM, Barnett M, Mehler LN, Becker A, Das R, Beckman J, et al. Acute pesticide-related illness among emergency responders, 1993–2002. Am J Ind Med 2006;49:383-93.
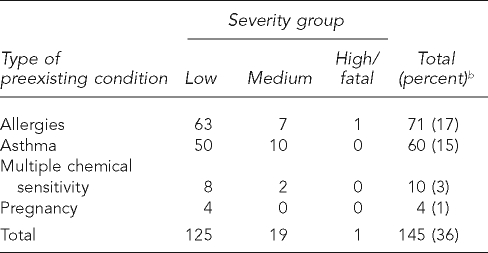
For reported preexisting conditions, 71 people had allergies (17%), 60 had asthma (15%), 10 had multiple chemical sensitivity (3%), and four were pregnant (1%) (Table 5). There was a significant association between the presence of any preexisting condition and illness severity (p=0.035), with a larger proportion of those with preexisting conditions having moderate or high severity illness.
Table 5.
Frequency of reported preexisting conditions for 407 cases of exposure to pyrethrin or pyrethroid pesticides by case severity grouping, Oregon and Washington, 2001–2005a
aReported by affected individual, clinician, or both
bPercent of 407 cases
For route of exposure to the pesticides, the predominant method was inhalation (63%), followed by skin (37%), eye (28%), and ingestion (8%). More than one exposure route could be reported for each case. There was no significant association found between route of exposure and illness severity (data not shown).
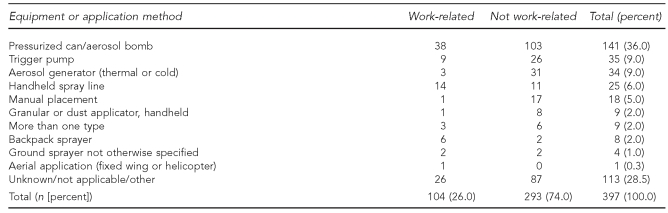
Most exposures (74%) were not occupational exposures (Tables 1, 6, 7, and 8). For the 293 nonoccupational cases, 136 (46%) occurred when the individual was mixing, applying, or otherwise handling the pesticide. Approximately half became exposed during routine living activities not involving handling of the pesticide (145 of 293, 49%) (Table 6). The most common equipment used for nonoccupational cases was a pressurized can or “bug bomb” (103 of 293, 35%) (Table 7). The vast majority of nonoccupational cases were residential exposures (255 or 293, 87%) (Table 8).
Table 6.
Activity of pesticide-exposed individual by work status, Oregon and Washington, 2001–2005 (n=397)a
aTen cases of individuals with unknown work status were excluded.
Table 7.
Equipment or application method of pesticide by exposed individual's work status, Oregon and Washington, 2001–2005 (n=397)a
aTen cases of individuals with unknown work status were excluded.
Table 8.
Location of exposure by work status of individuals exposed to pyrethrin or pyrethroid pesticides, Oregon and Washington, 2001–2005 (n=397)a
aTen cases of individuals with unknown work status were excluded.
For work-related cases (26%) (Tables 1, 6, 7, and 8), 71% of people were exposed while doing their normal work duties and were not handling pesticides (74 of 104) (Table 6). As with nonoccupational cases, the most common equipment or application method was a pressurized can or aerosol bomb (38 of 104, 36%) (Table 7). In contrast with nonoccupational cases, people in the occupational cases were most likely to be exposed at a non-manufacturing facility, such as a retail nursery or office building (41 of 104, 39%). The second most commonly reported location for occupational exposures was a private residence (22 of 104, 21%) (Table 8). More than half (13 of 22, 59%) of the workers exposed at residences were licensed pesticide applicators, and another third (7 of 22, 32%)—five emergency responders and two reporters—were exposed during a single incident (data not shown).
Pesticide-related illness may result in time lost from work, school, or regular activities. Where lost-time status was known, 13% (31 of 242) of people with low severity cases reported one day or more of lost time; for those with moderate to high severity cases, the percentage increased to 52% (14 of 27). Because days of lost time are part of the severity ranking, it is not surprising that lost-time status was significantly associated with severity grouping (p<0.0001) (data not shown).
Case reports
The following two cases, one from Oregon and one from Washington, are presented to illustrate the potential severity of pyrethrin or pyrethroid poisoning.
Case 1—Oregon.
In 2005, a licensed pesticide applicator utilized a crack and crevice technique with a mixture of two types of pesticide: a pyrethroid (esfenvalerate, 3.48%) and pyrethrins (1.00% formulated with piperonyl butoxide and N-octyl bicycloheptene dicarboxidmide) for pest control on the interior of a residence in Florence, Oregon. An exterior application of a permethrin was made after the interior application. Upon reentry into the home, approximately three and a half hours after the interior application, one of the residents, an elderly woman with a history of heart disease, experienced acute respiratory symptoms and cardiac arrhythmia. Resuscitation attempts were unsuccessful, and the woman died at the scene.
The woman's spouse, two neighbors, and five responders to the incident (three emergency medical technicians and two police officers) experienced less severe, but similar upper respiratory symptoms (dyspnea, mucous production, throat constriction, and coughing). Their symptoms resolved shortly after they exited the home or within several hours. The spouse of the deceased woman was hospitalized overnight for observation. Upon review by Oregon's Pesticide Analytical Response Center, it was concluded that the cardiac arrhythmia was most likely caused by respiratory distress, which “resulted from inhalation exposure to the pesticides that were applied to the interior of her home.” This conclusion was in agreement with the Lane County Medical Examiner, who concluded that her death was due to “sudden cardiac arrhythmia following exposure to pyrethroid insecticide in an elderly woman with significant heart disease.”15
Case 2—Washington.
In June 2005, a 53-year-old man with a history of reactive airways disease and allergies to multiple common therapeutic drugs sprayed his car with an aerosol house and garden insecticide containing the type I pyrethroids phenothrin and d-cis,trans allethrin. He left his car with the windows closed until the following day. He did not clean surfaces or thoroughly ventilate the car before driving it into town the next day. He reported that difficulty breathing developed within an hour of reentering the car. His symptoms progressed to coughing and respiratory congestion.
Over the next five days, he reported intermittent fever, increasing congestion with productive cough, and persistent wheezing. He was seen by his primary care provider five days after his exposure and was prescribed steroids and antibiotics in addition to his normal inhalers. When he did not improve, he was admitted to the hospital 10 days after his exposure for failure of outpatient treatment. On admission, he was noted to have bilateral and diffuse rhonchi and expiratory wheezing. He was treated with intraveneous steroids, antibiotics, bronchodilators, and oxygen supplementation as needed. He was discharged three days later in stable condition with a diagnosis of severe asthma exacerbation. Other ingredients identified on the product material safety data sheet were 0.1% sodium nitrite, 3%–7% isoparaffinic hydrocarbon solvent, 3%–7% isobutane, 5%–10% propane, and 70%–80% water. There was no mention on the label that the insecticide product could exacerbate asthma or other respiratory conditions.
DISCUSSION
Both Oregon and Washington had overall increasing incidence rates for acute pesticide poisonings from pyrethrins and pyrethroids from 2001 to 2005, although Oregon's rates fluctuated and the highest rate occurred in 2002 (Table 2). The increasing incidence rates for both states may be explained by the phase-out of residential products containing chlorpyrifos in December 2001 and diazinon in December 2004. Pyrethroid products likely replaced these organophosphate substances in the urban and suburban environment. This cannot be verified, as neither state tracked pyrethrin or pyrethroid sales or usage during the time period of this analysis. However, the results of our study match those of other investigators.16
We found a significant association between the presence of any preexisting condition and case severity (p=0.035). However, only limited conditions are routinely recorded (pregnancy, asthma, allergies, and multiple chemical sensitivity), and data are often incomplete. Preexisting conditions in people exposed to pyrethrins or pyrethroids have limited documentation in the literature. Newton and Breslin found exacerbation of asthma symptoms after inhalation of pyrethroids.17 Wagner described the death of an 11-year-old girl with asthma who shampooed her dog with a formulation containing 0.2% pyrethrins.18 A recent population-level study found no association between mosquito control spraying of pyrethroids for West Nile virus and emergency department (ED) asthma visits in New York City in 2000.19 More complete data collection on preexisting conditions, or the possibility of documenting new or different conditions, might be warranted and may clarify the results shown in this analysis.
Type II pyrethroids are more toxic to mammals than type I pyrethroids, and more clinical reports of poisonings have been attributed to them.4 However, our results showed an association between active ingredients in type 1 pyrethroids and higher severity cases (p=0.0117). This finding might be explained by noting that the type I and type II classification schemes are based on doses of pyrethroids that cause obvious neurotoxicity; these may not apply to low dose exposures.20 Further, many cases in our dataset involved effects to the skin, eyes, or respiratory system; because type II pyrethroids are more potent neurotoxins than type I, neurotoxicity might not be the underlying cause of reported symptoms. It is also possible that inert ingredients in the aerosol or fogger formulations may have contributed to the reported health effects. We did not analyze the number of inert ingredients or any synergists that may have been present in the pyrethrin or pyrethroid products used. Piperonyl butoxide is the most commonly used synergist with pyrethroids;5 it inhibits detoxification by mixed function oxidase systems.21 In this study, individuals in the cases we analyzed were exposed to an average of 2.4 active ingredients and an unknown number of inert ingredients. Information on inert ingredients was not available because they are considered trade secrets; thus, it is unknown if inert ingredients could be associated with the reported signs or symptoms. Hoffman et al. found that pure bifenthrin (a type I pyrethroid) was not toxic to a culture of T-cells, but that the commercial preparation was toxic, leading those authors to suggest that inert ingredients could lead to loss of cell viability.22 One of the products used in the Florence, Oregon, case is known to have <25% chlorinated hydrocarbons (1,1,1-trichloroethane) (Personal communication, Dr. Daniel Sudakin, National Pesticide Medical Monitoring Program, July 2005). Deaths in humans following acute exposure to high concentrations of 1,1,1-trichloroethane are usually due to respiratory arrest or cardiac arrhythmias.23 However, many of the signs and symptoms noted in Table 4 correspond to those attributable to pyrethrin or pyrethroid exposure in the literature.6,24 More research on the role of synergists or inert substances on illness severity is warranted.
Death from exposure to pyrethrins or pyrethroids has been reported. He et al. reviewed 573 cases of acute pyrethroid poisoning in the Chinese medical literature and found seven deaths—one from misdiagnosis of organophosphate poisoning and subsequent atropine treatment, two from occupational exposures, and four accidental or intentional cases.7 Wax and Hoffman reported on a woman with mild asthma who died after washing her dog with a pyrethrin-containing flea shampoo.25 Calvert et al. in 2007 described a fatal case of a man with a history of asthma who was exposed to a rodenticide and a pyrethrin insecticide; his death followed complications from steroidal treatment.11 Bradberry et al. reported fewer than 10 deaths from occupational or intentional exposures.5 In the Oregon case described previously, death was due neither to occupational exposure or ingestion. It appears to be the first recorded case where death followed reentry into a home where a pyrethroid had been applied and the home had not been ventilated prior to reentry.
Twelve percent of Washington's cases were classified as moderate to high severity, while only 4% of Oregon's cases received the same classification. This may be due to differences in case ascertainment rather than Washington having more severe cases than Oregon. Washington receives a higher proportion of its cases from health-care providers than does Oregon. It is logical to think that those people whose symptoms are severe enough to seek medical attention are more likely to later be classified as moderate to high severity cases than those who do not seek medical attention. Oregon has experienced a decline in the number of pesticide poisonings confirmed or suspected by clinicians that are directly reported to the health department, from 13 in 2001 to one in 2005, and this may have lowered the number of cases that were classified as “definite” using the NIOSH standardized case definition. The infrequency with which Oregon receives cases from medical providers suggests a lack of knowledge among Oregon's medical community that pesticide poisoning is a reportable condition.
Limitations
There are limitations present that could have affected our analyses. Although pesticide poisoning is a reportable condition in both Washington and Oregon, there is likely considerable underreporting of incidents to the health department. A study in the Yakima region of Washington found that 60% of workers with pesticide-related diagnoses were captured by Washington's surveillance system.26 Cases were unlikely to be included in our dataset if the symptomatic person did not seek health care, the health-care provider did not recognize symptoms as pesticide related, or the health-care provider failed to report the case through the Poison Control Center, industrial insurance claims, or directly to the health department. There is also potential for differences in the type of information gathered for individual variables. For example, if a person in Oregon initially contacted the state Poison Control Center and subsequently sought care in an ED, the initial medical care that was sought most likely would be noted as Poison Control Center (38% of Oregon cases); in Washington, this would be recorded as ED (43% of Washington cases). Discovering this difference between how the two states classify initial medical care was one positive aspect of this analysis that allows evaluation of the surveillance system and provides opportunities for improvement.
The potential for misclassification must also be considered. Affected individuals may have reported an exposure or symptoms days or weeks after an incident. In addition, data were not always complete for cases, and the signs reported by the individuals themselves may not have been verified by medical record review. These limitations could have resulted in a misclassification of case severity. Further, the rates of poisoning presented in this article probably underestimate the true extent of the problem, as some people may not have received medical attention and would not have been entered into the surveillance system. Some people may have been treated, but the health-care provider may not have correctly attributed the illness to a pesticide exposure. There is also potential for false positives, because nonspecific symptoms may have been coincidental and not actually caused by the pesticide exposure. However, the standardized case definition provided an objective approach to assess relationships between pyrethrin or pyrethroid exposures and health effects.
A strength of this analysis is the regional approach that was undertaken. Pooling data from states that have similar surveillance systems but different pesticide data-entry systems allowed us to describe the scope and nature of pyrethrin and pyrethroid illnesses for a larger population, but also to note differences between the states. The use of a standardized case definition and severity index for both states that has been well documented provided another assurance that cases were being classified in similar ways.
Outcomes from the fatal Oregon case and pyrethrin-pyrethroid surveillance in general have a scope beyond the Pacific Northwest. Oregon supplied data from the Florence, Oregon, case described previously to the Washington Department of Agriculture, via the WDOH Pesticide Program. This information was used at a state meeting of the Federal Insecticide, Fungicide, and Rodenticide Act Issues Research and Evaluation Group Working Committee, Pesticide Operations and Management. Poor or problematic label language, especially on foggers, was discussed. The Oregon fatality was described as a death due to faulty directions, and it was decided that the Label Accountability Workgroup (a group led by the Office of Pesticide Program's Field and External Affairs Division and including representatives from EPA's regional offices, the Office of General Counsel, and the Office of Enforcement and Compliance Assurance) would assess and report on the scope, costs, and consequences of problem labels to EPA management.27
CONCLUSIONS
The data presented in this article show the scope and magnitude of acute illness associated with pyrethrin and pyrethroid insecticides in both Oregon and Washington from 2001 through 2005. Further, the data underscore the need for state-based surveillance programs—they are a valuable tool used to estimate the magnitude of the problem, identify new or emerging issues, identify risk factors and areas for intervention, and communicate research results. Based on the data findings from this analysis as well as the presented case studies, we propose the following list of recommendations to these specific groups:
EPA: (1) Change product labels for unrestricted (over-the-counter) pesticides to warn sensitive subpopulations to avoid exposure, lengthen the amount of time people are advised to wait before entering a treated area, and define ventilation; (2) require commercial applicators to initiate mechanical ventilation for indoor applications of pyrethroid products instead of instructing the occupant to enter and open windows; and (3) define optimal mechanical ventilation.
Emergency response workers: Because these individuals have an increased risk of pesticide poisoning compared with workers in nonagricultural industries,28 we recommend that they (1) evaluate protective equipment and response protocols, and don respiratory protection when entering enclosed environments; and (2) know how to locate information on chemical hazards.
State agencies or health departments: (1) Continue to monitor the health effects of indoor use of pyrethrins and/or pyrethroids, especially in mixtures and for sensitive populations (such as younger or older individuals and those with preexisting health conditions); (2) develop outreach to organizations that educate asthma and allergy patients on potential risks of these pesticides, and develop sets of recommended precautions for asthma and chronic obstructive pulmonary disease patients; and (3) educate applicators and consumers about the importance of reading pesticide product labels and following all directions. Special attention should be given to the importance of ventilating indoor areas before reentry.
Health-care providers: (1) Be aware that pyrethroids, pyrethrins, and many of the solvents in aerosols and insect foggers are respiratory irritants and have the potential to cause severe and prolonged asthmatic reactions; (2) be aware that known or suspected cases of pesticide exposure or poisoning are reportable conditions in some states (e.g., Oregon, Washington, California, and Florida), and be encouraged to report these to public health authorities; and (3) obtain an adequate history of any environmental and occupational exposures that could cause or exacerbate disease.
Acknowledgments
The authors thank Drs. Geoffrey Calvert, Jae Douglas, and Cynthia Lopez for reviewing an earlier version of this article, and Lynden Baum for preparing Washington's data.
Footnotes
This research was supported by cooperative agreement U60 OH008472 (Oregon) from the National Institute for Occupational Safety and Health (NIOSH).
The findings and conclusions in this article are those of the authors and do not necessarily reflect those of NIOSH.
REFERENCES
- 1.Kiely T, Donaldson D, Grube A. Pesticides industry sales and usage: 2000 and 2001 market estimates. Washington: Environmental Protection Agency, Office of Pesticide Programs (US); 2004. [cited 2007 Nov 6]. Also available from: URL: http://www.epa.gov/oppbead1/pestsales/01pestsales/market_estimates2001.pdf. [Google Scholar]
- 2.Sudakin DL. Pyrethroid insecticides: advances and challenges in biomonitoring. Clin Toxicol (Phila) 2006;44:31–7. doi: 10.1080/15563650500394647. [DOI] [PubMed] [Google Scholar]
- 3.Ray DE, Fry JR. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol Ther. 2006;111:174–93. doi: 10.1016/j.pharmthera.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Ray DE, Forshaw PJ. Pyrethroid insecticides: poisoning syndromes, synergies, and therapy (review) J Toxicol Clin Toxicol. 2000;38:95–101. doi: 10.1081/clt-100100922. [DOI] [PubMed] [Google Scholar]
- 5.Bradberry SM, Cage SA, Proudfoot AT, Vale JA. Poisoning due to pyrethroids. Toxicol Rev. 2005;24:93–106. doi: 10.2165/00139709-200524020-00003. [DOI] [PubMed] [Google Scholar]
- 6.Sutton PM, Vergara X, Beckman J, Nicas M, Das R. Pesticide illness among flight attendants due to aircraft disinsection. Am J Ind Med. 2007;50:345–56. doi: 10.1002/ajim.20452. [DOI] [PubMed] [Google Scholar]
- 7.He F, Wang S, Liu L, Chen S, Zhang Z, Sun J. Clinical manifestations and diagnosis of acute pyrethroid poisoning. Arch Toxicol. 1989;63:54–8. doi: 10.1007/BF00334635. [DOI] [PubMed] [Google Scholar]
- 8.Wilks MF. Pyrethroid-induced paresthesia—a central or local toxic effect? J Toxicol Clin Toxicol. 2000;38:103–5. doi: 10.1081/clt-100100923. [DOI] [PubMed] [Google Scholar]
- 9.Environmental Protection Agency (US) Recognition and management of pesticide poisonings. 5th ed. Washington: EPA Office of Pesticide Programs (US); 1999. [Google Scholar]
- 10.Calvert GM, Plate DK, Das R, Rosales R, Shafey O, Thomsen C, et al. Acute occupational pesticide-related illness in the US, 1998–1999: surveillance findings from the SENSOR-pesticides program. Am J Ind Med. 2004;45:14–23. doi: 10.1002/ajim.10309. [DOI] [PubMed] [Google Scholar]
- 11.Calvert GM, Petersen AM, Sievert J, Mehler LN, Das R, et al. Acute pesticide poisoning in the U.S. retail industry, 1998–2004. Public Health Rep. 2007;122:232–44. doi: 10.1177/003335490712200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (US) Severity index for use in state-based surveillance of acute pesticide-related illness and injury, 2005. [cited 2007 Dec 11]. Available from URL: http://www.cdc.gov/niosh/topics/pesticides/pdfs/pest-sevindexv6.pdf.
- 13.Barnett M, Calvert GM. Pesticide-related illness and injury surveillance: a how-to guide for state-based programs. Cincinnati: Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (US); 2005. [cited 2007 Dec 11]. Also available from: URL: http://www.cdc.gov/niosh/docs/2006-102. [Google Scholar]
- 14.SAS Institute, Inc. SAS: Version 9.1 for Windows. Cary (NC): SAS Institute, Inc.; 2003. [Google Scholar]
- 15.Oregon Department of Agriculture. Pesticide analytical response center concludes its review of 2005 Florence fatality. Salem (OR): Oregon Department of Agriculture; 2006. [press release]; 2006 Mar 22. [Google Scholar]
- 16.Power LE, Sudakin DL. Pyrethrin and pyrethroid exposures in the United States: a longitudinal analysis of incidents reported to poison centers. J Med Toxicol. 2007;3:94–9. doi: 10.1007/BF03160917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton JG, Breslin AB. Asthmatic reactions to a commonly used aerosol killer. Med J Aust. 1983;1:378–80. doi: 10.5694/j.1326-5377.1983.tb99416.x. [DOI] [PubMed] [Google Scholar]
- 18.Wagner SL. Fatal asthma in a child after use of an animal shampoo containing pyrethrin. West J Med. 2000;173:86–7. doi: 10.1136/ewjm.173.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpati AD, Perrin MC, Matte T, Leighton J, Schwartz J, Barr RG. Pesticide spraying for West Nile virus control and emergency department asthma visits in New York City, 2000. Environ Health Perspect. 2004;112:1183–7. doi: 10.1289/ehp.6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123–36. doi: 10.1289/ehp.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osimitz TG, Breathnach R. The safety assessment of piperonyl butoxide. In: Krieger RI, editor. Handbook of pesticide toxicology. 2nd ed. San Diego: Academic Press; 2001. p. 1461. [Google Scholar]
- 22.Hoffman N, Tran V, Daniyan A, Ojugbele O, Pryor SC, Bonventre JA, et al. Bifenthrin activates homotypic aggregation in human T-cell lines. Med Sci Monit. 2006;12:BR87–94. [PubMed] [Google Scholar]
- 23.Agency for Toxic Substances and Disease Registry. Toxicological profile for 1,1,1-trichloroethane. Atlanta: Department of Health and Human Services (US); 2006. [cited 2007 Dec 13]. Also available from: URL: http://www.atsdr.cdc.gov/toxprofiles/tp70.html#bookmark07. [Google Scholar]
- 24.Proudfoot AT. Poisoning due to pyrethrins. Toxicol Rev. 2005;24:107–13. doi: 10.2165/00139709-200524020-00004. [DOI] [PubMed] [Google Scholar]
- 25.Washington State Department of Health. Improving data quality in pesticide illness surveillance. 2004. Jun 17, [cited 2007 Nov 7]. Available from: URL: http://www.doh.wa.gov/ehp/oehas/publications_pdf/improvingdataqualitypesticideillnessssurveillance-2004.pdf.
- 26.Wax PM, Hoffman RS. Fatality associated with inhalation of a pyrethrin shampoo. J Toxicol Clin Toxicol. 1994;32:457–60. doi: 10.3109/15563659409011049. [DOI] [PubMed] [Google Scholar]
- 27.State FIFRA [Federal Insecticide, Fungicide, and Rodenticide Act] Issues Research – Evaluation Group (SFIREG) Working Committee, Pesticides Operations and Management. Meeting minutes, 2007 Oct 1–2. [cited 2007 Nov 29]. Available from: URL: http://aapco.ceris.purdue.edu/htm/minutes07.htm.
- 28.Calvert GM, Barnett M, Mehler LN, Becker A, Das R, Beckman J, et al. Acute pesticide-related illness among emergency responders, 1993–2002. Am J Ind Med. 2006;49:383–93. doi: 10.1002/ajim.20286. [DOI] [PubMed] [Google Scholar]