Abstract
The cancer stem cell hypothesis proposes that cancers arise in stem/progenitor cells through disregulation of self-renewal pathways generating tumors which are driven by a component of “tumor initiating cells” retaining stem cells properties. The HER2 gene is amplified in 20–30% of human breast cancers and has been implicated in mammary tumorigenesis as well as in mediating aggressive tumor growth and metastasis. We demonstrate that HER2 overexpression drives mammary carcinogenesis, tumor growth and invasion through its effects on normal and malignant mammary stem cells. HER2 overexpression in normal mammary epithelial cells increases the proportion of stem/progenitor cells as demonstrated by in vitro mammosphere assays and the expression of stem cell marker ALDH as well as by generation of hyperplastic lesions in humanized fat pads of NOD/SCID mice. Overexpression of HER2 in a series of breast carcinoma cell lines increases the ALDH expressing “cancer stem cell” population which displays increased expression of stem cell regulatory genes, increased invasion in vitro and increased tumorigenesis in NOD/SCID mice. The effects of HER2 overexpression on breast cancer stem cells are blocked by trastuzumab in sensitive, but not resistant, cell lines, an effect mediated by the PI3-kinase Akt pathway. These studies provide support for the cancer stem cell hypothesis by suggesting that the effects of HER2 amplification on carcinogenesis, tumorigenesis and invasion may be due to its effects on normal and malignant mammary stem/progenitor cells. Furthermore, the clinical efficacy of trastuzumab may relate to its ability to target the cancer stem cell population in HER2 amplified tumors.
INTRODUCTION
The HER2 gene is amplified in 20–30% of human breast cancers and is associated with aggressive metastatic disease (Slamon et al., 1987; Slamon et al., 1989). Although a number of signaling pathways including PI3-K/Akt have been shown to be regulated by HER2, the mechanisms by which these pathways contribute to the aggressive characteristics of these tumors remain unclear. The humanized anti-HER2 antibody trastuzumab (Herceptin) has been an important agent used to treat breast cancers displaying HER2 overexpression (Beuzeboc et al., 1999). Despite its benefits, a substantial fraction of patients treated in the adjuvant setting still relapse, one-third of patients with advanced disease fail to respond and the majority of initial responders demonstrate disease progression within one year (Miller, 2004; Seidman et al., 2001). The development of effective strategies for treating these trastuzumab resistant tumors would be facilitated by a clearer understanding of the mechanisms by which HER2 overexpression induces aggressive behavior in breast cancer and the molecular mechanisms of trastuzumab resistance.
There is increasing evidence that a wide variety of malignancies, including breast cancer, may be driven by a small subset of “tumor initiating cells” or “cancer stem cells” (CSC) that display stem cell properties. Our group and others have described a subpopulation of cells in human mammary carcinomas, with the phenotype CD44+/CD24−/lin- that display “cancer stem cell” properties (Al-Hajj et al., 2003). These cells comprising 1–5% of primary tumors, are able to form tumors in immunocompromised mice as well as to generate the phenotypic heterogeneity of the initial tumor. More recently, we have reported that the stem/progenitor cell population in both the normal mammary gland and in mammary carcinomas may also be identified by virtue of increased expression of the enzyme aldehyde dehydrogenase as assessed by the Aldefluor assay or by immunohistochemistry (Ginestier et al., 2007).
In addition to driving carcinogenesis, CSCs may contribute to therapeutic resistance. Indeed, it has recently been reported that CD44+/CD24− mammary stem cells are relatively resistant to radiation and chemotherapy in vitro and in mouse models (Phillips et al., 2006). The clinical relevance of these observations was confirmed by the demonstration of an increase in the CSC population following neo-adjuvant chemotherapy in patients with locally advanced breast cancer (Li et al., 2008)
We have recently reported a significant correlation between HER2 overexpression and expression of the stem cell marker ALDH1 in a series of 491 breast cancer patients (Ginestier et al., 2007). Since tumorigenesis, invasion and metastasis may be mediated by the tumor stem cell component we reasoned that the effects of HER2 overexpression on tumorigenesis might be due to the effects of this signaling pathway on regulating the mammary stem cell population. We report that HER2 overexpression increases the stem/progenitor cell population of both normal and malignant mammary cells. Furthermore, the effects of HER2 overexpression on mammary tumorigenesis and invasion are due to its effects on the CSC population. These effects correlate with increased HER2 and Akt phosphorylation and are inhibited by trastuzumab in sensitive but not in resistant cells. These studies indicate that HER2 signaling regulates the mammary stem/progenitor cell population driving carcinogenesis and tumor invasion.
RESULTS
Effects of HER2 overexpression on normal mammary stem/progenitor cells
We have previously demonstrated that normal human mammary cells generate non-adherent spherical colonies called mammospheres (Dontu et al., 2003). These mammospheres are composed of a small number of self-renewing cells capable of mammosphere initiation upon serial passage, as well as a larger number of progenitor cells capable of multi lineage differentiation (Dontu et al., 2003). To examine the role of HER2 in mammary stem cell self-renewal, we infected normal mammary epithelial cells with lentiviruses expressing either DsRed-HER2 or DsRed. Elevated HER2 protein levels in the HER2 lentivirus infected compared to DsRed infected cells was confirmed by Western blotting (Figure 1A). HER2 lentivirus infected cells showed over 90% DsRed positive cells when analyzed by flow cytometry (Supplemental Figure 1A). We have also analyzed HER2 surface expression in both non-infected and HER2-infected NMECs by flow cytometry. Over 90% of HER2-infected cells displayed HER2 surface expression as compared to less than 4% of non-infected NMECs (Supplemental Figure 1B).
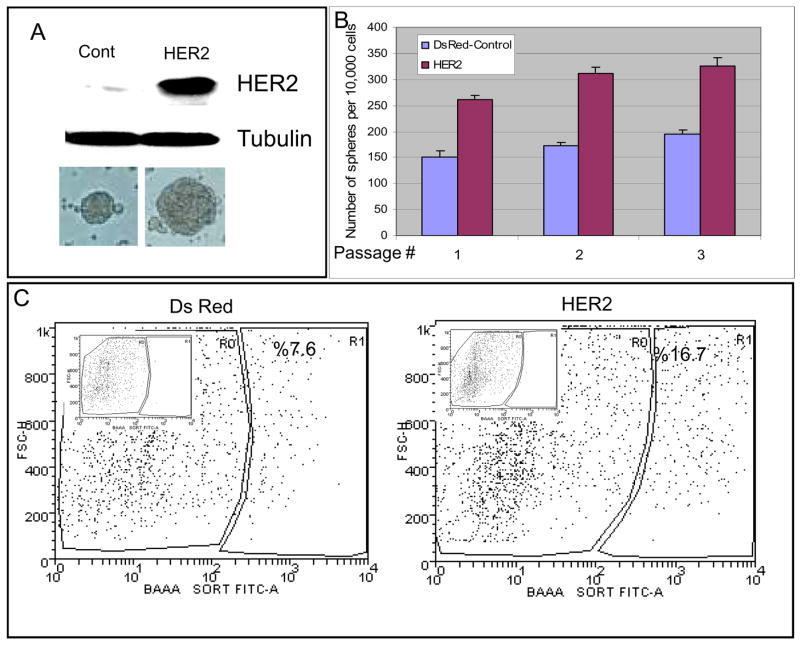
Figure 1. HER2 expression increases mammosphere formation and the Aldelfuor-positive cell population.
A. HER2 expression in normal mammary epithelial cells infected with HER2 or DsRed lentivirus. Increased size of mammospheres in HER2 expressing cells as compared to DsRed control. B. Serial passage of HER2 and DsRed control mammospheres. HER2 expression increases mammosphere forming capacity upon serial passage. P values for each passage are 5.6×10−37, 8.05×10−43, 3.4×10−32 respectively. C. HER2 or DsRed cells were assessed for ALDH activity utilizing the Aldefluor assay. HER2 expressing cells demonstrate greater than a two-fold increase in the Aldefluor-positive cell population compared to DsRed control cells. Insert show DEAB inhibited flow results for compensation. Data represented here are the mean of 5 independent experiments performed in triplicates.
When DsRed or HER2 transfected cells were cultured for seven days in suspension, both the number and size of mammospheres significantly increased in HER2 expressing normal mammary epithelial cells (NMEC) as compared to the DsRed controls (Figure 1A). We have previously demonstrated that the number of mammospheres generated upon serial passage provides an indirect measure of mammary stem cell self-renewal while mammosphere size reflects progenitor cell proliferation. We therefore examined the ability of primary mammospheres from both DsRed and HER2 expressing cells to form secondary and tertiary mammospheres. The number of secondary and tertiary mammospheres generated from the HER2 expressing cells was significantly higher than that of the control cells (Figure 1B).
HER2 overexpression expands the Aldefluor-positive cell population of normal mammary epithelial cells in vitro and increases outgrowths in NOD-SCID mice
We previously demonstrated that normal mammary epithelial cells that express aldehyde dehydrogenase as determined by the Aldefluor assay, display characteristics of stem/progenitor cells. (Ginestier et al., 2007). Figure 1C shows overexpression of HER2 in normal mammary epithelial cells increased the Aldefluor-positive population more than two-fold compared to DsRed infected cells. The effects of HER2 overexpression on mammosphere formation and aldehyde dehydrogenase activity may reflect direct effects of HER2 signaling on these properties. Alternatively, HER2 overexpression may effect stem cell self-renewal producing an increase in the stem cell population. In order to distinguish between these possibilities, we examined the mammosphere-forming capacity of the Aldefluor-positive and -negative cells overexpressing HER2. Only the Aldefluor-positive population was capable of mammosphere formation in HER2 expressing cells (Figure 2A). We also demonstrated that only a small fraction of NMECs express HER2 surface protein (Supplemental Figure 1B). Furthermore, we observed similar HER2 surface expression in Aldefluor-positive and Aldefluor-negative NMECs (Supplemental Figure 1C). This suggests that the effects of HER2 overexpression are due to the generation of increased proportion of primitive mammary cells capable of mammosphere formation.
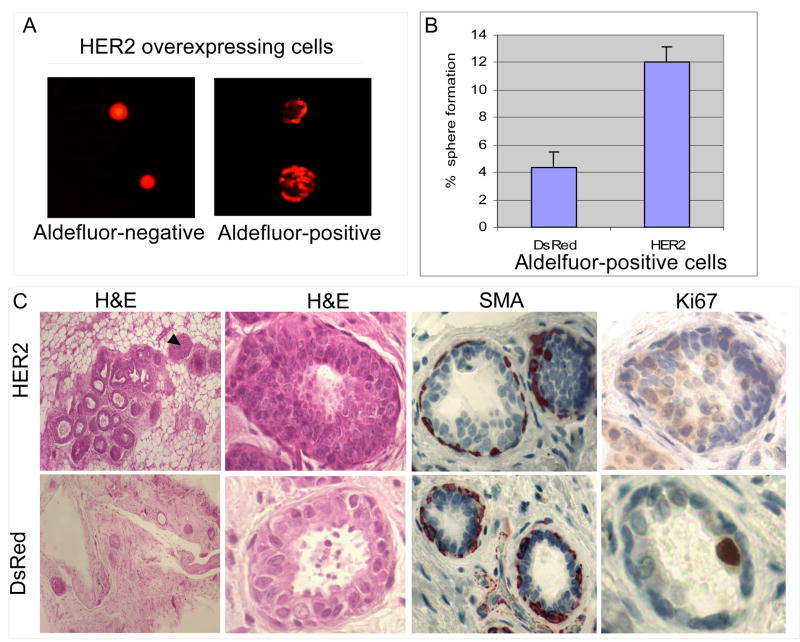
Figure 2. HER2 expression increases the frequency of mammosphere formation and of ductal structures in NOD-SCID mice.
A. When plated at clonal density, only the Aldefluor-positive cells are capable of mammosphere formation. B. A three-fold increase in mammosphere initiating single cells in the HER2 Aldefluor-positive cell population compared to the DsRed Aldefluor-positive population. C. Serial dilutions of HER2 or DsRed expressing NMECs were injected into the mammary fat pads of humanized NOD/SCID mice. HER2 overexpressing cells generated an increased number of mammary outgrowths which displayed hyperplasia (arrows) characterized by an 8 fold increase in Ki67 staining. Myoepithelial cells were demonstrated by smooth muscle actin (SMA) staining.
To determine whether HER2 overexpression increased the mammosphere forming capacity of the Aldefluor-positive population, we sorted single Aldefluor-positive cells expressing HER2 or DsRed in low attachment plates and examined the frequency of mammosphere formation. Figure 2B demonstrates a three-fold increase in the frequency of single HER2 expressing cells able to form mammospheres as compared to single DsRed cells. Together, these experiments suggest that HER2 overexpression increases the mammary stem cell population and also increases the capacity for sphere formation in this population.
We previously demonstrated that Aldefluor-positive normal human mammary cells have the capacity to generate outgrowths in the humanized mammary fat pads of NOD-SCID mice (Ginestier et al., 2007). We determined the effect of HER2 overexpression on mammary outgrowths in this model. Outgrowths generated from both control and HER2 overexpressing cells were composed of luminal and myoepithelial cells (Figure 2C). The myoepithelial cell layer was demonstrated by smooth muscle action staining (Figure 2C). In addition, there were areas of hyperplasia characterized by greater than an eight-fold increase in expression of the proliferation marker KI-67 in outgrowths generated from HER2 infected compared to DsRed cells (Figure 2C). We also tested the ability of Aldefluor-positive and Aldefluor-negative cells overexpressing HER2 or DsRed to form mammary outgrowths. Consistent with our previous report, only Aldefluor-positive cells produced outgrowths in this model (Table 1). Furthermore, HER2 overexpressing Aldefluor-positive cells produced outgrowths with as few as 250 cells (Table 1). These results are consistent with the in vitro experiments demonstrating that HER2 overexpression increases the stem cell pool as well as driving proliferation of this pool in vivo.
Table 1. Ductal outgrowths generated from HER2 or DsRed expressing cells.
Normal mammary epithelial cells infected with HER2 or DsRed lantiviruses were introduced into the humanized mammary fatpads of NOD-SCID mince. We observed a three fold increase in the number of outgrowths generated by HER2 overexpressing cells compared to dsRed controls. As few as 250 Aldefluor-positive HER2 expressing cells formed outgrowths while Aldefluor-negative HER2 or DsRed expressing cells failed to form outgrowths. The results represent mean of three independent experiments.
| Cell # | Unsorted | Aldefluor+ | Aldefluor− | ||||
|---|---|---|---|---|---|---|---|
| 10K | 5K | 5K | 500 | 250 | 5K | 250 | |
| DsRed | 8 | 2 | 4 | 0 | 0 | 0 | 0 |
| HER2 | 23 | 11 | 53 | 5 | 3 | 0 | 0 |
HER2 overexpression increases tumorigenicity of mammary carcinomas by modulating the stem/progenitor cell population
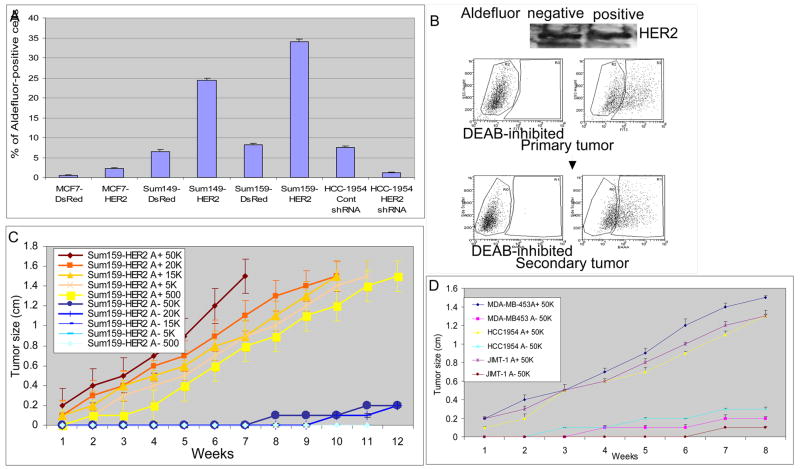
In order to determine the effect of HER2 overexpression on the cancer stem cell population, we stably infected three mammary carcinoma cell lines; MCF7, SUM149, and SUM159 with HER2 or DsRed lentiviruses. The efficiency of infection was demonstrated by Western blotting (Supplemental Figure 2A, B and C). HER2 overexpression resulted in a four to five-fold increase in the Aldefluor-positive population in each of these three mammary carcinoma cell lines (Figure 3A, Supplemental Figure 2A, B and C).
Figure 3. Effects of HER2 overexpression on Aldefluor-positivity and tumorigenicity of human mammary carcinoma cell lines.
A. Overexpression of HER2 increased the Aldefluor-positive populations three- to five-fold compared to control DsRed cells in indicated breast cancer cell lines. Knockdown of HER2 in HCC1954 cells using HER2 siRNA decreased the Aldefluor-positive cell population. B. Aldefluor-positive but not Aldefluor-negative cells display properties of tumor stem cells despite equivalent HER2 expression in both populations as shown by Western blotting. Tumors generated from the Aldefluor-positive population are composed of both Aldefluor-positive and -negative populations recapitulating the initial tumor phenotype. C. Growth curves of serial dilutions of Aldefluor-positive and -negative SUM159-HER2 cells. Aldefluor-positive cells form tumors at all cell concentrations whereas the Aldefluor-negative cells failed to do so. D. In HER2 amplified cell lines MDA-MB453, HCC1954 and JIMT-1, only Aldefluor-positive cells were tumorigenic.
To further examine whether continued HER2 expression was required for maintenance of the stem cell phenotype, we tested the effect of knockdown of HER2 by using a HER2 shRNA in HCC1954 cells, a cell line which displays HER2 amplification (Finn et al., 2007). We achieved approximately an 80% reduction in HER2 expression as assessed by Western blot utilizing this approach (Supplemental Figure 2D). Control shRNA transfected cells contained an Aldefluor-positive population of 6–10%, whereas knockdown of HER2 expression reduced the Aldefluor-positive population to less than 2% (Figure 3A). These results complement the gain of function studies and suggest that in HER2 amplified cells continued HER2 expression is required for maintenance of the stem cell population.
To determine if the effects of HER2 overexpression on tumorigenicity occurred in all transfected cells or was limited to stem/progenitor cells, we examined the ability of Aldefluor-positive and negative populations to form tumors in the humanized mammary fat pads of NOD/SCID mice. We demonstrated equal HER2 total and surface expression in Aldefluor-positive and Aldefluor-negative cell populations (Figure 3B and Supplemental Figure 3A) Despite equal levels of HER2 expression in Aldefluor-positive and negative SUM159-HER2 cells, only the Aldefluor-positive cells were tumorigenic in this model (Figure 3B and C). Figure 3C illustrates the ability of serial dilutions of Aldefluor-positive and negative populations of HER2 overexpressing cells to form tumors. As few as 500 Aldefluor-positive cells, reproducibly formed tumors whereas only limited tumor growth was seen when up to 50,000 Aldefluor-negative cells were injected. As was the case for MCF7-HER2 cells, we found that only the Aldefluor-positive population of the MCF7-HER2 cells was tumorigenic (Supplementary Figure 4A). The ability of Aldefluor-positive but not Aldefluor-negative cells to form tumors was also demonstrated in three HER2-amplified breast cancer cell lines (Figure 3D). Similar level of HER2 surface expression in Aldefluor-positive and Aldefluor-negative cell populations were demonstrated by flow cytometry and immunohistochemistry (Supplemental Figure 3B and C). The Aldefluor-positive population was not only capable of self-renewal as demonstrated by serial transplantation, but was also able to regenerate the phenotypic heterogeneity of Aldefluor-positive and negative populations found in the initial tumor (Figure 3B). Thus, the Aldefluor-positive-HER2-positive cell population meets the functional definition of “cancer stem cells” in its ability to both self-renew and to differentiate regenerating the cellular heterogeneity present in the original tumor.
To determine whether HER2 overexpression increased the tumorigenicity of the cancer stem cell component, we examined the tumorigenicity of the Aldefluor-positive populations of SUM159-DsRed or SUM159-HER2 cells. HER2 overexpression increased the rate of tumor growth in Aldefluor-positive cells approximately two-fold compared to control Aldefluor-positive cells (Supplemental 4B). Together, these results demonstrate that the effects of HER2 on tumorigenicity are due to its effects on the Aldefluor-positive cancer stem cell component. HER2 overexpression expands this population as well as increasing its tumorigenicity.
HER2 overexpression increases invasiveness of the stem/progenitor cell population
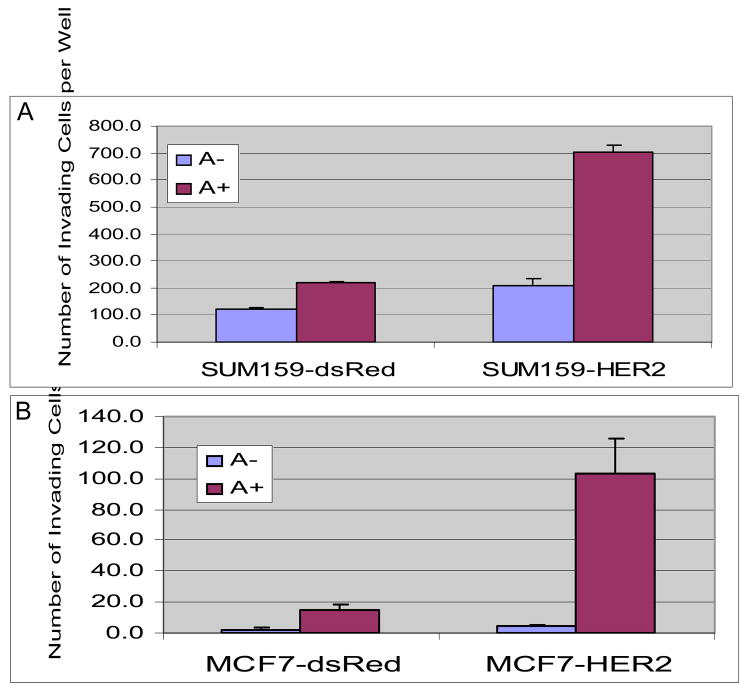
Previous studies have demonstrated that HER2 overexpression leads to increased invasion in in vitro matrigel assays. It has been suggested that tumor invasion and metastasis may be mediated by the cancer stem cell population (Sheridan et al., 2006; Wicha et al., 2006). To determine whether the effect of HER2 overexpression was due to a direct effect on tumor invasion or alternatively was due to an increase in cancer stem cells, we tested the ability of Aldefluor-positive and Aldefluor-negative populations of HER2 or DsRed infected MCF7 and Sum159 cells to invade matrigel. The Aldefluor-positive subpopulations were considerably more invasive than Aldefluor-negative populations (Figure 4A and B). Furthermore, overexpression of HER2 resulted in a three to six-fold increase in invasiveness in the Aldefluor-positive populations. These results suggest that HER2 overexpression not only increases the Aldefluor-positive stem cell component but also increases the invasiveness of this population.
Figure 4. Effects of HER2 overexpression on invasive properties of Aldelfuor-positive and -negative populations of breast cancer cell lines.
Aldefluor-positive populations from Sum159-DsRed, SUM159-HER2 (A), MCF7-DsRed or MCF7-HER2 cells (B) showed significantly higher invasiveness compared to Aldefluor-negative populations. Furthermore, HER2 overexpression resulted in a 5 to 6-fold increase in the invasive potential of Aldefluor-positive cells.
HER2 overexpression induces the expression of stem cell related genes and activates the PI3-K/Akt pathway
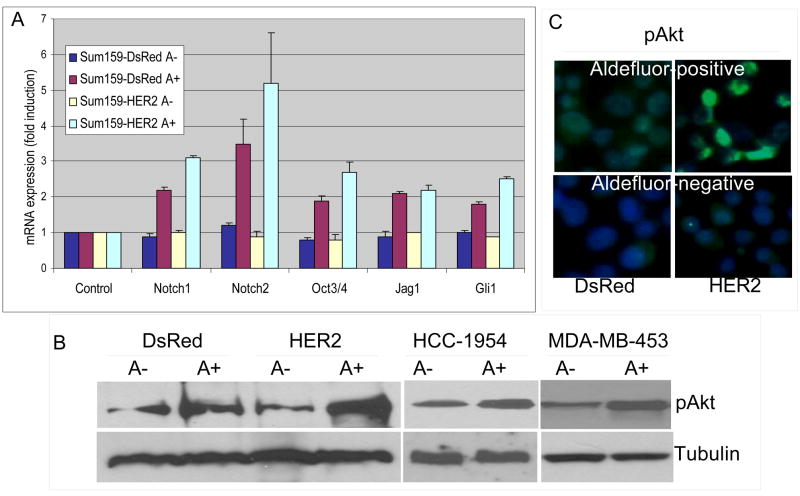
A number signaling pathways have been implicated in stem cell self-renewal (12). To determine whether HER2 affected the expression of genes involved in stem cell behavior, we examined the expression of Oct3/4, Notch1, Notch2, Jag1 and Gli1 in Aldefluor-positive and negative populations from SUM159-HER2 or DsRed cells. Expression of these stem cell related genes was significantly increased in Aldefluor-positive compared to Aldefluor-negative cell populations (Figure 5A). Furthermore, there was a significant increase in the expression of these genes in SUM159-HER2-Aldefluor-positive cells compared to Aldefluor-positive-DsRed cells. This suggests that HER2 overexpression not only increases the stem cell pool, but also increases the expression of stem cell related genes within this cell population.
Figure 5. Expression of stem cell related genes and Akt phosphorylation.
A. Aldefluor-positive cells expressed higher levels of all indicated stem cell related genes than Aldefluor-negative cells. The expression of these genes was further elevated by HER2 overexpression. B. Aldefluor-positive populations of indicated breast cancer cell lines showed higher Akt-phosphorylation when compared to Aldefluor-negative populations. C. Akt-phosphorylation as assessed by immunoflourescence in Aldefluor-positive and –negative SUM159-DsRed and SUM159-HER2 cells. Increased phospho-Akt is detected in Aldefluor-positive and HER2 overexpressing cells.
HER2 has been shown to signal through the PI3-kinase/Akt pathway. In order to determine the relationship between activation of this pathway and the stem cell phenotype, we examined the levels of Akt phosphorylation by Western blot in Aldefluor-positive and -negative cell populations isolated from control or HER2 overexpressing cell lines. Aldefluor-positive cells showed significantly higher Akt phosphorylation compared to Aldefluor-negative cells isolated from the same cell line (Figure 5B). Furthermore, the Aldefluor-positive population from SUM159-HER2 showed increased levels of phospho-Akt compared to the Aldefluor-positive population from SUM159-DsRed cells. These results were further confirmed by phospho-Akt immunoflourescence staining of Aldefluor-positive and -negative SUM159-DsRed and SUM159-HER2 cells (Figure 5C).
Trastuzumab decreases the Aldefluor-positive population in sensitive but not in resistant breast cancer cells through inhibition of PI3-K Akt signaling
We examined the effect of trastuzumab on the Aldefluor-positive populations in several breast cancer cell lines. Figure 6A (and Supplemental 5C) demonstrates that trastuzumab treatment resulted in a greater than 80% reduction in the population of Aldefluor-positive SUM159-HER2 cells. Interestingly, treatment of SUM159-HER2 cells decreased the Aldefluor-positive cell population to the same level found in DsRed control cells suggesting that this antibody inhibited HER2 signaling to baseline levels. The ability of trastuzumab to reduce the percent of stem cells was also confirmed by immunohistochemistry utilizing an ALDH-1 monoclonal antibody (Supplemental Figure 5D). We found approximately 50% reduction in HER2 surface expression following trastuzumab treatement of SUM159-HER2 cells (Supplemental Figure 3A). When we analyzed whether HER2 surface expression was equally reduced in both Aldefluor-positive and Aldefluor-negative populations, we found no significant difference in HER2 surface expression in both populations (Supplemental Figure 3A). In order to examine trastuzumab-induced cell death, we analyzed apoptosis following trastuzumab treatment using Annexin V staining. Trastuzumab did not induce apoptosis in these cells as assessed by flow cytometry (Supplemental Figure 6A).
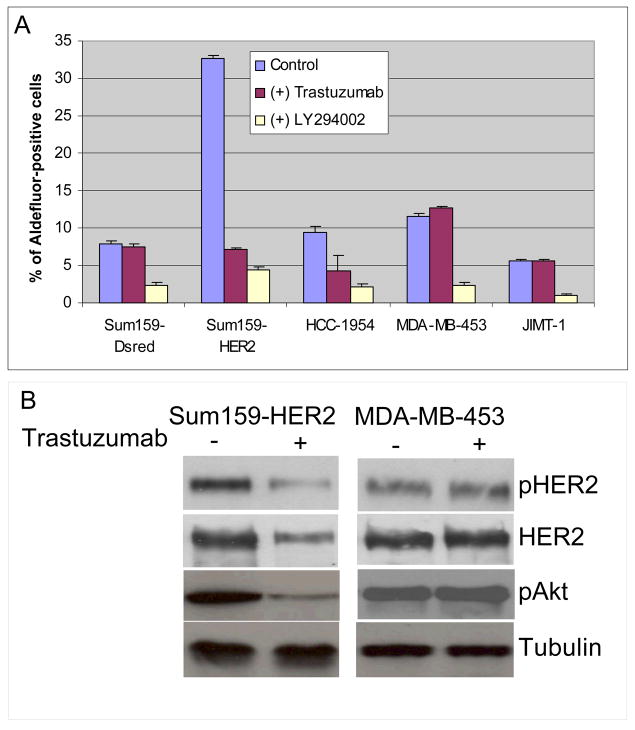
Figure 6. Effects of trastuzumab and LY294002 on the Aldefluor-positive population in breast cancer cell lines.
A. Trastuzumab reduced the proportion of Aldefluor-positive cells by as much as 80% in SUM159-HER2 and HCC1954 cells. In contrast, trastuzumab had no effect on the Aldefluor-positive population in MDA-MB-453 and JIMT-1 trastuzumab resistant cell lines. In contrast, LY294002 reduced the proportion of Aldefluor-positive cells in all cell lines. (B) Trastuzumab reduced phospho-HER2 and phospho-AKT expression in Sum159-HER2 cells but has no effect on expression of these proteins in trastuzumab resistant MDA-MB-453 cells.
To determine whether there was a correlation between trastuzumab’s effects on the stem cell phenotype and cellular sensitivity to this antibody, we examined the effect of trastuzumab on the Aldefluor-positive populations of HER2-amplified breast cancer cell lines, one HCC1954 which is trastuzumab sensitive, and MDA-MB-453 and JIMT-1 which are trastuzumab resistant. Trastuzumab reduced the Aldefluor-positive population more than 50% in trastuzumab sensitive HER2 amplified HCC-1954 cells (Figure 6A). In contrast, the antibody had no effect on the percent of Aldefluor-positive cells in the transtuzumab resistant cell lines MDA-MB-453 and JIMT-1 (Figure 6A, Suplementary Figure 5A and B).
In order to determine whether trastuzumab resistance was associated with Akt phosphorylation, we examined the effects of trastuzumab on Akt phosphorylation in trastuzumab sensitive and resistant HER2 overexpressing cells. As shown in Figure 6B, trastuzumab reduced Akt phosphorylation in the trastuzumab sensitive SUM159-HER2 cell line, but had no effect on Akt phosphorylation in the trastuzumab resistant MDA-MB-453 cell line, which displays constitutive Akt activation (Figure 6B).
Since HER2 activates the PI3-K/Akt pathway, we examined the effect of the PI3-K inhibitor LY-294002 on the Aldefluor-positive population in these cell lines. Treatment of trastuzumab sensitive and resistant cell lines with LY-294002 resulted in a significant decrease in the Aldefluor-positive populations (Figure 6A). Furthermore PI3-K inhibitor LY294002 induced apoptosis in 50% of cells as assessed by Annexin V staining (Supplemental Figure 6B).
Discussion
The “cancer stem cell hypothesis” proposes that cancers originate in tissue stem or progenitor cells through disregulation of the normally tightly regulated process of self-renewal. As a consequence cancers are composed of a cellular hierarchy with a limited number of “cancer stem cells” capable of self-renewal driving the malignant phenotype and differentiation generating tumor heterogeneity (Korkaya and Wicha, 2007). A frequent genetic alteration in human breast cancer is the amplification of the HER2 gene (Slamon et al., 1987). Although HER2 overexpression has been demonstrated to affect tumor growth, invasion and metastasis, the mechanisms involved remain undefined. We provide evidence that the effects of HER2 on mammary carcinogenesis, tumorigenicity and invasion result from effects of this signaling pathway on the cancer stem cell population. Overexpression of HER2 in normal mammary epithelial cells increases the stem/progenitor cell population as demonstrated by increased ALDH activity and mammosphere formation in vitro and generation of hyperplastic lesions in NOD/SCID mice. In contrast, Aldefluor-negative HER2 expressing cells neither formed mammospheres nor produced outgrowths in humanized NOD-SCID mice.
In women, HER2 overexpression frequently occurs in premalignant lesions including atypical ductal hyperplasia (ADH) and ductal carcinoma in situ (DCIS), suggesting that dysregulation of HER2 signaling plays a role in tumor initiation. Our findings suggest that HER2 amplification may lead to an expansion of mammary stem cells, providing targets for further carcinogenic events. This model could account for the demonstrated clonality of ADH and DCIS (Allred et al., 1994; Park et al., 2006; Xu et al., 2002). HER2 overexpression may also affect the behavior of mammary carcinomas by expanding their “cancer stem cell” populations. We show that overexpression of HER2 in a series of human breast cancer cell lines increases the percentage of cells with ALDH activity. The functional significance of this was demonstrated by the ability of Aldefluor-positive/HER2 cells to form tumors which could be serially passaged in NOD/SCID mice. Despite equivalent levels of HER2 expression in Aldefluor-positive and negative cell populations, Aldeflour-negative cells failed to form tumors even with 100-fold more cells. HER2 overexpression also increased the tumorigenicity within the Aldefluor-positive population. These experiments suggest that HER2 increases the cancer stem cell population as well as increasing tumorigenicity within this population. In contrast, HER2 overexpression does not affect the phenotype of more differentiated Aldefluor-negative cells.
A number of genes and signaling pathways have been identified which regulate the self-renewal of stem cells in a variety of developmental systems. These include Oct3/4 and genes involved in the Notch and Hedgehog signaling pathways (Dontu et al., 2004; Liu et al., 2006; Tokuzawa et al., 2003). We demonstrate that these genes show increased expression in Aldefluor-positive as compared to Aldefluor-negative Sum159 cells. Furthermore, Aldefluor-positive HER2-positive cells showed increased expression of these stem cell genes compared to Aldefluor-positive DsRed control cells. This suggests that in addition to increasing the Aldefluor-positive population, HER2 overexpression may also drive expression of stem cell regulatory genes within this population. HER2 has been shown to signal through the PI3-K Akt pathway generating a multidrug resistance phenotype (Knuefermann et al., 2003). We found that the Aldefluor-positive cell population displayed significantly higher Akt phosphorylation than Aldefluor-negative cells. AKT phosphorylation was further increased by HER2 overexpression in this cell population.
A recent report suggested that the subpopulation of breast cancer cells, previously characterized by the stem cell phenotype CD44+/CD24− (Al-Hajj et al., 2003) show higher levels of proinvasive genes and exhibit increased invasive properties (Sheridan et al., 2006). We demonstrate here that HER2 overexpression increases the invasiveness of both MCF7 and SUM159 cells. Furthermore, the Aldefluor-positive populations are significantly more invasive than the Aldefluor-negative populations. These results suggest that HER2 overexpression may have a direct effect on tumor invasion as well as an indirect effect resulting from increases in the invasive cancer stem cell population.
In the metastatic setting, trastuzumab increases the effectiveness of chemotherapy resulting in an increased response rate and duration of response (Burstein et al., 2001). Recent evidence suggests that CD44+/CD24− breast cancer stem cells may be relatively resistant to both chemotherapy and radiation. In contrast to the resistance of cancer stem cells to these conventional treatments, our results suggest that the effectiveness of trastuzumab may be directly related to its effects on the cancer stem cell population. A recent report demonstrating that the HER2/EGFR inhibitor, Lapatinib when used in neoadjuvant setting decreases the cancer stem cell component supports the clinical relevance of our findings (Li et al., 2008).
Despite the significant clinical benefits of trastuzumab, many patients treated in the adjuvant setting still relapse and most patients with advanced disease become trastuzumab-resistant within one year (Paik et al., 1990; Slamon et al., 1987). The development of secondary mutations such as deletion of the tumor suppressor PTEN may contribute to trastuzumab resistance (Nagata et al., 2004; Saal et al., 2007). We demonstrate that treatment of SUM159-HER2 cells with trastuzumab results in an 80% reduction of the Aldefluor-positive cell population. The effect of trastuzumab on Sum159-HER2 cells was associated with decreased expression of phospho-HER2 and phospho-Akt. In contrast to its effect on SUM159-HER2 cells, trastuzumab had no measurable effect on phospho-HER2, phopho-Akt, or on the proportion of the Aldefluor-positivity in two trastuzumab resistant cell lines. However, the PI3-K inhibitor LY294002 reduced the Aldefluor-positive population in both trastuzumab sensitive and resistant cell lines. These findings are consistent with previous reports showing increased Akt activity in trastuzumab-resistant cell lines (Kucab et al., 2005; Tanner et al., 2004) and suggest that the PI3-K/Akt pathway plays an important role in mediating the effects of HER2 signaling. It is also consistent with recent reports suggesting a role for Akt signaling in stem cell self-renewal (Welham et al., 2007) and in mediating treatment resistance (Ma et al., 2007; Saal et al., 2007). Most recently, Berns et al. identified the PI3K pathway as a major determinant of trastuzumab resistance in human breast tumors using a functional genomic approach (Berns et al., 2007).
In conclusion, our results suggest that the effects of HER2 amplification on mammary carcinogenesis, tumorigenicity and invasion result from effects of this signaling pathway on mammary cancer stem cells. These effects are due to the ability of HER2 to expand the stem/progenitor cell population as well as increasing the tumorigenicity and invasiveness of this population. Carcinogenesis may be initiated by expansion of stem cell pools which provide targets for further carcinogenic events. Increased HER2 expression in breast cancers, in turn, may increase the cancer stem cell component driving tumorigenesis invasion and metastasis. An elucidation of the molecular mechanisms by which HER2 signaling drives the stem cell pool may lead to further insights into the behavior of normal and malignant mammary stem cells. In addition, identification of components of these pathways may lead to new targets for cancer prevention and therapy.
Materials and Methods
Dissociation of normal mammary tissue and mammosphere assay
Mammary tissues from reduction mammoplasties was dissociated as previously described (Stingl et al., 1998). For mammosphere experiments, single cell suspensions of NMEC were plated on 1% agarose coated plates at a density of 1×105 and grown for 7–10 days. Subsequent cultures after dissociation of primary spheres were plated on ultra-low attachment plates at a density of 5×103 to 1×104. Mammosphere cultures were grown in a serum-free mammary epithelium basal medium as previously described (Dontu et al., 2003).
Lentiviral construction and cell infections
All lentiviral constructs were prepared by the University of Michigan Vector Core facility. The pLentiLox RSV-GFP-HER2 construct was generated by ligating the NheI & XbaI HER2 fragment from pCI-HER2 (generous gift from K. Ignatoski, Urology Medical School, University of Michigan Ann Arbor, MI) into NheI site of pLentiLox RSV lentiviral-GFP vector. After confirmation by restriction digestion and DNA sequencing, large scale pLentiLox-HER2 virus was produced and used for infecting NMECs and breast cancer cell lines. pLentiLox RSV- dsRed-HER2 vector was generated by replacing the GFP with a DsRed insert.
Implantation of cells in NOD/SCID mice
Fat pads of three weeks old NOD/SCID mice were cleared and replaced with 1:1 mixture of irradiated and non-irradiated human fibroblasts. Within two to three weeks, human fibroblasts generated a humanized mammary fat pad in mice. These resulting fat pads were injected with NMECs infected with DsRed or HER2 lentivirus. An estrogen pellet was also implanted subcutaneously in each mouse. The mice were sacrificed after 4 to 8 weeks and the fat pads were analyzed for outgrowths.
Breast cancer cells at serial dilutions were directly injected into the fat pads of 5 weeks old NOD/SCID mice with an estrogen pellet implantation. The kinetics of tumor growth in these mice were analyzed at indicated time points.
Aldefluor Assay and Flow cytometry
To measure and isolate cells with high ALDH activity, the Aldefluor assay was performed according to manufacturer’s (Stemcell Technologies, Durham, NC) guidelines. Dissociated single cells were suspended in Aldefluor assay buffer containing the ALDH substrate, Bodipy-aminoacetaldehyde (BAAA) at 1,5 μM and incubated for 40 minutes at 37 °C. To distinguish between ALDH-positive and -negative cells, a fraction of cells was incubated under identical condition in the presence of a 10-fold molar excess of the ALDH inhibitor, diethylamino benzaldehyde (DEAB). This results in a significant decrease in the fluorescence intensity of ALDH-positive cells and was used to compensate the flow cytometer.
Cell lines and HER2, DsRed lentiviral infection
MDA-MB-453 and HCC1954 cells were maintained in RPMI supplemented with 10% fetal bovine serum and antibiotic/antimycotic (10.000 units/ml penicillin G sodium, 10.000 μg/ml streptomycin sulfate and 25 μg/ml amphotericin B) and the JIMT-1 cells were maintained in DMEM supplemented with 10% serum and antibiotic/antimycotic. SUM159, SUM149 and MCF7 cells were infected with the lentivirus expressing human HER2 or control lentiviruses expressing either GFP or DsRed. Following 12–16 hours of incubation, the viruses were removed and replaced with fresh medium.
Immunoblotting
Cells were lysed in a laemmli buffer, boiled and loaded onto SDS-polyacrylamide gels. Following electrophoresis, proteins were transferred onto Nitrocellulose Membranes (Pierce, Rockford, IL) using semi dry Trans-Blot (Bio Rad Laboratories, Hercules, CA). Blots were first incubated in TBS blocking buffer containing either 2% Milk or 2% BSA (for Phospho-specific antbodies) for 1–2 hours at room temperature and then with the respective primary antibodies diluted in TBST (containing 0.1%Tween20 and 2% BSA) overnight at 4°. Subsequently, blots were washed and incubated with appropriate secondary antibodies (GE Healthcare, UK) in TBST and detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). Antibodies to human HER2, c-erbB-2/HER-2/neu Ab-17 (Clone e2–4001+3B5) mouse monoclonal antibody and anti-phospho-erbB-2/HER-2 (Tyr1248) antibody were from Upstate (Temecula, CA), and the phospho-Akt1 (p-Akt1/2/3 (Ser 473) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunohistochemisty and immunofluorescent staining were carried out as described previously (6)
Supplementary Material
HER2 surface expression in control and HER2 lentivirus infected cells. A. HER2 lentivirus infected cells were analyzed by flow cytometry based on DsRed expression demonstrating greater than 90% lentivirus infection efficiency. B. A small fraction of normal mammary epithelial cells show HER2 surface expression while more than 90% cells display HER2 surface expression in HER2 lentiviral infected cells. C. Aldefluor-positive and Aldeflluor-negative cells show equal HER2 surface expression as assessed by flow cytometry.
HER2 overexpression increases the Aldefluor-positive cell population in three mammary carcinoma cells lines. HER2 expression in MCF7-HER2 (A), SUM149-HER2 (B), SUM159-HER2 (C) and their respective parental lines as assessed by Western blotting 48 hours post-transfection. A, B and C. All three stable HER2 overexpressing lines demonstrated greater than a three-fold increase in the Aldefluor-positive populations compared to parental non-HER2 expressing cell lines. D. HER2-amplified breast cancer cells consist of about 7% Aldefluor-positive population and the knock-down of HER2 using shRNA decreased Aldefluor-positive cell population to less than 2%.
HER2 surface expression in Aldefluor-positive and Aldefluor-negative populations of breast cancer cell lines. A. SUM159-HER2 breast cancer cell lines were analyzed for HER2 surface expression in both Aldefluor-positive and Aldefluor-negative cell populations before and after trastuzumab treatment. More than 80% of control treated cells from both populations of SUM159-HER2 cells displayed HER2 surface expression. Following the trastuzmab treatment, we observed a 50% reduction in HER2 surface expression in both Aldefluor-positive and Aldefluor-negative cells. B. All the cells from both populations of HCC1954 showed HER2 surface expression. C. This was further demonstrated by HER2 immunohistochemical staining of Aldefluor-positive and Aldefluor-negative populations of HCC1954 sorted by Aldefluor assay.
Aldefluor-positive cells from Sum159-HER and MCF7-HER2 exhibit increased tumorigenicity compared to parental lines. A. Aldelfuor-positive MCF7-HER2 cell population but not –negative population are tumorigenic. B. The kinetics of tumor growth of Aldefluor-positive cells from Sum159-DsRed and SUM159-HER2 cells were tested. The Aldefluor-positive SUM159-HER2 cells showed a 2 fold increased tumorigenicity compared to SUM159-DsRed cells.
Inhibition of HER2 signaling with trastuzumab does not effect the Aldefluor-positive population in resistant breast cancer cell lines. Flow cytometry analyses showing that the Aldefluor-positive population in MDA-MB-453 (A)and JIMT-1 (B) breast cancer lines does not change following 7 days of trastuzumab treatment. C. and D. However, SUM159-HER2 cells are sensitive to trastuzumab as evidenced by a significant decrease in ALDH expression assessed by the Aldefluor assay an and immunoflourescent staining.
The PI3-K inhibitor LY294002 but not trastuzumab induce apoptosis in SUM159-HER2 cells. SUM159-HER2 cells were incubated with trastuzumab and apoptosis assessed by Annexin V and PI analysis by flow cytometry. Cells treated with trastuzumab showed no increased apoptosis compared to control treated cells. Whereas LY294002 treatement induced apoptosis in 50% of cells.
Acknowledgments
We would like to thank Dr. Thomas Giordano and the University of Michigan Cancer Center Flow Cytometry core for their assistance, Drs. Bruce Boman, Emmanuelle Charafe-Jauffret, Gabriela Dontu, Suling Liu, Christophe Ginestier for their advice and critical review of this manuscript. The HER2 construct is a generous gift from Dr. Ignatoski. This work was supported by NIH Grants CA129765 and CA101860 and in part by the University of Michigan Cancer Center NIH support Grant 5 P 30 CA46592.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred DC, O’Connell P, Fuqua SA, Osborne CK. Immunohistochemical studies of early breast cancer evolution. Breast Cancer Res Treat. 1994;32:13–8. doi: 10.1007/BF00666202. [DOI] [PubMed] [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Beuzeboc P, Scholl S, Garau XS, Vincent-Salomon A, Cremoux PD, Couturier J, et al. [Herceptin, a monoclonal humanized antibody anti-HER2: a major therapeutic progress in breast cancers overexpressing this oncogene?] Bull Cancer. 1999;86:544–9. [PubMed] [Google Scholar]
- Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2001;19:2722–30. doi: 10.1200/JCO.2001.19.10.2722. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–26. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur M, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–12. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Wicha MS. Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs. 2007;21:299–310. doi: 10.2165/00063030-200721050-00002. [DOI] [PubMed] [Google Scholar]
- Kucab JE, Lee C, Chen CS, Zhu J, Gilks CB, Cheang M, et al. Celecoxib analogues disrupt Akt signaling, which is commonly activated in primary breast tumours. Breast Cancer Res. 2005;7:R796–807. doi: 10.1186/bcr1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu M-F, et al. Therapeutic resistance and tumor-initiation: Molecular pathways involved in breast cancer stem cell self-renewal. J Natl Cancer Inst. 2008 in press. [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133(+) HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2007 doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- Miller KD. The role of ErbB inhibitors in trastuzumab resistance. Oncologist. 2004;9(Suppl 3):16–9. doi: 10.1634/theoncologist.9-suppl_3-16. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–12. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- Park K, Han S, Kim HJ, Kim J, Shin E. HER2 status in pure ductal carcinoma in situ and in the intraductal and invasive components of invasive ductal carcinoma determined by fluorescence in situ hybridization and immunohistochemistry. Histopathology. 2006;48:702–7. doi: 10.1111/j.1365-2559.2006.02403.x. [DOI] [PubMed] [Google Scholar]
- Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–9. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–95. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Kuusk U, Emerman JT. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation. 1998;63:201–13. doi: 10.1111/j.1432-0436.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- Tanner M, Kapanen AI, Junttila T, Raheem O, Grenman S, Elo J, et al. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther. 2004;3:1585–92. [PubMed] [Google Scholar]
- Tokuzawa Y, Kaiho E, Maruyama M, Takahashi K, Mitsui K, Maeda M, et al. Fbx15 is a novel target of Oct3/4 but is dispensable for embryonic stem cell self-renewal and mouse development. Mol Cell Biol. 2003;23:2699–708. doi: 10.1128/MCB.23.8.2699-2708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welham MJ, Storm MP, Kingham E, Bone HK. Phosphoinositide 3-kinases and regulation of embryonic stem cell fate. Biochem Soc Trans. 2007;35:225–8. doi: 10.1042/BST0350225. [DOI] [PubMed] [Google Scholar]
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–90. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895–6. [DOI] [PubMed] [Google Scholar]
- Xu R, Perle MA, Inghirami G, Chan W, Delgado Y, Feiner H. Amplification of Her-2/neu gene in Her-2/neu-overexpressing and -nonexpressing breast carcinomas and their synchronous benign, premalignant, and metastatic lesions detected by FISH in archival material. Mod Pathol. 2002;15:116–24. doi: 10.1038/modpathol.3880503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HER2 surface expression in control and HER2 lentivirus infected cells. A. HER2 lentivirus infected cells were analyzed by flow cytometry based on DsRed expression demonstrating greater than 90% lentivirus infection efficiency. B. A small fraction of normal mammary epithelial cells show HER2 surface expression while more than 90% cells display HER2 surface expression in HER2 lentiviral infected cells. C. Aldefluor-positive and Aldeflluor-negative cells show equal HER2 surface expression as assessed by flow cytometry.
HER2 overexpression increases the Aldefluor-positive cell population in three mammary carcinoma cells lines. HER2 expression in MCF7-HER2 (A), SUM149-HER2 (B), SUM159-HER2 (C) and their respective parental lines as assessed by Western blotting 48 hours post-transfection. A, B and C. All three stable HER2 overexpressing lines demonstrated greater than a three-fold increase in the Aldefluor-positive populations compared to parental non-HER2 expressing cell lines. D. HER2-amplified breast cancer cells consist of about 7% Aldefluor-positive population and the knock-down of HER2 using shRNA decreased Aldefluor-positive cell population to less than 2%.
HER2 surface expression in Aldefluor-positive and Aldefluor-negative populations of breast cancer cell lines. A. SUM159-HER2 breast cancer cell lines were analyzed for HER2 surface expression in both Aldefluor-positive and Aldefluor-negative cell populations before and after trastuzumab treatment. More than 80% of control treated cells from both populations of SUM159-HER2 cells displayed HER2 surface expression. Following the trastuzmab treatment, we observed a 50% reduction in HER2 surface expression in both Aldefluor-positive and Aldefluor-negative cells. B. All the cells from both populations of HCC1954 showed HER2 surface expression. C. This was further demonstrated by HER2 immunohistochemical staining of Aldefluor-positive and Aldefluor-negative populations of HCC1954 sorted by Aldefluor assay.
Aldefluor-positive cells from Sum159-HER and MCF7-HER2 exhibit increased tumorigenicity compared to parental lines. A. Aldelfuor-positive MCF7-HER2 cell population but not –negative population are tumorigenic. B. The kinetics of tumor growth of Aldefluor-positive cells from Sum159-DsRed and SUM159-HER2 cells were tested. The Aldefluor-positive SUM159-HER2 cells showed a 2 fold increased tumorigenicity compared to SUM159-DsRed cells.
Inhibition of HER2 signaling with trastuzumab does not effect the Aldefluor-positive population in resistant breast cancer cell lines. Flow cytometry analyses showing that the Aldefluor-positive population in MDA-MB-453 (A)and JIMT-1 (B) breast cancer lines does not change following 7 days of trastuzumab treatment. C. and D. However, SUM159-HER2 cells are sensitive to trastuzumab as evidenced by a significant decrease in ALDH expression assessed by the Aldefluor assay an and immunoflourescent staining.
The PI3-K inhibitor LY294002 but not trastuzumab induce apoptosis in SUM159-HER2 cells. SUM159-HER2 cells were incubated with trastuzumab and apoptosis assessed by Annexin V and PI analysis by flow cytometry. Cells treated with trastuzumab showed no increased apoptosis compared to control treated cells. Whereas LY294002 treatement induced apoptosis in 50% of cells.