Abstract
Hepcidin is a 25-amino-acid iron peptide hormone originated from its two precursors of prohepcidin (60-amino-acid) and preprohepcidin (84-amino-acid). Serum prohepcidin levels have been widely used to evaluate iron overload in clinical and preclinical studies. However, its usefulness is often questioned and its stepwise conversion mechanism remains largely unknown. Using New York University Women’s Health Study subjects, we measured serum levels of prohepcidin with ELISA and hepcidin with mass spectrometry as well as ferritin and soluble transferrin receptor 1 (sTfR1) in 45 normal healthy postmenopausal women over a 1-year period with 2 samples per subject. We found that serum prohepcidin levels are correlated with the serum sTfR1 levels (r=0.45, p<0.01) but not to ferritin levels (r=0.08, p=0.60), suggesting that serum prohepcidin is not a biomarker of iron overload that was originally thought and designed for. Interestingly, serum hepcidin levels are associated with serum ferritin levels (r=0.64, p<0.0001) but not with sTfR1 levels (r=0.04, p=0.69), indicating that hepcidin is a measure of iron overload. Although hepcidin is a downstream product of prohepcidin, the amounts of hepcidin and prohepcidin are not related to each other (r=−0.007, p=0.90) under normal physiological conditions. The interrelationships between sTfR1 and prohepcidin or between ferritin and hepcidin suggest that ferritin- and sTfR1-sensed hepcidin conversion system exists in human body and maybe regulated at the post-translational level.
Keywords: Iron, homeostasis, transferrin receptor, ferritin, hepcidin
Introduction
Hepcidin is a peptide hormone synthesized in the liver and is the principal regulator of systemic iron homeostasis. Hepcidin controls plasma iron concentration and tissue iron distribution by inhibiting intestinal iron absorption, iron recycling by macrophages, and iron mobilization from hepatic stores [1,2]. Hepcidin influences iron absorption through direct binding to ferroportin at the basolateral membrane, leading to decreased export of iron to the circulation system [3]. The functional hepcidin molecule is derived from the 2-step conversion of an 84-amino-acid long peptide, the preprohepcidin, by N-terminus cleavage of a 24-amino-acid signal peptide to give first rise to prohepcidin, followed by a second cleavage of a 35-amino-acid peptide to yield the active 25-amino-acid hepcidin [4,5]. Prohepcidin is expressed at the basolateral membrane of hepatocytes and is found in the blood [6]. Serum levels of prohepcidin have been widely used to diagnose iron overload. However, clinical and preclinical studies have failed to show positive correlations between serum prohepcidin concentrations and serum iron overload markers such as serum ferritin levels [6–9]. Until now, it has been unclear whether serum prohepcidin is a measure of active hepcidin or simply a non-functional precursor [10], and mechanisms controlling its stepwise conversion in the circulation remain unknown.
Currently, prohepcidin can be easily measured in serum using the commercially available enzyme-linked immunosorbent assay (ELISA) [6]. The active form of hepcidin can be measured in serum by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS) [11]. Our aim was to examine the relationship between active hepcidin and prohepcidin in sera of normal healthy subjects and to assess whether serum ferritin and soluble transferrin receptor 1 (sTfR1) play a part in hepcidin regulation at the post-translational level.
Materials and Methods
The New York University (NYU) Women’s Health Study
Between March 1985 and June 1991, the NYU Women's Health Study enrolled a cohort of 14 274 healthy women, aged 34–65 years, at a breast cancer screening center in New York City and details about blood collection and completion of a self-administered questionnaire were previously described [12,13]. Forty-five women were selected at random from the pool of eligible women within the NYU Women’s Health Study as previously described [14]. This pool consisted of the NYU Women’s Health Study participants who were postmenopausal at entry, had given blood on 3 or more occasions at yearly intervals, had a yield of 11 or more aliquots at each visit, had not been diagnosed with cancer or cardiovascular disease, and had not been selected as a control in any case-control study nested within the cohort. The Institutional Review Board at the NYU School of Medicine approved this study. Two yearly samples were retrieved for each woman.
Laboratory analyses for serum prohepcidin, ferritin, and sTfR1
Serum samples were identified solely by a sample number so that laboratory personnel were not aware of the identity of the contributing participants. All samples from a subject were always assayed in the same batch. Levels of prohepcidin were determined by an ELISA assay based on a competitive principle (DRG International, Inc., Mountainside, NJ). sTfR1 in sera was determined by an ELISA technique using two different monoclonal antibodies specific for sTfR1 (R & D System, Minneapolis, MN). Ferritin in sera was determined according to a previously published protocol [15].
Analyses of serum hepcidin by mass spectrometry
Serum hepcidin measurements by SELDI-TOF MS were performed as previously described [11]. In brief, the hepcidin SELDI test involves the chromatographic retention of hepcidin and hepcidin variants using an immobilized metal affinity chromatographic ProteinChip® Array pre-loaded with copper ions. After binding the sample under optimized conditions of pH and ionic strength, non-specifically associated proteins are removed by washing with the binding buffer. The retained proteins are then detected by SELDI-TOF MS and the specific hepcidin variants are identified within the mass spectrometry by their unique mass/charge ratio. For the quantitation of hepcidin, the relative peak intensity of the hepcidin variant is compared against a standard curve generated by spiking in synthetic hepcidin peptide (American Peptide Company, Sunnyvale, CA) into a reference serum. Data were expressed as µg/ml concentration.
Statistical Methods
Correlation among prohepcidin, hepcidin, ferritin, and sTfR1 were analyzed using the Spearman rank correlation.
Results
Women had a median age (range) at first blood donation of 62.3 years (49.5 – 68.1 years), with a median time since menopause of 12.6 years (2.2 – 25.3 years). The median weight and body mass index were 66.0 kg (43.0 – 86.0 kg) and 24.6 kg/m2 (19.2 – 35.9 kg/m2), respectively. Serum samples were in storage at −80°C for a median of 16.6 years (15.8 – 18.1 years) at the time when we performed all the measurements.
Figure 1 presents a linear standard curve obtained from peak intensity at 2792 m/z with the synthetic peptide ranging from 0 to 2 µg/mL (A) and representative protein mass spectra of a standard and a serum sample from one of the study subjects (B). We found interassay precisions of 24.8%, 11.2%, 8.7%, 7.0%, and 1.4% at 0.125, 0.25, 0.5, 1, and 2 µg/mL, respectively.
Figure 1. Standard curve of synthetic hepcidin and representative protein mass spectra of synthetic hepcidin and a serum sample.
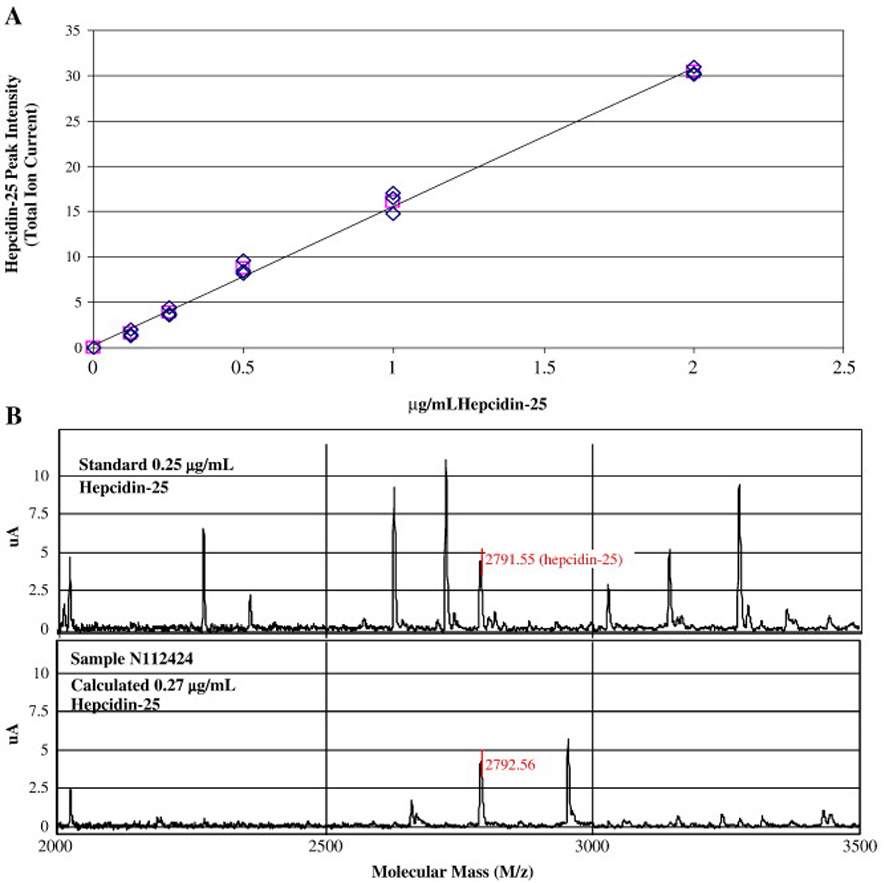
(A) Mean slope from a set of 4 standard curves with hepcidin-25; (B) Top: synthetic hepcidin; Bottom: Serum sample from one of the study subjects.
Table 1 shows the Spearman rank correlation coefficients among serum levels of prohepcidin, sTfR1, ferritin, and hepcidin over the two visits. Combining the data of both visits, we found that prohepcidin is positively correlated to sTfR1 (r=0.45, p<0.01) but not to ferritin (r=0.08, p=0.60). Active hepcidin is positively associated with ferritin (r=0.64, p<0.0001) but not with sTfR1 (r=0.06, p=0.70). To our surprise, serum prohepcidin levels are not correlated at all with serum active hepcidin levels (r=−0.007, p=0.90). Serum ferritin levels are not correlated with serum sTfR1 levels (r=0.06, p=0.67). In addition, we found that the reliability coefficient for active hepcidin was fairly low (r=0.49, 95% confidence interval 0.22–0.68). This is in contrast to the high temporal reliability coefficients that we previously observed for ferritin, sTfR1, and prohepcidin, which were 0.78 (95% CI: 0.67–0.86), 0.79 (95% CI: 0.69–0.87), and 0.89 (95% CI: 0.84–0.94), respectively [14].
Table 1.
Results of Spearman rank correlation among prohepcidin, hepcidin, ferritin, and sTfR1 (N = 45)
| Prohepcidin |
Ferritin |
sTfR1 |
Hepcidin |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 |
Visit 2 |
Visit 1 |
Visit 2 |
Visit 1 |
Visit 2 |
Visit 1 |
Visit 2 |
|||||||||
|
r |
p |
r |
p |
r |
p |
r |
p |
r |
p |
r |
p |
r |
p |
r |
p |
|
| Prohepcidin | 1 | 1 | 0.079 | 0.60 | 0.08 | 0.60 | 0.42* | 0.004 | 0.49* | 0.007 | −0.025 | 0.87 | 0.01 | 0.94 | ||
| Ferritin | 0.079 | 0.60 | 0.08 | 0.60 | 1 | 1 | 0.09 | 0.56 | 0.04 | 0.79 | 0.57* | < 0.0001 | 0.71* | < 0.0001 | ||
| sTfR1 | 0.42* | 0.004 | 0.49* | 0.007 | 0.09 | 0.56 | 0.04 | 0.79 | 1 | 1 | 0.04 | 0.77 | 0.08 | 0.62 | ||
| Hepcidin | −0.025 | 0.87 | 0.01 | 0.94 | 0.57* | < 0.0001 | 0.71* | < 0.0001 | 0.04 | 0.77 | 0.08 | 0.62 | 1 | 1 | ||
Discussion
Hepcidin is a key regulator of body iron metabolism and, thus, there is an enormous interest in quantifying circulating hepcidin in clinical samples. Because SELDI-TOF MS is not easily accessible for hepcidin measurements, ELISA-based technique to measure prohepcidin has been widely used as an amenable tool with the assumptions that the concentrations of prohepcidin correlate with those of hepcidin and both of them have similar clinical implications in iron overload. However, previous studies failed to show prohepcidin changes as a function of iron status changes, which raised the question of the usefulness of prohepcidin in evaluating iron overload [6,9,16]. For example, it has been shown that serum prohepcidin concentrations in blood samples from hereditary hemochromatosis patients were not significantly different from those of normal healthy subjects and were not related to differences in iron stores as measured by ferritin [9]. Even in hemochromatosis patients undergoing phlebotomy, the range of serum prohepcidin was small, despite large differences in serum ferritin concentrations [9]. Serum prohepcidin concentrations in patients with sickle cell anemia with abnormally high serum ferritin concentrations were no different than in controls [16]. Similarly, no association was found between serum prohepcidin and iron status measures such as serum iron, transferrin saturation, or serum ferritin [6].
In view of the discordance between prohepcidin and iron overload, we measured serum levels of prohepcidin with ELISA and hepcidin with SELDI-TOS MS, as well as ferritin and sTfR1 in 45 health post-menopausal subjects. Surprisingly, our study shows that prohepcidin levels are correlated to sTfR1, suggesting that prohepcidin may be associated with hypoferremia or erythropoiesis. In contrast, active hepcidin is positively associated with ferritin but not with sTfR1, indicating that active hepcidin is a biomarker of iron overload. This positive association is in agreement with the data showing a significant and positive correlation between active hepcidin and ferritin in hemodialysis patients as well as in healthy controls [17,18]. Although our sample size is relatively small, the associations between active hepcidin and ferritin but not sTfR1, and between prohepcidin and sTfR1 but not ferritin are reproducible with repeated visits by the same subject. Moreover, subjects in our study were normal healthy post-menopausal women, so that levels of prohepcidin and hepcidin in these individuals should not be affected by disease conditions such as inflammation, anemia, iron overload, hemodialysis, or cancer [17,19– 22]. No impact of estrogen or menstruation should be at play either because all women were post-menopausal and not taking hormone replacement therapy [23]. Therefore, levels of these four iron proteins should reflect only the physiological iron status in the healthy body.
Iron homeostasis is strictly controlled at the transcriptional level in the body and at posttranscriptional level in the cells [2,3]. Intracellular iron balance is achieved through posttranscriptional regulation of TfR1 and ferritin mRNA levels by iron regulator proteins [1,24]. Cellular iron deficiency increases iron uptake by over-expressing membrane TfR1 and down-regulating ferritin in the cells [25]. Hepcidin is a negative regulator of body iron uptake [2]. At the systemic level, iron overload up-regulates preprohepcidin mRNA levels and iron deficiency down-regulates preprohepcidin in the liver [26–28]. By incubating HepG2 cells with sera from iron deficiency anemia and thalassemia major patients, which contain elevated sTfR1, levels of preprohepcidin mRNA expression were significantly decreased [29]. Bone morphogenetic proteins have been found to use hemojuvelin as a coreceptor to regulate preprohepcidin expression and to be more potent than IL-6 in stimulating preprohepcidin transcription [30,31]. Recently, it has been identified that transmembrane serine protease 6 (TMPRSS6) is an essential component detecting iron deficiency and blocking preprohepcidin transcription [32]; germline mutations in the TMPRSS6 gene can cause iron-refractory iron deficiency anemia [33].
In addition to its regulation at the transcriptional level, post-translational cleavage of prohepcidin to hepcidin in human hepatocytes is mediated by the prohormone convertase furin [34]. Our results suggest that stepwise conversions of preprohepcidin after its secretion from liver may exist in the circulation (Figure 2). Serum sTfR1 is usually considered to be markers of hypoferremia and/or erythropoiesis. Ferritin is related to iron overload and/or inflammation. Under hypoferremic condition or high erythropoietic activity, increased sTfR1 may not only inhibit mRNA and protein synthesis of preprohepcidin in the liver [26–28], but also could mediate conversion of preprohepcidin to prohepcidin in sera. That is probably why in our study sTfR1 was positively associated with prohepcidin. This regulation could be a synchronized event in order to decrease the overall mRNA and protein levels of preprohepcidin.
Figure 2. Proposed ferritin- and sTfR1-sensed hepcidin conversion mechanism.
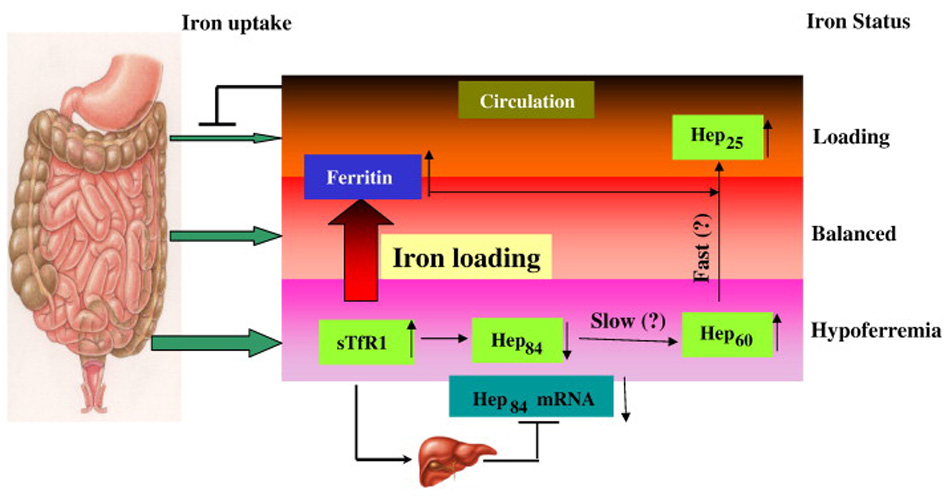
Iron uptake may be controlled by ferritin- and sTfR1-sensed preprohepcidin conversion mechanism in the circulation at the post-translational level. Regulation of preprohepcidin in the liver at the transcription level is also shown. Hep84: preprohepcidin; Hep60: prohepcidin; Hep25: hepcidin.
Our data also showed that the reliability coefficient for prohepcidin is higher (r=0.89) than that of active hepcidin (r=0.48) over a 1-year period. The high reliability of prohepcidin indicates that this protein is fairly stable over time within an individual, relative to others. On the contrary, the lower reliability of hepcidin indicates variations over time for serum hepcidin levels. In view of the facts that 1) synthetic hepcidin causes rapid dose-dependent hypoferremia and immediate excretion of hepcidin metabolites in the urine; 2) furin quickly cleaves prohepcidin to hepcidin in hepatocytes; and, 3) both hepcidin and ferritin are acute phase proteins in response to inflammation [19,34–36], it is reasonable to assume that the step 2 conversion of prohepcidin to hepcidin is fast. Thus, the step 1 conversion of preprohepcidin to prohepcidin may be the rate-limiting step (Figure 2). This step 1 conversion is probably the first line of defense to prepare the body to get ready when iron overload arises. When iron loading does occur, the step 2 conversion can quickly take place leading to the inhibition of iron absorption.
Overall, our results indicate that prohepcidin is a biomarker reflecting hypoferremic conditions and possibly erythropoietic activity, which was previously unexpected in its clinical and preclinical use. On the other hand, active hepcidin is a biomarker for iron overload such as hemochromatosis. Our results also suggest that a ferritin- and sTfR1-sensed hepcidin conversion mechanism may exist to regulate systemic iron homeostasis at the post-translational levels. Further studies should elucidate the mechanisms of how human body senses the needs for hepcidin conversions and what factors play a role in these processes.
Acknowledgement
This work was funded by the US National Institute of Health (NIH CA132684) and in part by NIH grants ES00260, CA34588, and CA16087.
Abbreviations
- sTfR1
soluble transferrin receptor 1
- TMPRSS6
transmembrane serine protease 6.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 2.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 3.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 4.Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 5.Park CH, Valore EV, Waring AJ, et al. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 6.Kulaksiz H, Gehrke SG, Janetzko A, et al. Pro-hepcidin: expression and cell specific localisation in the liver and its regulation in hereditary haemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. 2004;53:735–743. doi: 10.1136/gut.2003.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eleftheriadis T, Kartsios C, Liakopoulos V, et al. Does hepcidin affect erythropoiesis in hemodialysis patients? Acta Haematol. 2006;116:238–244. doi: 10.1159/000095873. [DOI] [PubMed] [Google Scholar]
- 8.Luukkonen S, Punnonen K. Serum pro-hepcidin concentrations and their responses to oral iron supplementation in healthy subjects manifest considerable inter-individual variation. Clin Chem Lab Med. 2006;44:1361–1362. doi: 10.1515/CCLM.2006.241. [DOI] [PubMed] [Google Scholar]
- 9.Roe MA, Spinks C, Heath AL, et al. Serum prohepcidin concentration: no association with iron absorption in healthy men; and no relationship with iron status in men carrying HFE mutations, hereditary haemochromatosis patients undergoing phlebotomy treatment, or pregnant women. Br J Nutr. 2007;97:544–549. doi: 10.1017/S0007114507336829. [DOI] [PubMed] [Google Scholar]
- 10.Brookes MJ, Sharma NK, Tselepis C, et al. Serum pro-hepcidin: measuring active hepcidin or a non-functional precursor? Gut. 2005;54:169–170. doi: 10.1136/gut.2004.047639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemna EH, Tjalsma H, Podust VN, et al. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53:620–628. doi: 10.1373/clinchem.2006.079186. [DOI] [PubMed] [Google Scholar]
- 12.Toniolo PG, Pasternack BS, Shore RE, et al. Endogenous hormones and breast cancer: a prospective cohort study. Breast Cancer Res Treat. 1991;18 Suppl 1:S23–S26. doi: 10.1007/BF02633522. [DOI] [PubMed] [Google Scholar]
- 13.Zeleniuch-Jacquotte A, Shore RE, Koenig KL, et al. Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. Br J Cancer. 2004;90:153–159. doi: 10.1038/sj.bjc.6601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeleniuch-Jacquotte A, Zhang Q, Dai J, et al. Reliability of serum assays of iron status in postmenopausal women. Ann Epidemiol. 2007;17:354–358. doi: 10.1016/j.annepidem.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali MA, Akhmedkhanov A, Zeleniuch-Jaquotte A, et al. Reliability of serum iron, ferritin, nitrite, and association with risk of renal cancer in women. Cancer Detection and Prevention. 2003;27:116–121. doi: 10.1016/s0361-090x(03)00027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezeh C, Ugochukwu CC, Weinstein J, et al. Hepcidin, haemoglobin and ferritin levels in sickle cell anaemia. Eur J Haematol. 2005;74:86–88. doi: 10.1111/j.1600-0609.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 17.Kato A, Tsuji T, Luo J, et al. Association of prohepcidin and hepcidin-25 with erythropoietin response and ferritin in hemodialysis patients. Am J Nephrol. 2008;28:115–121. doi: 10.1159/000109968. [DOI] [PubMed] [Google Scholar]
- 18.Tomosugi N, Kawabata H, Wakatabe R, et al. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006;108:1381–1387. doi: 10.1182/blood-2005-10-4043. [DOI] [PubMed] [Google Scholar]
- 19.Kemna E, Pickkers P, Nemeth E, et al. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 20.Lee P, Peng H, Gelbart T, et al. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward DG, Roberts K, Brookes MJ, et al. Increased hepcidin expression in colorectal carcinogenesis. World J Gastroenterol. 2008;14:1339–1345. doi: 10.3748/wjg.14.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai J, Jian J, Bosland M, et al. Roles of hormone replacement therapy and iron in proliferation of breast epithelial cells with different estrogen and progesterone receptor status. The Breast. 2008;17:172–179. doi: 10.1016/j.breast.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouault TA, Stout CD, Kaptain S, et al. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991;64:881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- 25.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 26.Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18:394–400. doi: 10.1681/ASN.2006070802. [DOI] [PubMed] [Google Scholar]
- 27.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 29.Kemna EH, Kartikasari AE, van Tits LJ, et al. Regulation of hepcidin: Insights from biochemical analyses on human serum samples. Blood Cells Mol Dis. 2007 doi: 10.1016/j.bcmd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 31.Truksa J, Peng H, Lee P, et al. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valore EV, Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis. 2008;40:132–138. doi: 10.1016/j.bcmd.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konijn AM, Hershko C. Ferritin synthesis in inflammation. I. Pathogenesis of impaired iron release. Br J Haematol. 1977;37:7–16. [PubMed] [Google Scholar]
- 36.Rivera S, Nemeth E, Gabayan V, et al. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106:2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]


